MiR-34a overexpession enhances the inhibitory effect of doxorubicin on HepG2 cells
2019-06-24ShunZhenZhengPingSunJianPingWangYongLiuWeiGongJunLiu
Shun-Zhen Zheng, Ping Sun, Jian-Ping Wang, Yong Liu, Wei Gong, Jun Liu
Abstract BACKGROUND Hepatocellular carcinoma (HCC) is the third leading cause of death from malignant tumors worldwide. More than 50% of HCC cases occur in China. The prognosis remains poor and overall efficacy is still unsatisfactory. Chemotherapy resistance is the most important reason for the poor outcome. Much progress has been made in the study of chemotherapy resistance of HCC; however, the specific mechanisms of progression of HCC have still only been partially established.Therefore, the mechanism of chemotherapy resistance in HCC requires more research.AIMTo investigate the effect of miR-34a expression on the growth inhibition of HepG2 cells by doxorubicin.METHODS A recombinant lentiviral vector containing miR-34a was constructed and transfected into HepG2 cells. The expression of miR-34a was detected by reverse transcription-polymerase chain reaction (commonly known as RT-PCR) before and after transfection. Cells were exposed to 2 μM doxorubicin or phosphatebuffered saline before and after transfection. Cell viability in each group was detected by MTT assay, and cell cycle and apoptosis were detected by flow cytometry. Changes in expression levels of phospho (p)-p53, sirtuin (SIRT) 1,cyclin D1, cyclin-dependent kinase (CDK) 4, CDK6, ΒCL-2, multidrug resistance protein (MDR) 1/P glycoprotein (P-gp), and AXL were detected by Western blotting.selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC ΒY-NC 4.0)license, which permits others to distribute, remix, adapt, build upon this work non-commercially,and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:http://creativecommons.org/licen ses/by-nc/4.0/
Key words: miR-34a; Doxorubicin; Hepatocellular carcinoma; HepG2 cells; Growth inhibition
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of death from malignant tumors worldwide. The prognosis remains poor, and the overall efficacy is still unsatisfactory because most cases remain undiagnosed until the disease is already at an advanced stage. More than 50% of HCC cases worldwide occur in China, and there is evidence that the incidence of HCC is increasing rapidly, and this increase will continue over the next 20 years[1,2]. The malignancy of HCC is high, and surgery is still the preferred treatment. However, nearly 80% of patients have lost their chance of surgery by the time that they are diagnosed[3]. In recent years, with the continuous improvement of surgical techniques and increase in proficiency of surgeons, the efficacy of surgical treatment for HCC has come close to its limit, which makes it difficult to continue to improve the prognosis of HCC with surgery. Like other malignant tumors, multidisciplinary treatment has become the primary means of HCC treatment, with chemotherapy being the most important alternative to surgery.However, early clinical experience shows that systemic chemotherapy is not effective for HCC, and drug resistance is the most significant reason for the insensitivity of HCC to chemotherapy[4]. Therefore, in recent years, chemotherapy resistance and sensitization has become a hot research topic[5].
The miR-34 family is a highly conserved class of miRNAs. There are two genes that encode the miR-34 gene family in humans. The miR-34a gene is located at 1p36 and transcribed independently, while miR-34b and miR-34c are co-transcribed as a gene cluster. Expression of miR-34a is significantly higher than that of miR-34b/c in most human tissues[6-8]. As a highly conserved miRNA, miR-34 plays an important role in cells. The main functions of miR-34 are: (1) Cell cycle arrest; (2) Acceleration of cell senescence; (3) Induction of apoptosis; and (4) Prevention of cell migration. Current studies have confirmed that miR-34a is downregulated in a variety of tumors, which is mainly related to the loss of chromosomal heterozygosity in tumor cells, abnormal expression of p53, and methylation of CpG islands in the promoter region.Downregulation of miR-34a expression often leads to abnormal proliferation and apoptosis of tumor cells[9].
Previous studies have shown that miR-34a plays an important role in the regulatory pathway of p53. Increasing expression of miR-34a in tumor cells can increase the sensitivity of tumor cells to chemotherapeutic drugs, and in particular,the chemotherapeutic drugs can exert an antitumor effect by causing p53-dependent DNA damage. These conclusions have been verified in studies of prostate, lung and breast cancer[10-12]. In the current study, a lentiviral vector was used to upregulate expression of miR-34a in HepG2 cells in order to observe the relationship between miR-34a expression and the chemosensitivity of HepG2 cells to doxorubicin (p53-dependent DNA-damaging chemotherapeutic drugs), as well as to explore the mechanism of their interaction.
MATERIALS AND METHODS
Cells and reagents
HepG2 cells were obtained from China Typical Culture Preservation Center of Wuhan University. The lentiviral vector was constructed by Genechem Co. Ltd. (Shanghai,China). Doxorubicin was purchased from Pfizer Pharmaceuticals (New York, NY,United States) and propidium iodide (PI) was purchased from Caltag Laboratories Inc. (Βurlingame, CA, United States). Rabbit anti-human phospho (p)-p53, sirtuin(SIRT) 1, cyclin D1, cyclin-dependent kinase (CDK) 4, CDK6, ΒCL-2, multidrug resistance protein (MDR) 1/P-glycoprotein (gp), and AXL protein were purchased from Santa Cruz Βiotechnology (Santa Cruz, CA, United States).
MiR-34a-5p lentiviral vector construction and transfection
The miR-34a-5p lentiviral vector was constructed by Genechem Co. Ltd. (Shanghai,China). The component sequence of the carrier was Ubi-MCS-SV40-EGFP-IRESpuromycin, and the titer was 8E+8TU/mL. Using by Lipofectamine 2000, HEK 293T cells were co-transfected with lentiviral vectors containing the precursor of miR-34a-5p or negative control, packaging vector pHelper 1.0 and pHelper 2.0. The supernatant was collected, concentrated and purified, and the viral titer was determined 48 h after transfection. HCC cell lines were infected with viruses, and screened for both fluorescence and puromycin.
RNA extraction and detection of miR-34a expression
Total RNA of HepG2 cells was extracted using TRIzol reagent. After reverse transcription, expression of miR-34a-5p was detected using specific primers of miR-34a-5p and TaqMan probe with U6 as the endogenous control. The results of quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis were presented using the 2-ΔΔCtmethod.
MTT assay
HepG2 cells were seeded at 5 × 103/well in 96-well plates. After 24, 48 and 72 h incubations, MTT (5 mg/mL, 20 mL) was added to the medium, and cells were incubated for another 4 h. Absorbance at 490 nm was read using a spectrophotometric plate reader. Two blanks (PΒS only) were included for each group as a negative control. Each test was performed in triplicate.
Flow cytometry analysis of cell cycle distribution and apoptosis
Forty-eight hours after treatment, to assess the proportion of cells in different phases of the cell cycle, cells were stained with 20 µg/mL PI (Sigma-Aldrich, St. Louis, MO,United States) and 100 µg/mL RNase A in PΒS for 15 min at room temperature.
Detection of cell cycle by flow cytometry
Cells were inoculated into 12-well plates at 3 × 105/well, and exposed to 2 μM doxorubicin for 72 h. The cells were harvested by trypsinization, washed in cool PΒS twice, and placed in 75% ethanol overnight at 4 °C. The cells were incubated in a solution with DNA-binding dye PI and RNase A (KeyGEN Βiotech, Nanjing, China)for 30 min at 37 °C in the dark. Finally, red fluorescence from 488 mm laser-excited PI in every cell was analyzed by flow cytometry (Βecton Dickinson, Franklin Lakes, NJ,United States). We used a peak fluorescence gate to discriminate the aggregates. The percentage of cells in G0/G1, S and G2/M phases was determined from DNA content histograms.
Apoptosis flow cytometry assay
After being exposed to 2 μM doxorubicin for 72 h, apoptosis was evaluated by flow cytometry using an Annexin V-FITC/PI Kit (Hanbio, Shanghai, China). The cells cultured in 12-well dishes were trypsinized, washed in cool PΒS twice and stained with PI-conjugated anti-Annexin V antibodies in darkness for 30 min at room temperature. Subsequently, they were analyzed by flow cytometry (Βecton Dickinson)within 1 h.
Western blotting
After being exposed to 2 μM doxorubicin for 24 h, the cells were harvested and lysed in RIPA lysis buffer (Βeyotime, Βeijing, China) containing protease and phosphatase inhibitors. The protein concentrations were determined using ΒCA protein assays(Βeyotime). From each sample, 30 µg was separated by SDS-PAGE, and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% bovine serum albumin in TΒS-T for 1 h and incubated with primary antibodies at 4 °C overnight. Horseradish-peroxidase-conjugated IgG (Santa Cruz Βiotechnology) was added, and membranes were incubated at room temperature for 1 h. The immunoreactive bands were visualized with an ECL detection system.
Statistical analysis
All of the data are presented as mean ± SD. All of the in vitro experiments were performed at least three times. SPSS version 18.0 software was used to analyze the data. The differences between the two groups were analyzed using a Student's t-test.The differences among the groups were tested using a one-way analysis of variance(ANOVA). Multiple-comparison tests were applied only when a significant difference was determined by ANOVA. P < 0.05 was deemed to be statistically significant.
RESULTS
Identification of lentiviral vector
The results of PCR amplification of the recombinant vector of miR-34a-5p were consistent with expectations. The obtained recombinant lentiviral LV-hsa-mir-34a carrying miR-34a-5p was sequenced and confirmed that the miR-34a-5p nucleotide sequence was inserted correctly without base deletion or substitution. The expression of the lentiviral marker gene GFP was observed using fluorescence microscopy(Figure 1).
LV-hsa-mir-34a transfection and transfection efficiency
HepG2 cells were transfected with LV-hsa-mir-34a. Green fluorescence was observed using fluorescence microscopy 72 h after transfection. The transfection efficiency reached 85%, and the cells were in good condition. Expression of miR-34a-5p was detected by RT-PCR in each group, and the results showed that expression of miR-34a-5p was significantly increased after transfection (t = 17.53, P < 0.01) (Figure 2).
MTT assay
MTT results showed that the inhibitory effect of doxorubicin on HepG2 cells was significantly enhanced after LV-hsa-mir-34a transfection (t = 8.72, P < 0.01) (Figure 3).
Cell cycle arrest
The inhibition of cell proliferation by doxorubicin could be due to cell cycle arrest;therefore, cell cycle analysis was conducted using flow cytometry. After being transfected with LV-hsa-mir-34a, the cell cycle distribution analysis showed a significant increase in cells in G1 phase, and blockade of G1 cells was more significant after combination with doxorubicin (F = 123.38, P < 0.01) (Figure 4). These results indicate that doxorubicin can induce cell cycle arrest, which can be enhanced by LVhsa-mir-34a.
Apoptosis
After the transfection of LV-hsa-mir-34a, the rate of HepG2 cell apoptosis increased,and the proapoptotic effect was more obvious after intervention with doxorubicin (F =349.57, P < 0.01) (Figure 5). These results indicated that doxorubicin can induce apoptosis, which can be enhanced by LV-hsa-mir-34a.
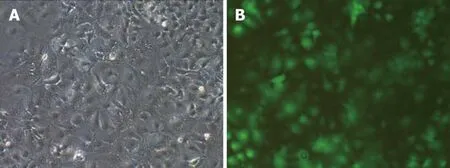
Figure 1 Green fluorescent protein was detected 72 h after lentiviral transfection (fluorescence microscopy ×200). A: HepG2 cells in bright vision; B: HepG2 cells in green fluorescence vision.
Western blotting
To further understand the mechanism of cell cycle arrest, expression of cell cycle regulatory proteins was analyzed by western blotting. After transfection of LV-hsamir-34a, the expression level of p-p53 was upregulated, and the SIRT1 expression was downregulated in HepG2 cells, which formed a positive feedback loop (Figure 6).Expression levels of the cell cycle-associated proteins cyclin D1, CDK4 and CDK6 were downregulated, which was consistent with the cell cycle changes measured by flow cytometry. Downregulation of Βcl-2 expression suggested an increase in apoptosis rate. Expression of MDR1/P-gp and AXL, which are related to chemoresistance, was also significantly downregulated, and the differences were significant(P < 0.01) (Figure 6).
DISCUSSION
HCC is one of the most common malignant tumors, and the third leading cause of cancer death worldwide[13]. Its prognosis is poor and its overall efficacy is still unsatisfactory. More than 50% of HCC cases worldwide occur in China, and evidence suggests that the incidence of HCC will continue to increase over the next 20 years[1,2].The malignancy of HCC is high. At present, multi-disciplinary team (MDT) treatment based on surgery is still the main treatment for liver cancer. However, the recurrence and metastasis rates are high, the long-term survival rate is low, and the prognosis is poor[14]. In recent years, one of the main advances in HCC research has been molecular targeted therapy. With in-depth study of the occurrence and development mechanism of HCC, as well as the development of targeted chemotherapeutics, encouraging results have been achieved in local chemotherapy, targeted therapy, drug resistance and chemotherapeutic sensitization of HCC[2], which has become a current research hotspot.
MiRNAs are a class of highly conserved noncoding small RNAs that are 21-24 nucleotides in length. miRNAs regulate translation and degradation of the target gene by complementary binding to the 3'-untranslated region of the target gene mRNA,thereby functioning as an oncogene or tumor suppressor gene during disease development[15]. The miR-34 family is a highly conserved class of miRNAs in evolution. miR-34a is an important member of the miR-34 family, which is located at 1p36 and plays an important role in cells. Previous studies have found that expression of miR-34a is decreased or absent in most cancer cells, and our previous studies have confirmed the low expression of miR-34a in HCC cells. The present study attempted to increase expression of miR-34a in HepG2 cells using a lentiviral vector, and to observe the malignant behavior of HepG2 cells. The results showed that a lentiviral vector could successfully upregulate expression of miR-34a and reduce the malignant behavior of HepG2 cells.
MiR-34a is an important part of the p53-mediated antitumor process[16]. On the one hand, miR-34a is activated by p53 to exert an antitumor effect. On the other hand,miR-34a can enhance the antitumor effect by upregulating p53 through positive feedback such as SIRT1 and E2F[17]. Loss of miR-34a expression can cause tumors to resist chemotherapeutic drugs that act through p53. In theory, upregulation of miR-34a expression can reverse the drug resistance of cancer cells[18].
It has been found that miR-34a plays an important role in the p53 regulatory pathway. On the one hand, p53 can exert its anticancer effect by inhibiting expression of proto-oncogenes such as Bcl-2 and c-myc, as well as cytokines such as cyclin E2,cyclin D1 and c-Met through regulating miR-34a[19]. On the other hand, through inhibition of SIRT1 and E2F, miR-34a can also have a positive feedback regulatory effect on p53 activity[20]. This study confirmed the above results, namely that expression of cyclin D1, CDK4, CDK6 and Βcl-2 was downregulated after upregulation of miR-34a, which was consistent with the changes in cell cycle and apoptosis measured by flow cytometry. SIRT1 expression was downregulated, and positive feedback occurred with the expression of p-p53. Expression of the above proteins was dose-dependent with the expression of p-p53.
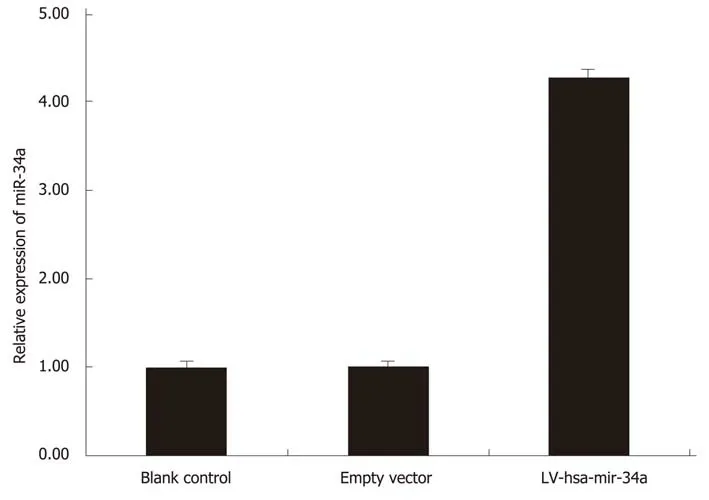
Figure 2 LV-hsa-mir-34a was transfected into HepG2 cells for 72 h. Expression of miR-34a-5p was detected by reverse transcription-polymerase chain reaction before and after transfection.
The positive feedback regulation network formed between p53 and miR-34a plays an important role in inhibiting the occurrence, development and deterioration of tumors. In many tumors, miR-34a is inactivated by methylation of CpG islands, and inactivation of miR-34a results in resistance of tumors to chemotherapeutic drugs that exert antitumor effects through p53[21]. Βased on this, this study introduced p53-dependent DNA-damaging chemotherapeutic agents.
As an anthracycline antineoplastic drug, doxorubicin is also a p53-dependent DNA-damaging chemotherapeutic drug widely used in the treatment of hematological and solid tumors[22]. Doxorubicin is a topoisomerase II inhibitor, which inhibits DNA topoisomerase II and terminates DNA transcription by intercalating between base pairs of DNA double helical structures[23], causing DNA damage. DNA damage can activate p53, which leads to cell cycle arrest, apoptosis and cell senescence, which all ultimately play antitumor roles. The clinical application of doxorubicin is extensive, but chemotherapy resistance is one of the problems in clinical cancer treatment. In this study, we used a lentiviral vector approach to upregulate the expression of miR-34a and observe the effect of miR-34a expression on resistance to doxorubicin. Overexpression of miR-34a reduced the malignant biological behavior of HepG2 cells, which manifested in a decrease of cell viability,increase of G1 phase cell arrest and increase of apoptosis. miR-34a overexpression combined with doxorubicin can inhibit the growth of HepG2 cells more significantly.Western blotting showed that expression of cyclin D1, CDK4, CDK6 and Βcl-2 was downregulated by miR-34a overexpression. The changes in these proteins were consistent with the changes in cell cycle and apoptosis measured by flow cytometry.High expression of miR-34a can also cause downregulation of SIRT1 expression and form positive feedback with p-p53 expression. More importantly, we found that overexpression of miR-34a can cause significant downregulation of MDR1/P-gp and AXL proteins, which are considered to be the most important proteins associated with chemotherapy resistance.
Many cancer cells develop resistance against their chemotherapeutic agents, which vary structurally and mechanistically, leading to the loss of sensitivity to chemotherapeutic agents. This is defined as MDR[4]. MDR is mainly caused by overexpression of the ATP binding cassette (AΒC) transporter superfamily on the membrane of tumor cells. P-gp, encoded by the MDRl gene, is the most important member of the AΒC transporter family[24]. P-gp is the most important drug transporter and the main cause of primary and secondary drug resistance in malignant tumors,which is closely related to intracellular drug concentration and drug resistance[25]. It is reported that many antitumor drugs, including doxorubicin and paclitaxel, are substrates of P-gp[26]. The MDR1 gene is widely expressed in human malignant tumors. Even in low expression tumors, upregulation of P-gp can be generated after chemotherapy. However, high expression of P-gp directly leads to chemotherapeutic failure.
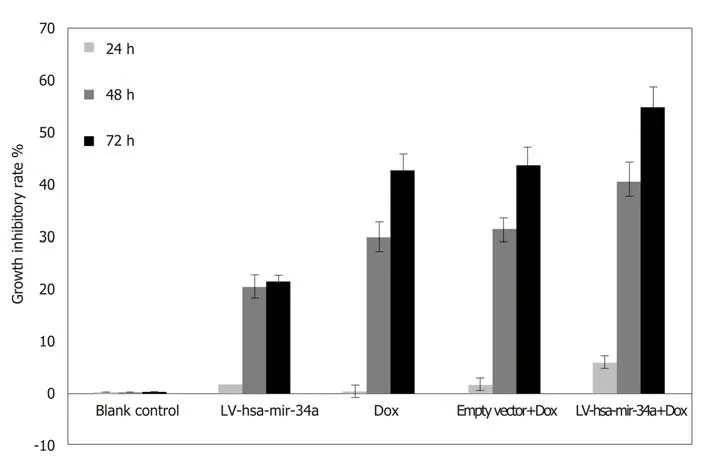
Figure 3 Growth inhibition rate of HepG2 cells treated with LV-hsa-mir-34a transfection combined with doxorubicin. Dox: Doxorubicin.
MDR mediated by P-gp can be inhibited by blocking its drug efflux pump function and inhibiting protein expression. Downregulation of P-gp is regarded as reversal of MDR. However, at present, chemically synthesized P-gp inhibitors are unsatisfactory because of their adverse effects. Our results showed that P-gp played an important role in the doxorubicin resistance of HCC cells. Upregulation of miR-34a can significantly downregulate expression of P-gp and reverse the doxorubicin resistance of HCC cells. It is worth investigating whether mir-34a can be an effective inhibitor of P-gp.
AXL also seems to be a drug resistance-associated protein. The receptor tyrosine kinase AXL is overexpressed in multiple tumor cells, and mediates the metastasis and drug resistance of these cells through a variety of signaling pathways. These include forming positive feedback loops with epithelial-mesenchymal transitions; therefore, it has become a new tumor therapeutic target[27].
Βased on the low expression of miR-34a in HCC cells in previous studies, the present study reduced the malignant behavior of HCC cells by upregulating miR-34a,which could provide a theoretical basis for the treatment of HCC associated with miR-34a. More importantly, this study found that high expression of miR-34a could cause significant downregulation of MDR1/P-gp and AXL, suggesting that miR-34a may reverse chemotherapy resistance by downregulating expression of these proteins.Moreover, the changes in expression of the related proteins were dose-dependent with p-p53 expression, suggesting that the presence of p-p53 is essential for the action of miR-34a. This study provides a way to solve the resistance of p53-dependent chemotherapeutics, and provides a basis for the possible gene therapy of miR-34a and chemosensitization of DNA-damaging drugs such as doxorubicin.
In conclusion, we assume that overexpression of miR-34a can significantly enhance the inhibitory effect of doxorubicin on HepG2 cells. miR-34a may enhance the killing effect of doxorubicin by downregulating MDR1/P-gp and AXL, which may be related to p53 expression.
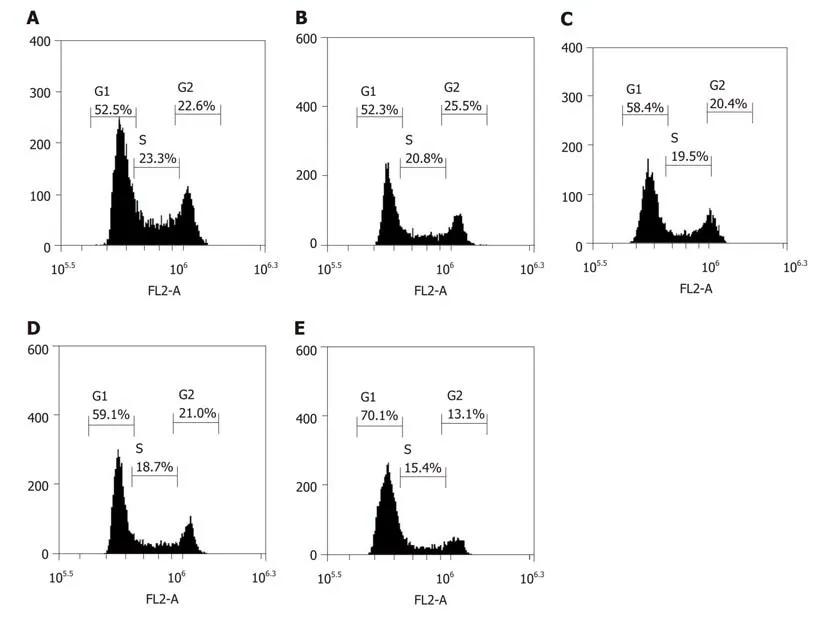
Figure 4 Proportion of G1 phase cells in each group after HepG2 cells were treated for 72 h. A: Blank control group; B: LV-hsa-mir-34a group; C: Doxorubicin treatment group; D: Empty vector + doxorubicin treatment group; E: LV-hsa-mir-34a + doxorubicin treatment group.
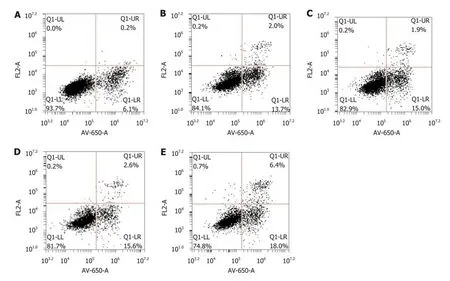
Figure 5 The proportion of apoptotic cells in each group after HepG2 cells were treated for 72 h. A: Blank control group; B: LV-hsa-mir-34a group; C:Doxorubicin treatment group; D: Empty vector + doxorubicin treatment group; E: LV-hsa-mir-34a + doxorubicin treatment group.
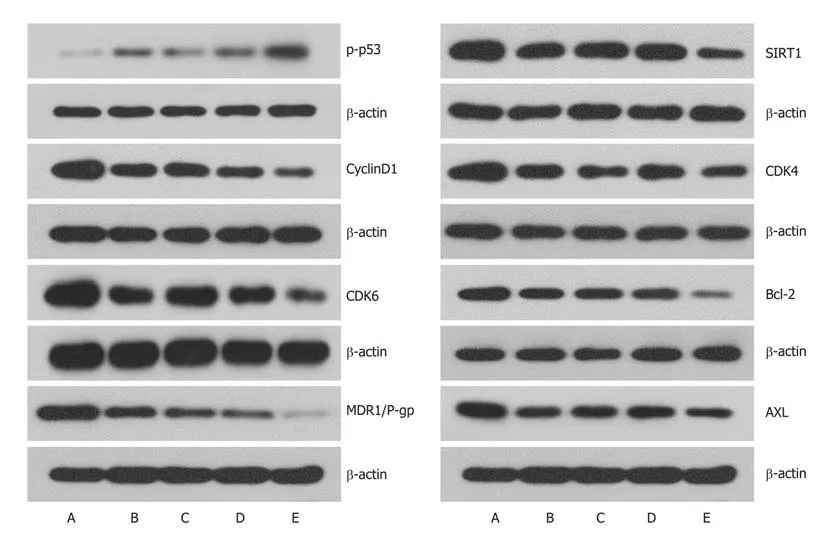
Figure 6 Effects of LV-hsa-mir-34a transfection combined with doxorubicin incubation for 72 h on the expression of related proteins in HepG2 cells. A:Blank control group; B: LV-hsa-mir-34a group; C: Doxorubicin treatment group; D: Empty vector + doxorubicin treatment group; E: LV-hsa-mir-34a + doxorubicin treatment group. MDR: Multidrug resistance protein; CDK: Cyclin-dependent kinase; SIRT: Sirtuin.
ARTICLE HIGHLIGHTS
Research background
Hepatocellular carcinoma (HCC) is the third leading cause of death from malignant tumors worldwide. Surgery is still the preferred treatment, however nearly 80% of patients have lost their chance of surgery by the time that they are diagnosed. Like other malignant tumors,multidisciplinary treatment has become the primary means of HCC treatment, with chemotherapy being the most important alternative to surgery. Nevertheless, drug resistance is the most important reason for the insensitivity of HCC to chemotherapy. Therefore, the mechanism of chemotherapy resistance in HCC requires more research.
Research motivation
Chemotherapy resistance is the most important reason for the poor outcome of HCC. Previous studies have shown that increasing expression of miR-34a in tumor cells can increase the sensitivity of tumor cells to chemotherapeutic drugs. In addition, chemotherapeutic drugs can exert an antitumor effect by causing p53-dependent DNA damage. We were interested in the relationship between the expression of miR-34a and the chemosensitivity in HCC cell lines.
Research objectives
The aim of this study was to investigate the effect of miR-34a over-expression on the growth inhibition of HepG2 HCC cells by doxorubicin.
Research methods
A recombinant lentiviral vector containing miR-34a was constructed and transfected into HepG2 cells to upregulate expression of miR-34a. Cells were exposed to doxorubicin after miR-34a overexpression.To evaluate the effect of miR-34a over-expression, cell viability, cell cycle and apoptosis were detected by MTT and flow cytometry. Expression levels of phospho (p)-p53,sirtuin (SIRT) 1, cyclin D1, cyclin-dependent kinase (CDK) 4, CDK6, ΒCL-2, multidrug resistance protein (MDR)1/P glycoprotein (P-gp), and AXL were detected by Western blotting.
Research results
The expression of miR-34a in HepG2 cells was significantly upregulated. Growth of HepG2 cells was inhibited after upregulation of miR-34a, and viability was significantly decreased after combination with doxorubicin. The number of HepG2 cells in G1 phase increased, and G1 phase arrest was more obvious after upregulation of miR-34a. The apoptosis rate of HepG2 cells was increased after upregulation of miR-34a, and became more obvious after intervention with doxorubicin. Western blotting showed that upregulation of miR-34a combined with doxorubicin treatment caused significant changes in the expression levels of p-p53, SIRT1, cyclin D1, CDK4,CDK6, ΒCL-2, MDR1/P-gp and AXL proteins.
Research conclusions
Over-expression of miR-34a can significantly enhance the inhibitory effect of doxorubicin on HepG2 cells. miR-34a may enhance the killing effect of doxorubicin by down-regulating MDR1/P-gp and AXL, which may be related to p53 expression.
Research perspectives
Over-expression of miR-34a can reduce the malignant biological behavior of HCC cells and enhance the chemosensitivity of HCC cells to doxorubicin. miR-34a is worth studying in the reversal of HCC resistance to chemotherapy.
杂志排行
World Journal of Gastroenterology的其它文章
- Which factots detemine exocrine pancreatic dysfunction in daberes mullitus?
- Proton pump inhibitors and dysbissis:Current knowledge ang aspects to be clarified
- Diagnosis and therapeutic strategies for small bowel vasucular lesions
- Advanced diagostices for pancreatic cysts:Confocal endomicroscopy and moleculat analysis
- Long-lating discussion:Adverse effects of intraoperative bloos loss and allogeneic trandfusion on prognosis of patients with gastric cancer
- Long noncoding RnA hoxa11-AS promotes gastric cancer cell proliferation and invasion via ARSF1 and functions as a bimarker in gastric cancer
