Interleukin-22 receptor 1 is expressed in multinucleated giant cells:A study on intestinal tuberculosis and Crohn's disease
2019-06-13ZiQiYuWenFeiWangYouChaoDaiXinChunChenJianYongChen
Zi-Qi Yu, Wen-Fei Wang, You-Chao Dai, Xin-Chun Chen, Jian-Yong Chen
AbstractBACKGROUND It is challenging to distinguish intestinal tuberculosis from Crohn’s disease due to dynamic changes in epidemiology and similar clinical characteristics. Recent studies have shown that polymorphisms in genes involved in the interleukin (IL)-23/IL-17 axis may affect intestinal mucosal immunity by affecting the differentiation of Th17 cells.AIM To investigate the specific single-nucleotide polymorphisms (SNPs) in genes involved in the IL-23/IL-17 axis and possible pathways that affect susceptibility to intestinal tuberculosis and Crohn's disease.METHODS We analysed 133 patients with intestinal tuberculosis, 128 with Crohn’s disease,and 500 normal controls. DNA was extracted from paraffin-embedded specimens or whole blood. Four SNPs in the IL23/Th17 axis (IL22 rs2227473, IL1β rs1143627,TGFβ rs4803455, and IL17 rs8193036) were genotyped with TaqMan assays. The transcriptional activity levels of different genotypes of rs2227473 were detected by dual luciferase reporter gene assay. The expression of IL-22R1 in different intestinal diseases was detected by immunohistochemistry.RESULTSThe A allele frequency of rs2227473 (P = 0.030, odds ratio = 0.60, 95% confidence interval: 0.37-0.95) showed an abnormal distribution between intestinal tuberculosis and healthy controls. The presence of the A allele was associated with a higher IL-22 transcriptional activity (P < 0.05). In addition, IL-22R1 was expressed in intestinal lymphoid tissues, especially under conditions of intestinal tuberculosis, and highly expressed in macrophage-derived Langhans giant cells.The results of immunohistochemistry showed that the expression of IL-22R1 in patients with Crohn's disease and intestinal tuberculosis was significantly higher than that in patients with intestinal polyps and colon cancer (P < 0.01).CONCLUSIONHigh IL-22 expression seems to be a protective factor for intestinal tuberculosis.IL-22R1 is expressed isn Langhans giant cells, suggesting that the IL-22/IL-22R1 system links adaptive and innate immunity.
Key words: Crohn’s disease; Intestinal tuberculosis; Single-nucleotide polymorphism;Interleukin-22; Interleukin-22 receptor 1; Multinucleated giant cells upon this work non-commercially,and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
INTRODUCTION
Intestinal tuberculosis (ITB) is chronic granulomatous inflammation caused by Mycobacterium tuberculosis (M. tuberculosis) invading the gut. Crohn’s disease (CD) is a chronic non-specific intestinal inflammatory disease. Although the pathogenesis of CD is still not clear, it is believed that genetic and environmental factors cause excessive mucosal immune responses to normal intestinal flora, causing intestinal pathological damage[1]. Asia was previously considered a low endemic area for CD,but the incidence has increased considerably over recent decades due to industrialization and the Westernization of lifestyle[2]. Moreover, due to the increase in floating population and HIV and M. tuberculosis infections, as well as the prevalence of multidrug-resistant strains, the prevention and control of tuberculosis (TB) are facing severe challenges[3,4]. It is challenging to distinguish ITB from CD due to dynamic changes in epidemiology and similar clinical characteristics, especially in developing countries[5,6]. After a misdiagnosis of ITB as CD, treatment with immunosuppressive agents may aggravate the TB infection and cause systemic dissemination. Upon misdiagnosis of CD as ITB, anti-TB treatment results in serious drug side effects, and appropriate treatment is delayed.
There is a large amount of mucosa-associated lymphoid tissue in the intestine,which plays a vital role in the pathogenesis of ITB and CD[7,8]. Th17 cells, named for their production of interleukin (IL)-17, have recently attracted much attention in the study of mucosal immunity. Th17 cells are a subset of CD4+T lymphocytes[9,10], which are critical in mucosal defence and autoimmune diseases[11]. Previous studies have reported that the lamina propria of the small intestine has abundant Th17 cells and that its unique microenvironment is beneficial for the differentiation of Th17 cells[12,13].
The coordination of Th17 cell differentiation and maintenance is complex (Figure 1);naïve T cells induce RORγt expression by activating the STAT3 signalling pathway in response to IL-23, IL-6, and TGF-β after dendritic cell presentation or antigen stimulation, thereby promoting the differentiation of Th17 cells into CD4+IL-17+cells[14-19]. IL-1β also plays an important role in this process[20-23]. The regulatory network through which Th17 cells exert physiological functions is called the IL-23/IL-17 axis.
Important active products of Th17 cell function are IL-17 and IL-22. Because of the unique role of IL-22 in the intestinal barrier system, we are particularly interested in it. IL-22 is a member of the IL-10 family and is produced by Th-17 cells, γδ T cells,natural killer T cells, and innate lymphoid cells[24,25]. IL-22 binds to a heterodimeric receptor complex consisting of IL-22R1 and IL-10R2 to activate the JAK-STAT signalling pathway[26,27]. IL-22–IL-22R1 interactions can induce the expression of genes encoding molecules involved in tissue inflammation, immunosurveillance, and homeostasis[28-31], suggesting that the IL-22–IL-22R1 pathway plays a key role in maintaining normal barrier homeostasis, especially in intestinal mucosal immunity.The protective function of IL-22 is evidenced by the observation that IL22-/-mice are susceptible to Citrobacter rodentium infection[32].
Mechanical, chemical, immune, and biological barriers together constitute the intestinal mucosal barrier; each barrier plays a substantial role in the defence of the enteric mucous membrane. IL-22 maintains these barriers through a variety of mechanisms. To maintain the mechanical barrier, IL-22 activates STAT3 to enhance the transcription of anti-apoptotic and pro-proliferative genes, thereby promoting mucosal healing[33], and regulates the integrity of intestinal epithelial cells by upregulating tight junction proteins in the human intestinal epithelium[34,35]. To maintain the chemical barrier, IL-22 stimulates Mucin 1 expression and glycosylation to produce a firm inner mucus layer that prevents bacterial invasion[36,37]. Regarding the immune barrier, IL-22 induces the production of innate antimicrobials, including defensins, Reg family molecules, and S100 proteins[38]. Regarding the biological barrier, IL-22 plays an important role in the shaping of body immunity and colonic flora balance[39,40].
Polymorphisms in genes involved in the IL-23/IL-17 axis have been reported to influence inflammatory bowel disease (IBD) susceptibility. A genome-wide association study based on a Japanese population showed that multiple genes in this pathway are associated with IBD[41], and similar studies have been conducted in Korean populations[42]. Our previous studies in sizable samples of Chinese Han populations found that through an single-nucleotide polymorphism (SNP) selection strategy, IL22 rs2227473 was associated with susceptibility to TB[43]; eleven polymorphic loci were also screened in the IL6 promoter region, and only rs1800795 was found to be associated with TB susceptibility[44]. Similarly, IL1β promoter polymorphism rs1143627 is associated with susceptibility to M. tuberculosis infection,the genotype of which affects the severity of TB[45]. Therefore, we aimed to study ITB and CD and to determine whether SNPs in genes involved in the IL-23/IL-17 axis differ between these two diseases.
MATERIALS AND METHODS
Study participants
This case-control study was conducted between January 2008 and December 2017 at the Jiangxi Provincial People’s Hospital (Nanchang, China). A total of 133 patients with ITB, 128 with CD, and 500 healthy controls (HCs) were included. All of the patients and HCs were randomly selected, and all of the subjects were local people from Jiangxi Province. The inclusion criteria for patients with ITB were as follows:Age 18 years or older; ITB diagnosed by clinical manifestations, endoscopy, X-ray imaging, pathological changes, positive acid-fast staining, and/or response to anti-TB treatment; and no other infectious, chronic, or autoimmune diseases. The inclusion criteria for patients with CD were as follows: Age 18 years or older; CD diagnosed based on clinical symptoms, X-ray findings, serological examination results,endoscopic findings, and histopathology following the Chinese Society of Gastroenterology consensus on the diagnosis and management of IBD; and no infectious or non-infectious colitis. The inclusion criteria for HCs were as follows: Age 18 years or older; negative TB-specific interferon gamma release assay; no clinical manifestations of TB (no abnormalities in chest radiology); and no other infectious,chronic, malignant, or autoimmune diseases. The present study protocol was reviewed and approved by the institutional review board of Jiangxi Provincial People's Hospital and complied with the principles of the Declaration of Helsinki.
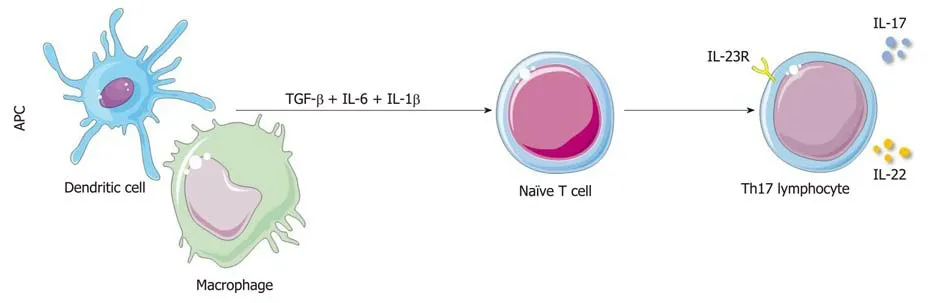
Figure 1 Schematic diagram of the Th17 cell differentiation pathway. APC: Antigen-presenting cells; TGF-β: transforming growth factor β; IL: Interleukin; IL-23R:Interleukin-23 receptor 1.
Genotyping of polymorphisms in IL-23/IL-17 axis genes
We obtained formalin-fixed paraffin-embedded (FFPE) specimens from ITB and CD patients. We sectioned each FFPE sample into three sections at 20-μm thickness and transferred the sections into Eppendorf tubes for immediate storage at - 20 °C.Lithium heparin anti-coagulated whole blood (2 mL) was collected from HCs and stored at 4 °C. Genomic DNA was extracted from paraffin-embedded specimens from the ITB and CD patients described above using an FFPE DNA Kit (Omega, United States) and from whole blood from HCs using a DNeasy Blood and Tissue Kit(QIAGEN, United States). Four SNPs in genes involved in the IL23/Th17 axis (IL22 rs2227473, IL1β rs1143627, TGFβ rs4803455, and IL17(A) rs8193036) were genotyped with TaqMan assays in a 20-μL reaction volume using the ABI 7500 Real-time PCR system (ABI, United States). The cycling parameters were as follows: Pre-PCR processing at 60 °C for 1 min; hold at 95 C for 10 min; 40 cycles of 95 °C for 15 s and 60°C for 1 min; and post-PCR processing at 60 °C for 1 min. The results were analysed by using ABI 7500 PCR software.
Dual-luciferase assays
DNA fragments of the rs2227473_G (-1810G) allele of the IL22 gene were amplified by PCR using human genomic DNA and were subsequently modified by site-directed mutagenesis (Beyotime, China) to generate rs2227473_A (-1810A). All amplicons were verified by direct sequencing and inserted into a pGL3-basic reporter vector(Promega, United States) harbouring a genetically engineered firefly luciferase gene without a promoter. 293T cells (2 × 105) were cultured in antibiotic-free Dulbecco’s modified Eagle medium containing 10% foetal bovine serum (Gibco, Australia) and plated in 12-well plates 24 h before transfection. Plasmid constructs were transfected into 293T cells with Lipofectamine 2000 reagent (Life Technology, United States). At 24 h post-transfection, luciferase reagent was added to the culture medium in each well, and the resulting solution was mixed. After 15 min, firefly luminescence was measured with a Multi-Mode Microplate Reader (Bio-Tek, United States). Then, Stop and Glo Reagent was added to the original culture medium in each well, and the resulting solution was mixed. After 10 min, Renilla luminescence was measured.Relative promoter activity is expressed as the ratio of firefly luciferase to Renilla luciferase signal. Three independent transfections and duplicate luciferase assays were performed for each condition, and the pGL3-basic vector was used as a control.
Immunohistochemistry
To compare the immunohistochemistry results of IL-22R expression in different intestinal diseases, we randomly selected 20 FFPE specimens of colon polyps and colon cancer patients according to the following criteria. The inclusion criteria for patients with colonic polyps were as follows: Age 18 years or older; pathological diagnosis of colon polyps; and no other infectious, chronic, malignant, or autoimmune diseases. The inclusion criteria for patients with colon cancer were as follows: Age 18 years or older; pathological diagnosis was colon cancer; no prior radiotherapy or chemotherapy; and no other infectious or autoimmune diseases. Twenty FFPE gut biopsies of colon polyps, colon cancer, ITB, and CD were cut with a rotary microtome(Leica RM2235, Germany) as three serial sections (4 μm) on the microslide. Unstained sections were placed in a 67 °C drying oven for 2 h of deparaffinization, rehydrated through two changes of xylene and a graded alcohol series, and then rinsed in dH2O.Antigens were retrieved by immersing sections in a pressure cooker containing boiling 1 × R-BUFFER B Solution (Electron Microscopy Sciences, United States) for 3 min at 100 °C. Sections were then rinsed in dH2O and processed for immunostaining using the EXPOSE rabbit-specific HRP/DAB detection IHC kit (Abcam, United States)according to the manufacturer's protocol. The primary antibody was rabbit/IgG polyclonal anti-IL22 receptor alpha antibody ab5984 (1:200; Abcam, United States).Negative controls consisted of phosphate buffered saline instead of primary antibodies. Mouse kidney tissue was used as a positive control according to the manufacturer's protocol. Sections were counterstained with Modified Harris Haematoxylin solution (Solarbio, China), dehydrated through a graded alcohol series and xylene, and mounted with neutral balsam (Solarbio, China). Images were captured at 200 × magnification using a Nikon Eclipse Ni microscope with NISElements AR ver. 4.40.00 software (Nikon, Japan). The expression of IL-22 receptor 1(IL-22R1) in intestinal tissues was semi-quantitatively evaluated using Image-Pro Plus version 6.0 (Bethesda, United States). Integrated optical density (IOD) was measured in five fields from each slice randomly selected by two senior pathologists. The capture conditions were strictly maintained across all the photographs.
Statistical analysis
In this case-control study, we first compared the baseline characteristics of the study population. The categorical variables were analysed by the chi-square test or Fisher’s exact test, and the continuous variables were compared using an independent sample t-test. The goodness-of-fit χ2 test was performed to check if the genotype frequencies of case and control groups conformed to Hardy-Weinberg equilibrium. Power calculations were performed using Power and Sample Size Calculation Statistical Software[46]. Unconditional logistic regression analysis was used to compare the genotype and allele frequencies among the three groups. Covariates such as gender,age, smoking, and drinking were included to adjust the odds ratio (OR) value. The disease-associated additive genetic model was evaluated by the Cochran-Armitage trend test. For the disease with significant results in the additive model text, logistic regression analysis was used to analyse the three genetic models (dominant, recessive,and co-dominant) that the SNP may follow in the disease; the OR and 95% confidence interval (CI) were then calculated and adjusted by using the above covariates. Oneway ANOVA was used for comparisons among multiple groups. All statistical analyses were performed using SPSS (statistical software version 24). The statistical tests were two-tailed, and statistical significance was evaluated at P < 0.05.
RESULTS
Clinical characteristics of the study population
As shown in Table 1, no significant difference was found between the ITB and CD groups in mean age, smoking or drinking history, white blood cell count,haemoglobin, erythrocyte sedimentation rate (ESR), purified protein derivative skin test, or disease-related surgery (P > 0.05). However, both the T cell spot test positive rate and abnormal lung imaging findings showed a significant difference (P < 0.05)between the two groups, indicating their potential utility in identifying ITB and CD.
Genotype distributions of the SNPs in the case and control groups conform to the Hardy-Weinberg equilibrium
Retrieving rs2227473, rs1143627, rs4803455, and rs8193036 from the dbSNP database,we found that the MAFs (minor allele frequencies) of the East Asian population were 0.072, 0.547, 0.348, and 0.267, respectively. The statistical power of this polymorphism study exceeded 0.80. The genotype and allele frequency distributions of the SNPs rs2227473 and rs1143627 are shown in Tables 2-7. After adjusting for gender, age of diagnosis, and the history of drinking and smoking, the GA genotype (P = 0.046, OR =0.57, 95%CI = 0.33-0.99) and the A allele frequency (P = 0.030, OR = 0.60, 95%CI =0.37-0.95) of rs2227473 were significantly abnormally distributed in ITB patients compared with HCs; similarly, the CT genotype (P = 0.033, OR = 1.81, 95%CI = 1.05-3.13), the TT genotype (P = 0.011, OR = 2.17, 95%CI = 1.19-3.95), and the T allele frequency (P = 0.014, OR = 1.43, 95%CI = 1.07-1.89) of rs1143627 were significantly abnormally distributed in ITB patients compared with HCs. The genotype and allele frequency distributions of the SNPs rs4803455 and rs8193036 were not significantly different among the three groups, and the results are shown in the Supplementary material.
Cochran-Armitage trend test
After the Cochran-Armitage trend test, the additive model fit IL22 rs2227473 in ITB(χ2 = 5.47, P = 0.019) and CD (χ2 = 1.91, P = 0.167); and the additive model fit IL1β rs1143627 in ITB (χ2 =6.53, P = 0.011) and CD (χ2 = 2.54, P = 0.111). Therefore, we mainly analysed the genetic models that the above two SNPs may follow in ITB, and the results are shown in Tables 8 and 9. We found a significant difference between the wild-type and the mutant-type under the dominant model in rs2227473, and the difference was still statistically significant after adjusting for the covariates (P = 0.040,OR = 0.58, 95%CI = 0.34-0.98); rs1143627 had a significant difference in the dominant,recessive, and co-dominant genetic models.
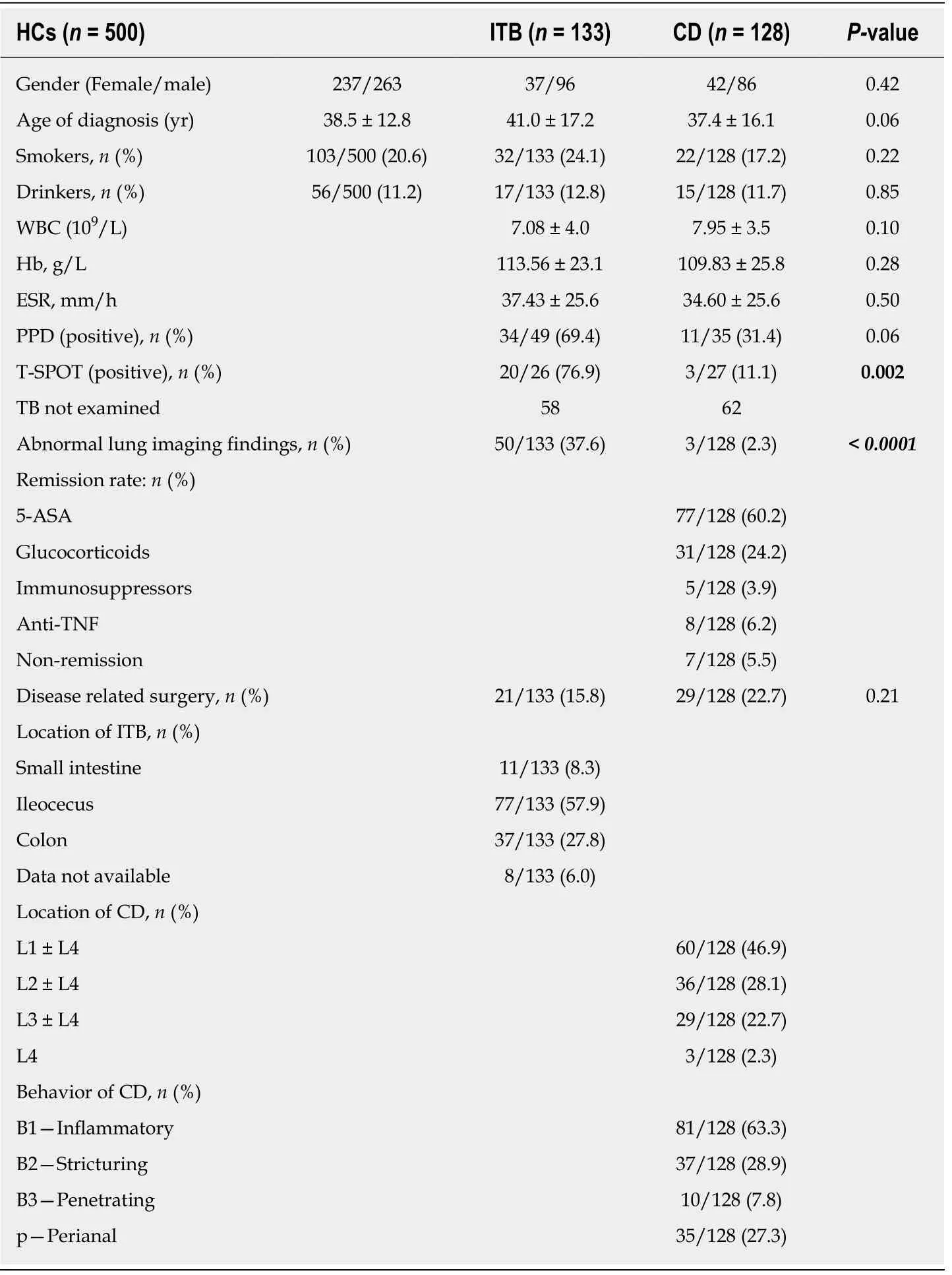
Table 1 Baseline characteristics of the study population
Association of haematological features with genotype
Some data, particularly ESR data, were missing because the corresponding examinations were not performed for the patients. There was no significant difference in the three clinical indicators among the different genotypes at the four examined loci in both ITB and CD patients (Supplementary material).
Dual-luciferase assayAs shown in Figure 2, the firefly/renilla luciferase values were significantly different between the recombinant vector and empty vector transfected groups, and pGL3-rs2227473_A was associated with significantly increased Firefly/Renilla luciferase values relative to those of pGL3-rs2227473_G.
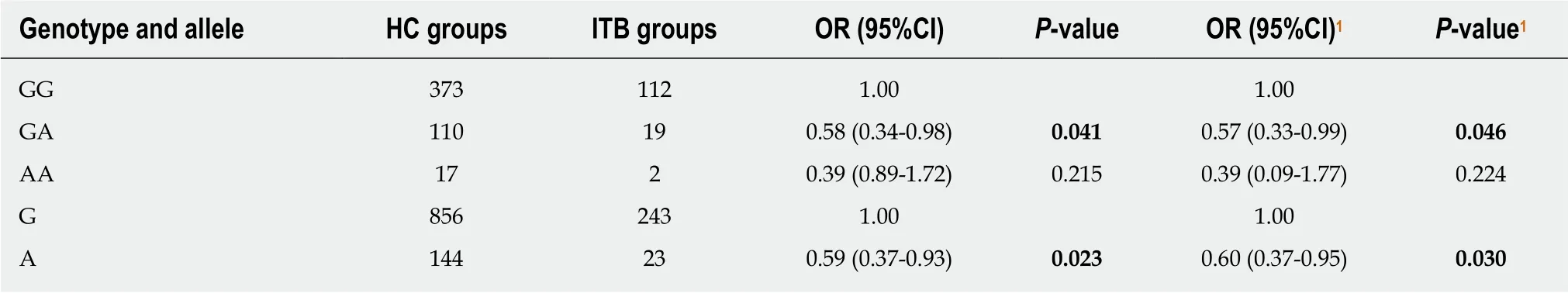
Table 2 Genotype and allele frequency distributions of rs2227473 in the intestinal tuberculosis and healthy control groups
Immunohistochemistry
As shown in Figure 3, IL-22R1 expression in CD and ITB was not restricted to epithelial cells; high IL-22R1 expression was observed in intestinal lymphoid tissues.However, it was difficult to ascertain whether expression was confined to the cell membrane because ITB samples showed many aggregated immune cells that were completely stained by diaminobenzidine (DAB).
High IL-22R1 expression in Langhans giant cells
Langhans giant cells are a typical component of tuberculous nodules, and cells in the central part of a tuberculous granuloma undergo caseous necrosis. As shown in Figure 4, epithelioid cells and Langhans multinucleated giant cells were observed around the necrotic area. Lymphocytes infiltrated the outer nodule regions, and fibroblasts and collagen fibres were distributed around these nodules. In addition,these multinucleated giant cells were ubiquitous and exhibited strong DAB staining.Average IOD (total IOD/total area) of IL-22R1 in gut tissues from colon polyps, colon cancer, ITB, and CD was calculated (Figure 5). Compared with colon polyps and colon cancer, both ITB and CD showed significantly higher IL-22R1 expression.
DISCUSSION
In this study, we analysed four SNP loci in genes involved in the IL23/IL17 axis and found that the frequency distributions of SNPs in IL22 (rs2227473) and IL1β(rs1143627) were significantly different between ITB patients and HCs. Although larger sample size is better in a genetic study, ITB and CD are both low-incidence diseases. Fortunately, all patients are local Han people, which provides a good genetic background for genetic research; and the genotype distributions of the four SNPs in the control groups conformed to the Hardy-Weinberg equilibrium, indicating that the study participants can be considered as random subjects from the population.Therefore, this polymorphism study has enough statistical power to prove its reliability. Our previous research confirmed that the rs1143627 T allele increases IL-1β production[45]. Therefore, we focused on IL22 (rs2227473) and found that the presence of the A allele is a protective factor for ITB; moreover, dual-luciferase assays indicated that the A allele is associated with high IL-22 expression.
However, as "a sheep in wolf's clothing"[47], IL-22 has dual roles as a protective and pathological factor at mucosal sites. The nature of IL-22 behaviour depends mainly on whether its expression is normal at different stages of the inflammatory response[25].Although IL-22 has a maintenance effect on the intestinal barrier, as described above,it also activates various pro-inflammatory factors[48]. In patients with CD, IL-22 expression levels in serum and intestinal tissues are significantly increased, with IL-22 expression being positively correlated with disease activity[49]. The role of IL-22 in TB remains unclear. Research by Wilson et al showed that IL-22 is dispensable for the development of immunity in host defence after infection with M. tuberculosis or Mycobacterium avium[50]. Given this observation, it is difficult to explain why IL-22 is abundant at disease sites in TB patients and to clarify why IL-22-producing γδ T cells can migrate into granulomas during M. tuberculosis infection[51,52]. This may require analysis based on different stages of the disease.
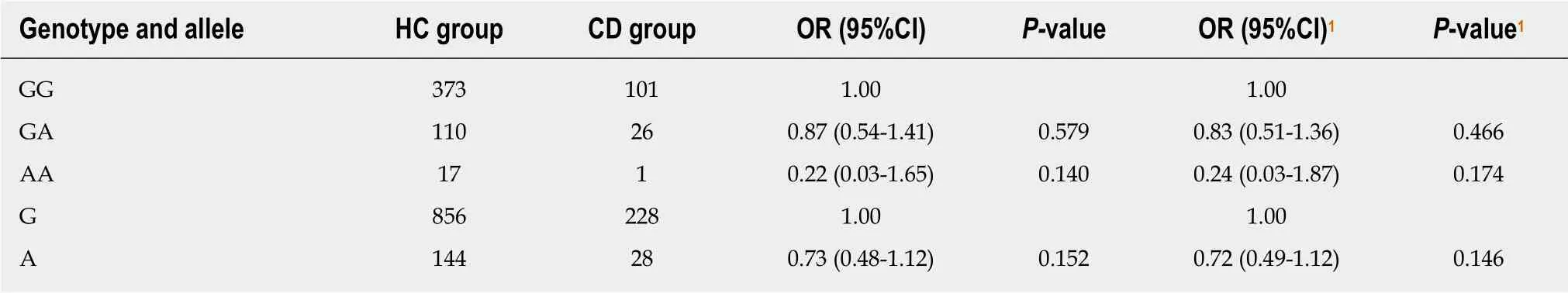
Table 3 Genotype and allele frequency distributions of rs2227473 in the Crohn's disease and healthy control groups
It is generally accepted that while IL-10R2 is ubiquitously expressed by most cell types, IL-22R1 expression is restricted to non-haematopoietic cells such as epithelial cells, hepatocytes, and fibroblasts[25,28,29,53]. However, in our study, we observed IL-22R1 expression in intestinal lymphoid tissues. Macrophage-derived Langhans giant cells,especially those from ITB patients, expressed high levels of IL-22R1. This finding suggests that IL-22 may affect adaptive immunity by regulating macrophages as well as enhancing innate immunity.
Three independent studies reported that M. tuberculosis induces IL-22R1 expression in infected macrophages[54]. In the first study, Rohan Dhiman et al found that human monocyte-derived macrophages had basal IL-22R1 expression that can be increased by stimulation with M. tuberculosis (H37Rv strain)[55]. Subsequently, Zeng et al[56]found that CD4+T cells infected with M. tuberculosis can differentiate into T effector cells that carry membrane-bound IL-22. Membrane-bound IL-22+CD4+ T effector cells matured in vivo and maintain membrane distribution during M. tuberculosis active infection.This finding may explain the immunohistochemistry results in this study which indicated that IL-22R1 expression in ITB did not appear to be confined to the cell membrane and that cells were likely to show widespread staining. Although it cannot be ruled out that these staining results may be caused by macrophage uptake of IL-22R1-positive epithelial cell debris, this possibility is unlikely considering that the positive cells were in an unusual aggregated state. A recent study from TB reported that IL-22-reactive cells are predominantly epithelial cells during acute M. tuberculosis infection in mice and humans, whereas epithelial cells and accumulated macrophages in tuberculous granulomas in the chronic disease stage possibly expressed IL-22R.Interestingly, as caseous necrosis in tuberculous granulomas leads to the gradual deterioration of cells, the accumulation of IL-22R1-positive macrophages also gradually decreases, and these cells mainly localize on the rim of necrotic granulomas[57]. This finding is consistent with the phenomenon we observed in the ITB immunohistochemistry analysis, suggesting that IL-22R1 expression levels in macrophages might be related to TB severity.
The IL-22-IL-22R axis also affects the pathogenesis of CD. Stephan et al found that IL-22R1 mRNA expression was upregulated after stimulation of intestinal epithelial cell culture system with pro-inflammatory cytokines such as lipopolysaccharide, IL-1β, or tumour necrosis factor (TNF), which, however, had no effect on IL-10R2 mRNA expression. This is also confirmed by the increased expression of IL-22R1 mRNA in the colonic biopsy of the inflammatory site of CD patients compared with the noninflammatory site; binding of IL-22 to its receptor complex activates multiple phosphorylated protein kinases based on STAT1/3 and affects downstream signalling, promoting intestinal epithelial cell recovery and intestinal barrier integrity[58].
IL-22 can directly activate macrophages by inducing the production of TNF, an extremely important cytokine in the immune response to TB[57]. TNF seems to be a central mediator in different chronic inflammatory disorders, including CD[59]. Widely used anti-TNF antibodies (such as infliximab) have positive effects in CD but cause unwanted immunosuppression, increasing the risk of M. tuberculosis infection. Many researchers have focused on systemic treatment targeting the IL-22/IL-22R1 axis because of the abovementioned role of IL-22 in the protective function of intestinal epithelial cells and the view that IL-22 does not affect the immune system[60,61].However, in patients with CD, we found that IL-22R1 was primarily expressed in epithelial cells, with IL-22R1 expression occurring within non-caseating granulomatous. This finding cautions against therapeutic targeting of the IL-22/IL-22R1 axis.
In conclusion, in this study of polymorphisms in genes within the IL-23/IL-17 axis,we found that the frequency of the G allele of IL22 (rs2227473) was significantly higher in ITB patients than in the HC population. Low IL-22 expression may be a risk factor for ITB. Further research revealed that the ITB and CD groups had higher IL-22R1 expression than the polyp and cancer groups. Importantly, we observed IL-22R1 expression in multinucleated giant cells; this finding is inconsistent with those of previous studies and may indicate that IL-22 is not dispensable in host immunity to TB. These data provide a premise for elucidating the role of the IL-22/IL-22R1 axis in the crosstalk between innate and adaptive immunity under conditions of chronic inflammation. Researchers should pay more attention to the immunological side effects of therapeutic modulation of the IL-22/IL-22R1 axis.
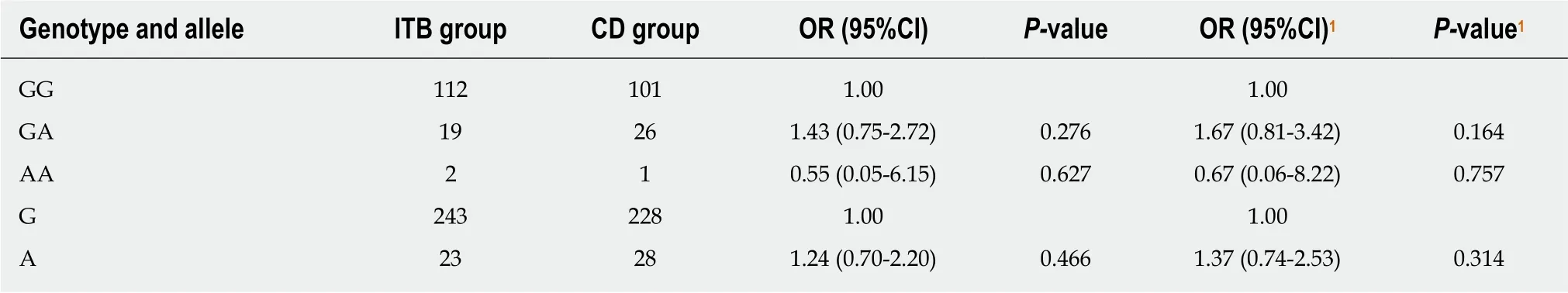
Table 4 Genotype and allele frequency distributions of rs2227473 in the intestinal tuberculosis and Crohn's disease groups
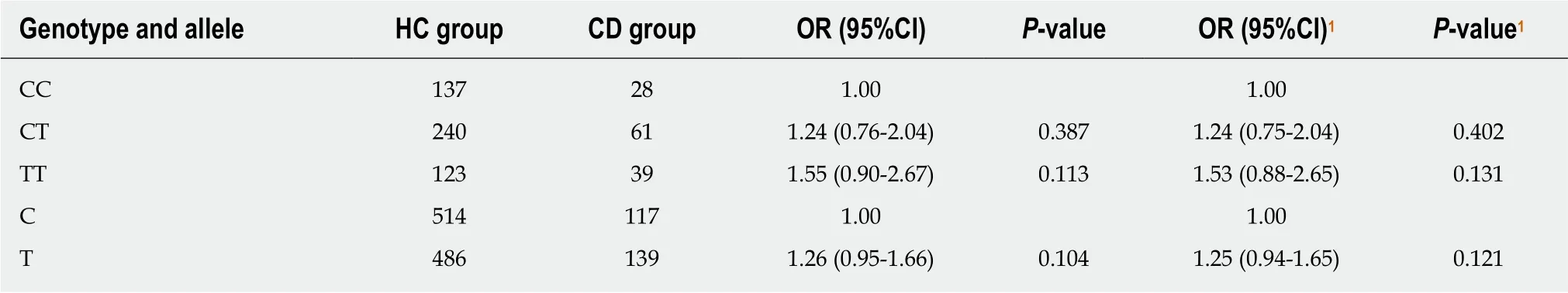
Table 6 Genotype and allele frequency distributions of rs1143627 in the Crohn’s disease and healthy controls groups
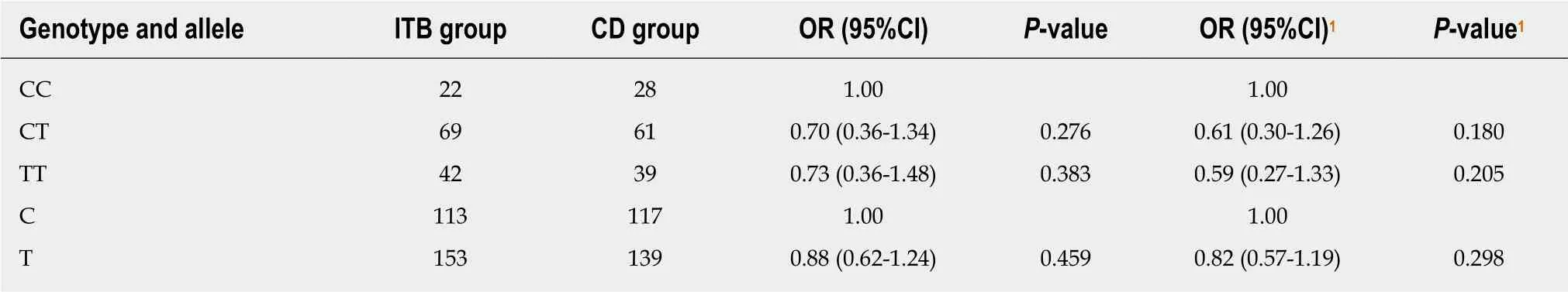
Table 7 Genotype and allele frequency distributions of rs1143627 in the intestinal tuberculosis and Crohn's disease groups
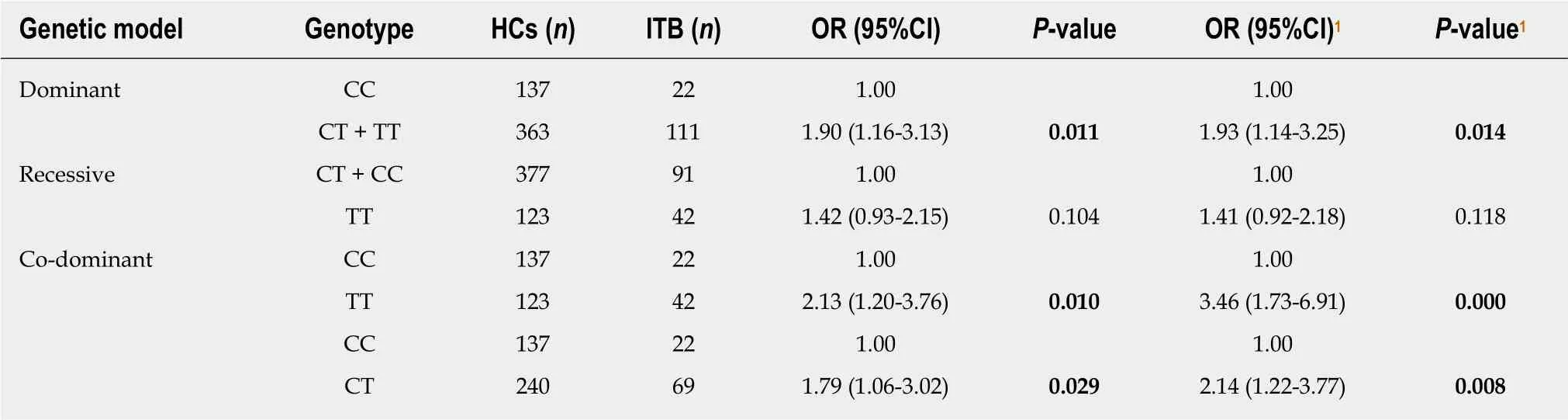
Table 9 Genetic model analysis of rs1143627 in the intestinal tuberculosis population
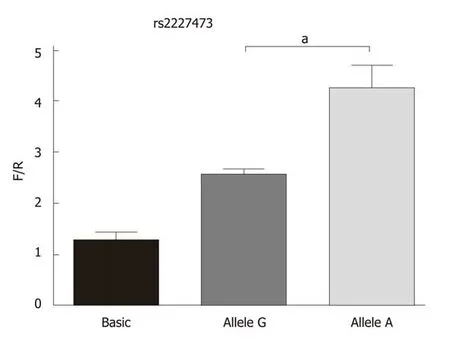
Figure 2 Fluorescence detected in cells transfected with the recombinant or empty vector. Allele G and allele A indicate the fluorescence results of cells transfected with the pGL3-rs2227473_G and pGL3-rs2227473_A plasmids, respectively. F/R: Firefly/renilla luciferase values; aP < 0.05.
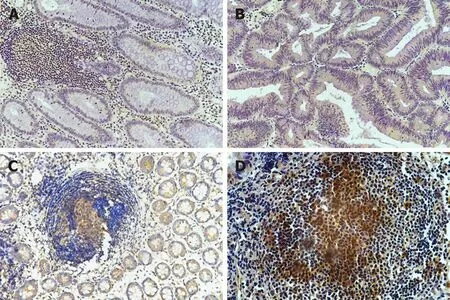
Figure 3 Immunohistochemical staining for interleukin-22 receptor 1 in intestinal biopsy tissues. A: Colon polyps; B: Colon cancer; C: Crohn's disease; D:Intestinal tuberculosis. Magnification, 100×.
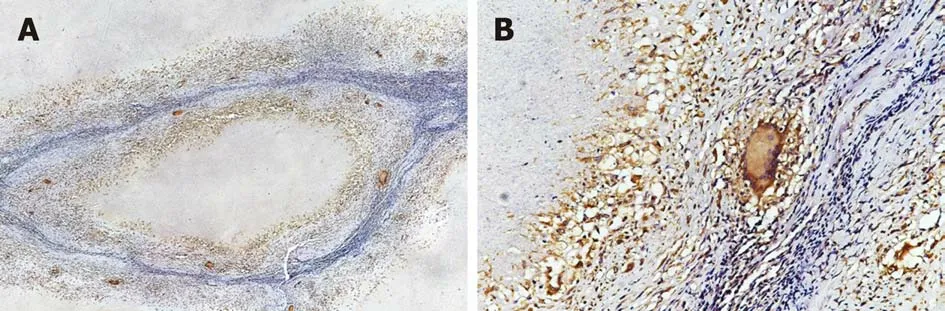
Figure 4 In typical caseous necrotic granulomas, Langhans giant cells with high interleukin-22 receptor 1 expression are present at the edge of granulomas.
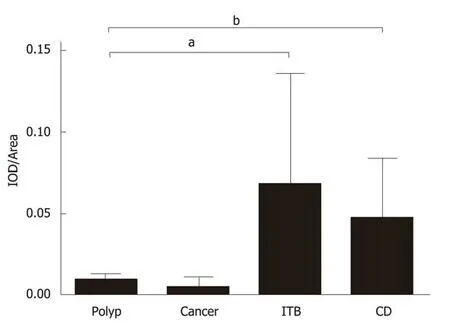
Figure 5 Total integrated optical density/total area of immunohistochemical images. IOD: Integrated optical density; CD: Crohn's disease; ITB: Intestinal tuberculosis; aP < 0.05, bP < 0.01.
ARTICLE HIGHLIGHTS
Research background
Intestinal tuberculosis (ITB) and Crohn’s disease (CD) are two kinds of intestinal granulomatous diseases with very similar clinical manifestations. In recent years, with the changes in epidemiology, these two diseases have often been difficult to identify during clinical work in developing countries. Misdiagnosis can have serious consequences for the patients. The impact of individual genetic diversity on ITB and CD susceptibility and the specific mechanism are still not clear.
Research motivation
Recent studies have shown that Th17 cells play a key role in mucosal immune responses.Differentiation of Th17 cells is regulated by the interleukin (IL)-23/IL-17 axis. We hypothesized that this axis-related gene functional polymorphism sites can affect the susceptibility to ITB and CD.
Research objectives
To determine the difference in genetic susceptibility between ITB and CD and to further elucidate the specific mechanism of action of differentially distributed genes in the occurrence and development of diseases, which will contribute to the identification of diseases and the implementation of individualized treatment.
Research methods
This case-control study was conducted by investigating ITB and CD patients who were admitted to the Jiangxi Provincial People's Hospital in the past 10 years. A total of 133 ITB, 128 CD, and 500 healthy patients were included, and their clinical data were recorded. Based on our previous findings, four single-nucleotide polymorphisms (SNP) loci located in genes involved in the IL-23/IL-17 axis were screened for genotyping of the subjects. Based on the results of the typing, we focused on the study of IL22 rs2227473. The dual luciferase reporter assay system was used to determine the transcriptional regulatory effect of rs2227473 on IL-22, and the IL-22-IL-22R axis was analysed by immunohistochemistry in different intestinal diseases.
Research results
According to our study, T cell spot test positive rate and abnormal lung imaging are helpful for identifying ITB and CD. Using unconditional logistic regression analysis to adjust covariates suggests that rs2227473 and rs1143627 polymorphisms affect ITB susceptibility instead of CD.Dual luciferase assay showed that the rs2227473 locus affects the transcriptional activity of the IL22 gene. Immunohistochemical staining suggests that the expression of IL-22R1 is significantly increased in ITB and CD compared with intestinal polyps and colon cancer. This differential expression may be used to identify intestinal diseases; more importantly, IL-22R1 is expressed in intestinal lymphoid tissues, especially in multinucleated giant cells. This finding breaks the notion that IL-22R1 expression is generally restricted to non-haematopoietic cells.
Research conclusions
IL22 SNP rs2227473 G allele is a risk factor for developing ITB. This finding is informative for further studies of the pathogenesis of tuberculosis. Furthermore, by analysing the baseline characteristics of the surveyed population, we discovered that the findings from T cell spot test and lung imaging were valuable for the differential diagnosis between ITB and CD, providing evidence-based support for clinical diagnosis. Finally, we found that IL-22R1 is expressed in multinucleated giant cells and that the IL-22-IL-22R axis may play an important role in the coordination of innate and adaptive immunity.
Research perspectives
The IL-23/IL-17 axis gene polymorphisms may affect the occurrence and development of tuberculosis by affecting the differentiation of Th17 cells and the expression of related cytokines.IL-22R1 plays an important role not only in maintaining mucosal barrier function in innate immunity but also in the adaptive immunity of inflammatory diseases, indicating that previous views on the safety of IL-22-IL-22R1 systemic therapy need to be reassessed.
ACKNOWLEDGEMENTS
The authors thank all of the staff for their excellent work in the Pathology Department of Jiangxi Provincial People's Hospital, Nanchang, China.
杂志排行
World Journal of Gastroenterology的其它文章
- From 2-dimensional to 3-dimensional: Overcoming dilemmas in intestinal mucosal interpretation
- Optimizing radiotherapy with immune checkpoint blockade in hepatocellular carcinoma
- Contribution of pancreatic enzyme replacement therapy to survival and quality of life in patients with pancreatic exocrine insufficiency
- Hepatocellular adenoma: An unsolved diagnostic enigma
- Trimethylamine N-oxide attenuates high-fat high-cholesterol dietinduced steatohepatitis by reducing hepatic cholesterol overload in rats
- Brucea javanica oil emulsion improves the effect of radiotherapy on esophageal cancer cells by inhibiting cyclin D1-CDK4/6 axis
