Spin glassy behavior and large exchange bias effect in cubic perovskite Ba0.8Sr0.2FeO3-δ∗
2019-06-18YuXuanLiu刘宇轩ZheHongLiu刘哲宏XuBinYe叶旭斌XuDongShen申旭东XiaoWang王潇BoWenZhou周博文GuangHuiZhou周光辉andYouWenLong龙有文
Yu-Xuan Liu(刘宇轩),Zhe-Hong Liu(刘哲宏),Xu-Bin Ye(叶旭斌),Xu-Dong Shen(申旭东),Xiao Wang(王潇),Bo-Wen Zhou(周博文),Guang-Hui Zhou(周光辉),and You-Wen Long(龙有文)
1Department of Physics and Synergetic Innovation Center for Quantum Effects and Applications of Hunan Province,Hunan Normal University,Changsha 410081,China
2Beijing National Laboratory for Condensed Matter Physics,Institute of Physics,Chinese Academy of Sciences,Beijing 100190,China
3School of Physics,University of Chinese Academy of Sciences,Beijing 100049,China
4Songshan Lake Materials Laboratory,Dongguan 523808,China
Keywords:high-pressure synthesis,exchange bias effect,spin glass
1.Introduction
Iron-based oxides with higher Fe valence states like Fe4+exhibit intriguing physical properties. For example,charge disproportionation is found to occur in CaFeO3and CaCu3Fe4O12,[1,2]which leads to crystal structural phase transition accompanied with metal-insulator transformation.In addition,Cu-Fe intermetallic charge transfer takes place in RCu3Fe4O12(R=La,Pr,Nd,Bi),[3-9]giving rise to a firstorder isostructural phase transition and a series of sharp variations in magnetism and electrical transport properties.BaFeO3usually crystallizes into a hexagonal phase by the high-temperature anneal method in oxygen flow.[10]Recently,a simple cubic perovskite phase of BaFeO3is reported to have been prepared when a lower-temperature reaction method is adopted through using ozone as an oxidizing agent,[11]although only a limited thickness of powder sample(ca.50 nm)can be obtained by this method.
In the 1950s,Meiklejohn and Bean[12]discovered the socalled exchange bias(EB)effect in the Co core and CoO shell structure.In general,this effect can occur when a sample is cooled in a magnetic field across the a critical temperature of a magnetic phase transition because the magnetic hysteresis will shift from the center point vertically or horizontally.[13]The EB effect promises to have potential applications in spin valves,[14]ultra high-density recording,[15]permanent magnets,etc.[16]Therefore,it has received much attention in many different material systems such as ferromagnetic(FM)-antiferromagnetic(AFM)bilayers,[17]nanostructured compounds(nanowires[18]and nanoparticles[19]),strongly correlated oxides,[20]heterostructures,etc.[21]Moreover,it is found that the competition between the FM interaction and the AFM interaction in a spin glassy system can significantly induce the EB effect.[22,23]
In this work,an oxygen deficient iron compound Ba0.8Sr0.2FeO2.81with a simple cubic perovskite structure is prepared.The random distribution of Fe3+and Fe4+causes competition between the FM interaction and AFM interaction,resulting in a spin glassy behavior which can be well explained by a critical slowing down model.More interestingly,a large exchange bias effect is observed in this compound.
2.Experimental details
The Ba0.8Sr0.2FeO2.81was prepared by a solid-state anneal method.The stoichiometric powders BaCO3(99.99%),SrCO3(99.99%),and Fe2O3(99.99%)used as starting materials were thoroughly mixed and ground in an agate mortar.The mixture was then heated in a tube furnace at 1323 K in O2flow for 24 h.The powder x-ray diffraction(XRD)was measured by a Huber diffractometer with Cu Kα1radiation at 40 kV and 30 mA at room temperature.The diffraction angle is selected in a range from 5°to 100°in steps of 0.05°.The XRD data were analyzed by Rietveld re finement with the GSASprogram.[24]The oxygen content was evaluated by thermogravimetric(TG)analysis on a Setaram TG-DTA system.The sample was heated up to 950 K in Ar flow with a heating speed of 10 K/min.Temperature dependence of magnetic susceptibility and field dependence of magnetization were measured by using a superconducting quantum interference device magnetometer(Quantum Design,SQUID-VSM).The alternating current(ac)magnetization,specific heat,and electrical transport properties were measured on a physical property measurement system(Quantum Design,PPMS-9 T).
3.Results and discussion
Figure 1(a)shows the XRD pattern of Ba0.8Sr0.2FeO2.81measured at room temperature.The Rietveld analysis demonstrates that the compound crystallizes into a simple cubic perovskite structure with space group Pm-3m.The lattice parameter we re fined is a=3.93943(1)˚A.This value is slightly smaller than that of BaFeO3(∼3.971˚A),due to the partial introduction of Sr at the A site.To identify the oxygen content,we perform TG measurement as shown in Fig.1(b).The compound is thermally stable below∼550 K.Above this temperature,there exists a remarkable weight loss,indicating the occurrence of oxygen release.Above 800 K,the product becomes stable again on heating up to 950 K,the maximum temperature that is used for TG measurement.According to the TG loss,as well as the final product(Ba0.8Sr0.2FeO2.5identi fied by XRD),the oxygen content is determined to be 2.81±0.02,suggesting the presence of a mixed Fe3.62+valence state.Therefore,the perovskite B site should be occupied by randomly distributed Fe3+and Fe4+in the current Ba0.8Sr0.2FeO2.81.
Figure 2(a)shows the temperature dependence of direct current(DC)magnetic susceptibility of Ba0.8Sr0.2FeO2.81measured at 0.1 T using zero- field-cooling(ZFC)and fieldcooling(FC)mode.Obviously,the ZFC curve displays a kink around 50 K.However,there is a large separation between the ZFC and FC susceptibility curves below this temperature.This feature can usually be caused by a canted long-range AFM ordering or a spin glassy effect.[25]However,when the specific heat as a function of temperature is measured(see Fig.2(b)),one cannot find any anomaly in the whole temperature region we measured(2 K-100 K),ruling out the possibility of long-range spin ordering in Ba0.8Sr0.2FeO2.81.Therefore,the compound should experience a spin glassy transition at a critical temperature Tg≈50 K.Note that the specific heat below 10 K can be well fitted by the formula Cp=βT3/2+αT3.The fitting gives β =5.31 × 10-3J·mol-1·K-5/2,and α =1.79 × 10-4J·mol-1·K-4,indicating that both FM interaction and AFM interaction play a role in specific heat,which is in agreement with the magnetic measurement result.As mentioned earlier,there exist random Fe3+and Fe4+ions in the cubic perovskite Ba0.8Sr0.2FeO2.81.The 180°Fe3+-O-Fe3+superexchange pathways can contribute to the AFM interaction,whereas the contribution to FM can be made by the Fe3+-O-Fe4+double exchange.The competition between the FM interaction and the AFM interaction is thus responsible for the spin glassy behavior observed in Ba0.8Sr0.2FeO2.81.Note that the field dependence of magnetization of Ba0.8Sr0.2FeO2.81presented in the inset of Fig.2(a)is also consistent with the spin glassy feature.

Fig.1.(a)The XRD pattern and the Rietveld re finement result of Ba0.8Sr0.2FeO2.81,where black circle and red line represent the observed and fitted data,respectively.Bottom blue line represents their difference,and pink ticks denote allowed Bragg re flections;(b)TG measurement result of Ba0.8Sr0.2FeO2.81.
Figure 2(c)shows the temperature dependence of resistivity measured on a pellet of Ba0.8Sr0.2FeO2.81.As the temperature decreases,the resistivity increases sharply,suggesting semiconducting or insulating electrical transport properties.Different electrical conductivity modes are attempted to fit the resistivity data. Like some manganites composed of mixed Mn3+and Mn4+,[26,27]the hopping model of small polarons can well reproduce the electrical transport of Ba0.8Sr0.2FeO2.81in a temperature range between 250 K and 300 K.The inset of Fig.2(c)shows the fitting result by using the function ρ = ρ0T exp(EA/KT).Here the EArepresents the activation energy,and K is the Boltzman constant.
The EAwe fitted is 224 meV.This value is slightly less than that obtained from the hexagonal BaFeO2.86(300 meV)and BaFeO2.9(260 meV),[28]which is probably due to the more straight Fe-O-Fe bonding in the current Ba0.8Sr0.2FeO2.81with a cubic perovskite structure.
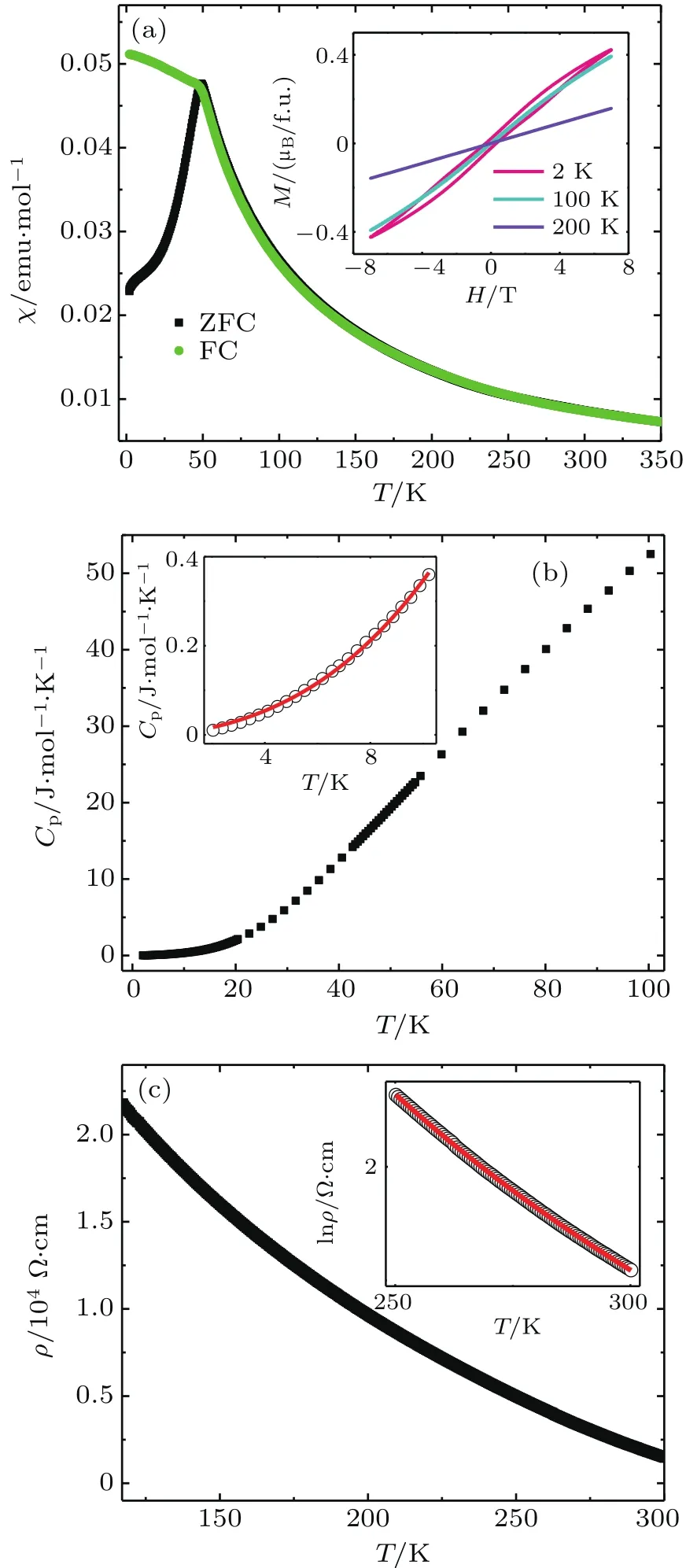
Fig.2.(a)Plots of temperature dependence of DC magnetic susceptibility measured at 0.1 T.The inset shows plots of magnetization versus magnetic field,measured at different temperatures for Ba0.8Sr0.2FeO2.81.(b)Temperature dependence of specific heat,measured between 2 and 100 K.Inset shows fitting result(red curve)using function Cp=βT3/2+αT3.(c)Temperature dependence of resistivity.Inset shows fitting result(red line)using hopping model of small polarons as described in the text.
To furthercharacterize the spin glassy state of Ba0.8Sr0.2FeO2.81,the AC magnetization values are measured at different frequencies.As shown in Fig.3,a frequencydependent cusp that shifts toward higher temperatures with the increasing of frequency is found to occur near the freezing temperature Tf(see the upper-left inset),providing convincing evidence for the formation of spin glass.Moreover,the spin glassy behavior of Ba0.8Sr0.2FeO2.81can be well described by the critical slowing down model[29]with the formula τf= τ0(Tf/Tg-1)-zv,where τ0is the relaxation time,the parameter zv is the dynamic critical exponent,and the maximum relaxation time τf=1/f.The bottom-right inset of Fig.3 presents the fitting result,yielding the parameters τ0=6.8(6)×10-14s and zv=11.03(4).These values are comparable to those observed in other spin glassy systems,like Eu0.4Sr0.6S,Sr2FeCoO6,[30-32]etc.

Fig.3.Plots of AC magnetization versus temperature,measured between 2 K and 100 K for Ba0.8Sr0.2FeO2.81.The upper-left inset shows enlarged view for the shift.Bottom right inset shows fitting by using critical slowing down model as described in the text.
Since the spin glassy behavior caused by the competition between the FM interaction and the AFM interaction is highly likely to induce a large exchange bias effect,the FC magnetization curves are measured at 2 K.For these measurements,the sample is cooled from 300 K to 2 K through the Tgunder different positive cooling fields(0 T-7 T).Figure 4(a)shows the related measurement results.The asymmetrical magnetic hysteresis loops that deviate from the origin point can be well observed.Speci fically,the magnetization curves shift towards the negative magnetic field axis and the positive magnetization axis, revealing the remarkable exchange bias effect .To quantitatively characterize the exchange bias effect of Ba0.8Sr0.2FeO2.81,the EB field(HEB=(|HL|-|HR|)/2)and the remnant magnetization shift(ME=(|MU|-MD|)/2)are calculated.Here,the HLand HRrespectively present the left and right cooling field intercept,and MUand MDare upside and downside magnetization intercept,as illustrated in the inset of Fig.4(a),measured at a cooling field HCF=2 T.Figure 4(b)shows the calculated HEBand MEas a function of HCF.The values of HEBsharply increase at lower cooling fields ranging from 0 T to 2 T,and then gradually decrease at highercooling fields.The maximum value of HEBweobtained at 2 T is 5.6 kOe(1 Oe=79.5775 A·m-1),which is significantly higher than those for most of other compounds.[33-35]Similarly,the MEalso sharply increases to 0.031µB/f.u.with field increasing up to 2 T.Above this cooling field,it decreases slightly.
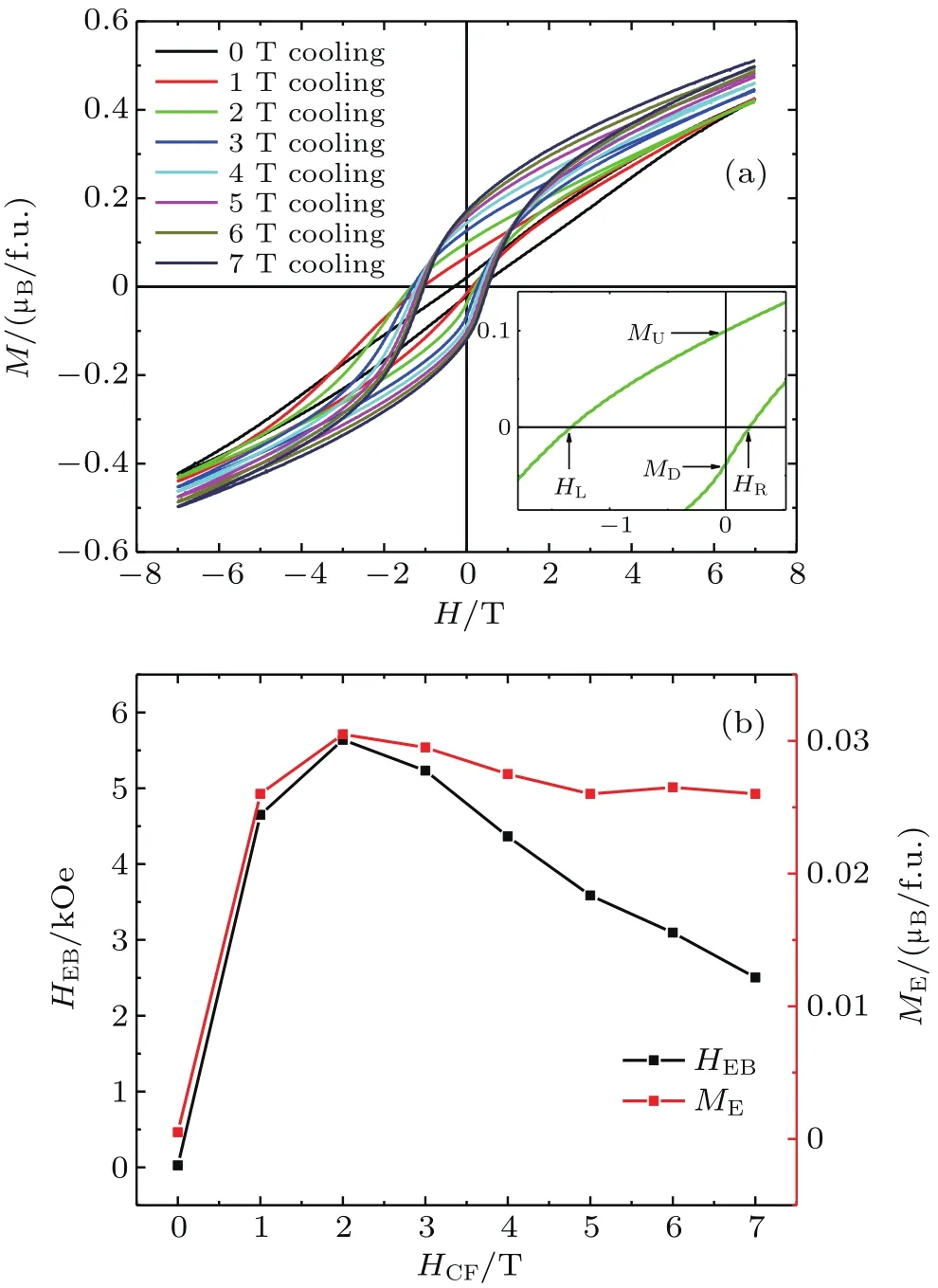
Fig.4.(a)Plots of field dependence of magnetization measured at 2 K after cooling at different fields from 300 K to 2 K for Ba0.8Sr0.2FeO2.81.Inset shows the enlarged view for the magnetic hysteresis loop measured at a 2-T cooling field.(b)Plots of HEBand MEas a function of HCFobtained at 2 K.
The magnitudes of HEBand MEare dependent on factors such as FM and AFM cluster size or thickness,inter facial roughness,and exchange coupling strength between the FM interaction and the AFM interaction.[13,36-38]In the current Ba0.8Sr0.2FeO2.81composed of disordered FM and AFM domains,at HCF=0 T,the spin glassy state caused by the competition between the FM interaction and the AFM interaction froze at Tg.When the applied cooling field increases up to 2 T,the magnetic anisotropy sharply increases due to the pinning effect of FM domains.Consequently,the values of HEBand MEincrease drastically in a cooling field range from 0 T to 2 T.Meanwhile,when the cooling field exceeds 2 T,the FM cluster size will develop considerably.Consequently,the exchange coupling effect becomes too weak to pin the FM spins,resulting in the HEBand MEdecreasing at higher HCF.
4.Conclusions
In summary of the present study,an oxygen deficient perovskite Ba0.8Sr0.2FeO3-δwith simple cubic Pm-3m space group is synthesized.The oxygen content is determined to be 2.81±0.02 by TG measurement.The electrical transport behavior can be well fitted by the hopping model of small polarons,and the activation energy we obtained is 224 meV.In magnetism,both DC and AC magnetic susceptibility measurements reveal the spin glassy features of Ba0.8Sr0.2FeO2.81with a glassy transition temperature at about 50 K.The spin glassy behavior can be attributed to the competition between the FM interaction and the AFM interaction originating from the Fe3+-O-Fe4+double exchange and Fe3+-O-Fe3+superexchange pathways,respectively.On using a positive magnetic field to cool Ba0.8Sr0.2FeO2.81from 300 K to 2 K through Tg,a large negative exchange bias effect is observed.Moreover,the exchange bias field increases sharply as HCFincreases up to 2 T,due to the FM pinning effect.The maximum HEBthat we obtained at 2 K is 5.6 kOe,which is located at a higher level than those observed in other compounds.
猜你喜欢
杂志排行
Chinese Physics B的其它文章
- Topological superconductivity in a Bi2Te3/NbSe2heterostructure:A review∗
- The universal characteristic water content of aqueous solutions∗
- Neutral excitation and bulk gap of fractional quantum Hall liquids in disk geometry∗
- Direct deposition of graphene nanowalls on ceramic powders for the fabrication of a ceramic matrix composite∗
- Hard carbons derived from pine nut shells as anode materials for Na-ion batteries∗
- Crystal structures and sign reversal Hall resistivities in iron-based superconductors Lix(C3H10N2)0.32FeSe(0.15<x<0.4)∗
