Brucea javanica oil emulsion improves the effect of radiotherapy on esophageal cancer cells by inhibiting cyclin D1-CDK4/6 axis
2019-06-13ZhongHuaQiuWeiWeiZhangHongHuaZhangGuiHuaJiao
Zhong-Hua Qiu, Wei-Wei Zhang, Hong-Hua Zhang, Gui-Hua Jiao
AbstractBACKGROUND Esophageal cancer is one of the most common cancers around the world, and it has high incidence and mortality rates. The conventional therapy for esophageal cancer is radiotherapy, although its effect is highly limited by the resistance of esophageal cancer cells. Thus, strong radiosensitizers can be very crucial during radiotherapy against esophageal cancer. Brucea javanica oil emulsion (BJOE) is a widely used drug against various cancers, such as liver, colon, and ovarian cancer. However, its anti-cancer effect and mechanism and the use of BJOE as a radiosensitizer have not been explored in esophageal cancer.AIM To evaluate the anti-cancer effect and mechanism of BJOE and explore the potential use of BJOE as a radiosensitizer during radiotherapy.METHODS The inhibitory effect of BJOE and its enhancement function with radiation on cell viability were examined with the calculated half-maximal effective concentration and half-maximal lethal concentration. The influence of BJOE on cell migration and invasion were measured with EC109 and JAR cells by wound-healing and transwell assay. Clonogenesis and apoptotic rate, which was measured by Hoechst staining, were investigated to confirm its enhancement function with radiation. To investigate the molecular pathway underlying the effect of BJOE,the expressions of several apoptosis- and cycle-related proteins was detected by western blotting.RESULTSOur results demonstrated that BJOE inhibited the growth of esophageal cancer cell lines more than normal cell lines, and it markedly reduced migration and invasion in esophageal cancer cells (EC109 and JAR). Moreover, it promoted cell apoptosis and enhanced the effect of radiotherapy against esophageal cancerous cells. In the viability test, the values of half-maximal effective concentration and half-maximal lethal concentration were reduced. Compared to the control, only around 1/5 colonies formed when using BJOE and radiation together in the clonogenic assay. The apoptotic rate in EC109 was obviously promoted when BJOE was added during radiotherapy. Our study suggests that the expression of the apoptosis-proteins Bax and p21 were increased, while the expression of Bcl-2 was stable. Further detection of downstream proteins revealed that the expression of cyclin D1 and cyclin-dependent kinase 4/6 were significantly decreased.CONCLUSION BJOE has a strong anti-cancer effect on esophageal cancer and can be used as a radiosensitizer to promote apoptosis in cancerous esophageal cells via the cyclin D1-cyclin-dependent kinase 4/6 axis.
Key words: Esophageal cancer; Brucea javanica oil emulsion; Radiosensitizer; Apoptosis;Cyclin D1-CDK4/6 axis the use is non-commercial. See:http://creativecommons.org/licen ses/by-nc/4.0/
INTRODUCTION
Esophageal cancer is the eighth most frequent cancer worldwide and the sixth most common cause of death from cancer. It affects more than 450000 people all over the world and its incidence is increasing[1,2]. China is one of the most severely affected areas and has the highest morbidity and mortality in 2015[3]. In China, esophageal squamous cell carcinoma (ESCC) accounts for more than 90% of the total cases of esophageal cancer[4]. Surgical treatment is a common clinical therapy for esophageal cancer. Postoperative radiotherapy also is an important treatment for reducing the risk of relapse of ESCC[5]. However, the 5-year overall survival rate still is low, only 20%-30%[6]. Therefore, it is urgent to identify a clinical treatment that is an effective radiosensitizer with a strong effect on inhibiting the relapse and proliferation of cancer cells.
The mechanism of radiotherapy against cancerous cells is the induction of cell apoptosis[7-9]. Bcl-2 and Bax are apoptosis-related proteins. Bcl-2 is an inhibitor of apoptosis[10], and Bax is a pro-apoptotic factor[11]. A previous study has shown that the low expression of Bcl-2 and high expression of Bax are related to stronger sensitivity to radiotherapy[12].
The cell cycle is closely related to apoptosis. Cyclin-dependent kinase (CDK),cyclin, and CKI (CDK inhibitor) are important factors that regulate the cell cycle.Cyclin D1 and CDK4/6 are primary mitogens in the G1 phase that is involved in cell division[13]. It has been reported that downregulating the expression of CDK4/6 and Cyclin D1 led to the block of G0/G1 transition, which causes cancerous cell apoptosis and suppression of the migration[14,15]. Inhibition of CDK4/6 is resistant to cancerous cells by increasing apoptosis[16,17]. Inhibition of CDK4/6 also can induce the degradation of cyclin D1[18]and then enhance the sensitivity of cancer cells to radiotherapy[19,20]. On the contrary, the overexpression of Cyclin D1 drives tumor cell proliferation[21]. P21 belongs to CKI, which is an inhibitor of CDK4/6. The overexpression of p21 can sensitize cancerous cells to apoptosis[22]. The expression of p21 is positively related to decreased proliferation in cancerous cells[23].
Brucea javanica (L.) Merr. (Simaroubaceae) is an evergreen shrub and is widely distributed in Southeast Asia and northern Australia. Brucea javanica oil emulsion(BJOE) is the fatty oil that is extracted from the desiccative ripe fruit of B. javanica. It possesses various kinds of biological activities, such as anti-fungal, anti-oxidative,anti-inflammatory, and anti-cancer[24-27]. Recent studies have found that BJOE could inhibit cell proliferation and induce cell apoptosis in various malignancies, such as liver, colon, and ovarian cancers. However, its function and mechanism in esophageal cancer was little known[28-31].
In this study, we investigated the effect of BJOE on viability, migration, and invasion of esophageal carcinoma cells. The effect of BJOE combined with radiotherapy on esophageal carcinoma cells was evaluated as well. To investigate its mechanism in esophageal cancer, the expression of apoptosis-related proteins (Bcl-2 and Bax) and cycle-related proteins (p21, cyclin D1 and CDK4/6) was examined, and it was demonstrated that BJOE, as a radiosensitizer, inhibited esophageal cancer cells via the cyclin D1-CDK4/6 pathway.
MATERIALS AND METHODS
Cell lines and cell culture
Esophagus cancer cell lines were used in the experiments. EC109, JAR, TE-1, and TE-10 cells were obtained from the China Center for Type Culture Collection (Wuhan,China). HEEC was purchased from BeNa culture Collection (BNCC337729, Beijing,China). EC109, JAR, TE-1, and TE-10 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Waltham, MA, United States), while HEEC cell was cultured in CM1-1 medium with 90% DMEM-H and 10% fetal bovine serum(Biological Industries, Cromwell, CT, United States). All cells were placed in a 37 °C incubator with 5% CO2.
Cell viability assay
EC109, JAR, TE-1, TE-10, and HEEC cells were treated with different amounts of BJOE(purity = 10%, Pharmaceutical factory of Shenyang Medical University, Shenyang,China) to detect cell viability by ATPlite assay (Perkin Elmer, Waltham, MA, United States) according to the manufacturer’s instructions.
Wound-healing migration and transwell invasion assay
Wound-healing migration and transwell invasion assay were both carried out on EC109 and JAR cells. With 8 μg/mL of BJOE treatment, cells were wounded using a 10 μL pipette tip. After 24 h, the cells that grew in the wounded area were calculated in the wound-healing migration assay. Using the same amount of BJOE, transwell invasion assay was carried out after 48 h with an optical microscope.
Clonogenic assay
Clonogenic assay was performed to determine the coordinated effect of radiotherapy and BJOE on EC109 and JAR cells. Briefly, after treatment (only radiotherapy or both radiotherapy and 8 μg/ml of BJOE), cells were trypsinized by 0.25% trypsin (Gibco),resuspended, and cultured in six-well cell culture plates (Corning-Costar, Corning,NY, United States). Later, cells were stained by Crystal Violet and the number of colonies was counted.
Detection of apoptosis
Radiotherapy, 8 μg/mL of BJOE, or both radiotherapy and 8 μg/mL of BJOE were used to treat EC109 cells, respectively. Hoechst Staining Kit (C0003, Beyotime,Shanghai, China) was used to detect apoptosis according to the manufacturer’s instructions.
Western blot
Anti-BCL-2 antibody (ab32124), Anti-P21 antibody (ab109520), Anti-BAX antibody(ab32503), Anti-cyclin D1 antibody (ab16663), Anti-WEE1 antibody (ab203236), Anti-CDK4 antibody (ab199728), Anti-CDK6 antibody (ab124821), and Anti-GAPDH antibody (ab181602) were used as primary antibodies (Abcam, Cambridge, United Kingdom), respectively. Protein were separated by 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto membrane of polyvinylidene difluoride (PVDF). Later, the membranes were incubated with primary and secondary antibodies, respectively. Enhanced chemiluminescence reagent was used to visualize protein.
Statistical analysis
Data were analyzed by using Graphpad (La Jolla, CA, United States). All experiments were repeated independently at least three times. Two-tailed t-test was used for comparison analysis between two populations, and the F-test analysis of variance and the post-hoc Tukey test were used for multiple comparisons analysis. All P values were determined, and statistical significance was set ataP < 0.05,bP < 0.01.
RESULTS
The killing effect of BJOE on esophageal carcinoma cells
To investigate the effect of BJOE on the growth of esophageal carcinoma cells, cell viability assays were performed with increasing concentrations of BJOE (from 0 to 128 μg/mL). We found that the viability of all types of cells decreased with the increasing BJOE concentration (Figure 1A). At a concentration range of 0 to 64 μg/mL BJOE, the killing effect on esophageal carcinoma cells was significantly stronger than that of normal human HEEC cells, in which its viability rate was slightly reduced. However,at 128 μg/mL, BJOE had a similar poisoning effect on esophageal carcinoma cells and normal human HEEC cells. Thus, at low and medium concentrations of BJOE,esophageal carcinoma cells were more sensitive to BJOE than normal cells. Based on the value of the half-maximal effective concentration (EC50) and the half-maximal lethal concentration (LC50) of all cell lines, this conclusion was further confirmed(Figure 1B). The average EC50 in esophageal carcinoma cells (EC109, JAR, TE-1, and TE-10) was 46.98 μg/mL, while the EC50 of HEEC was 82.68 μg/mL. Similarly, the average LC50 in esophageal carcinoma cells (4.05 μg/mL) was lower than that in HEEC (5.37 μg/mL). These results suggest that BJOE was able to inhibit effectively the growth of esophageal carcinoma cells but had slight influence on normal cells at low and medium concentrations.
The migration and invasion capacities of esophageal carcinoma cells were impaired by BJOE
Migration and invasion are the most important features of tumors and are also the main reasons of recurrence and death. To determine whether BJOE can inhibit the migration and invasion of esophageal carcinoma cells, we performed wound-healing assays and transwell invasion assays. Esophageal carcinoma cells lines EC109 and JAR were selected for the assays using 8 μg/mL BJOE, at concentration at which cell viability was reduced by about 30%. The wound-healing assays showed that BJOE significantly reduced the migration ability of EC109, while the wound width of JAR with BJOE treatment was just slightly broader than that of the control after 24 h(Figure 2A). However, results from the transwell invasion assays suggested that the invasion ability of both EC109 and JAR were significantly reduced by BJOE (Figure 2B).
The BJOE promotes apoptosis in esophageal carcinoma cells during radiotherapy
Radiotherapy is a common method to treat malignant tumors. To explore the potential of BJOE as a radiosensitizer, we analyzed the viability of EC109 and JAR on radiotherapy in combination with BJOE. As shown in Figure 3A, BJOE improved the killing effect of radiotherapy on EC109 and JAR, as the viability of EC109 and JAR cells were significantly decreased. Furthermore, the values of radiotherapy EC50 and radiotherapy LC50 of EC109 and JAR were also consistent with this finding. The average EC50 of cells treated with BJOE was 6.53 μg/mL, while the average EC50 of control was 8.32 μg/mL. Similarly, the average LC50 of cells treated with BJOE (3.25 μg/mL) was lower than control (4.01 μg/mL), which suggested that BJOE increased the sensitivity of esophageal carcinoma cells to radiotherapy (Figure 3B).
To confirm this finding, we performed the clonogenic assay. In cells treated with both radiotherapy and BJOE, there were only around a tenth (EC109) and a fifth (JAR)of normalized colonies formed compared with the control, which suggested that BJOE further enhanced the inhibition effect of radiotherapy on the growth of EC109 and JAR (Figure 3C). The apoptotic rates of EC109 were also measured during combined radiotherapy and BJOE treatment, and the results confirmed the enhancement of radiosensitivity. The apoptotic rate of EC109 after radiotherapy in combination with BJOE was increased more than radiotherapy or BJOE alone (Figure 3 D).
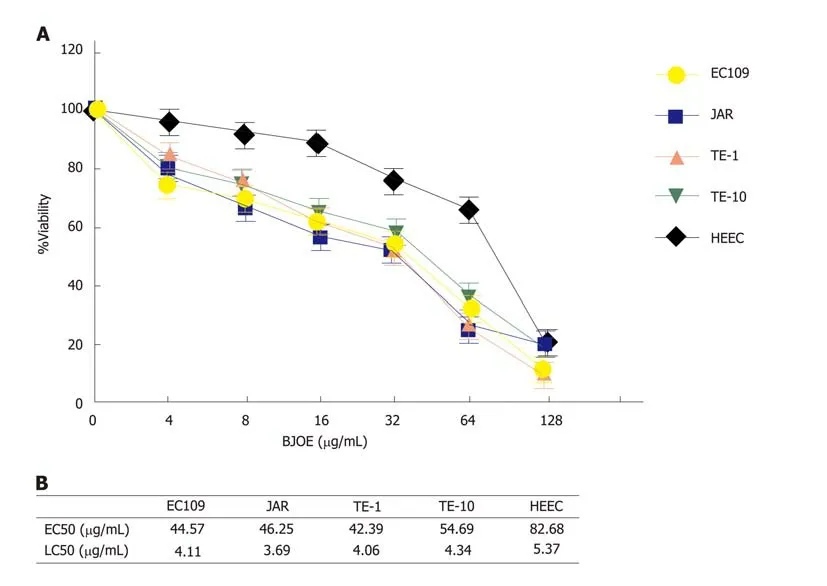
Figure 1 The inhibitory effect on esophageal carcinoma cells by Brucea javanica oil emulsion. A: Dose-response assays. The viability rates of HEEC and esophageal carcinoma cells lines were analyzed with BJOE. At least three repeats were carried out, and the mean ± SD is presented; B: The values of EC50 and LC50 of all cell lines. BJOE: Brucea javanica oil emulsion; SD: Standard deviation.
The BJOE radiosensitizes esophageal carcinoma cells via cyclin D1-CDK4/6 axis
To investigate the mechanism of BJOE in enhancing the effect of radiotherapy, we examined the expression level of the apoptosis-related proteins p21, Bcl-2, and Bax.Western blot analysis suggested that the expression of p21 and Bax increased dramatically, while the expression of Bcl-2 remained stable after radiotherapy and BJOE treatment (Figure 4A). Thus, it BJOE may promote the effect of radiotherapy by increasing the expression of p21 and Bax to induce cell apoptosis.
Since P21, a CKI, is involved in cell cycle regulation, we further examined the expression of cell cycle related proteins (WEE1, Cyclin D1, and CDK4/6) (Figure 4B).The result suggested that the expression of WEE1 did not correlate with radiotherapy and BJOE, while the expression of cyclin D1 and CDK4/6 were significantly inhibited,which was consistent with the previous studies. This result suggested that BJOE can radiosensitize esophageal carcinoma cells via the cyclin D1-CDK4/6 axis.
DISCUSSION
Esophageal cancers, including squamous cell carcinoma and adenocarcinoma, have high incidence and mortality rates[4]. Thus, it is crucial to develop effective drugs or treatments that reduce the risk of metastasis and recurrence in esophageal cancer.Traditional Chinese medicines or natural extracts provide a promising way and are of high effect and low toxicity compared to other types of drugs. BJOE is used to treat various cancers[27]and may have the potential as a radiosensitizer in esophageal cancer. Here, we investigate the inhibitory effect of BJOE on esophageal cancer and the enhancement of radiosensitivity of esophageal cancer induced by BJOE.
In this study, it was demonstrated that the growth of esophageal cancer can be inhibited significantly by BJOE. Especially at low and medium concentrations, the inhibitory effect of BJOE on esophageal cancerous cells was much higher than on normal cells. EC50 and LC50 values further verified the conclusion, suggesting it may be the suitable concentration of BJOE in the application. In addition, it was also demonstrated that 8 μg/mL BJOE significantly inhibits invasion and migration of esophageal cancerous cells, while the inhibitory effect of migration of JAR was lower than that of EC109. This may be because the resistance of different cells varies or that the concentration might not be suitable for JAR. These findings suggest that BJOE is a promising treatment for esophageal cancer.
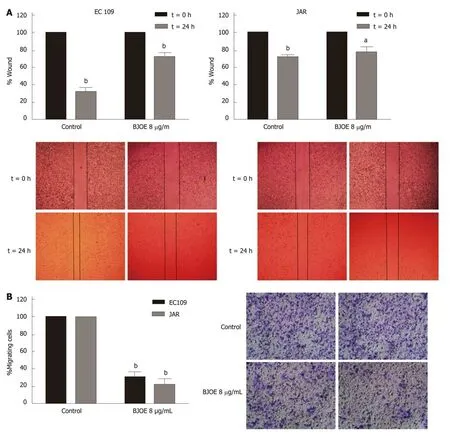
Figure 2 The migration and invasion of esophageal carcinoma cells treated with Brucea javanica oil emulsion. Wound healing assays (A) and transwell invasion assays (B) were used in 8 μg/mL of Brucea javanica oil emulsion. At least three repeats were carried out, and the mean ± SD was presented, aP < 0.05; bP <0.01. BJOE: Brucea javanica oil emulsion; SD: Standard deviation.
Viability and clonogenic assays were employed to determine whether BJOE could enhance radiosensitivity of esophageal cancer. Our results showed that BJOE markedly inhibited the viability and proliferation of EC109 and JAR cells during radiotherapy. In addition, it was shown by Hoechst 33, 258 staining that BJOE combined with radiotherapy can significantly promote apoptosis of esophageal cancerous cells, indicating the possibility that the radiosensitivity of esophageal cancerous cells was increased due to the apoptosis induced by BJOE.
Cell apoptosis is a type of programmed cell death and is regulated by multiple genes[32], making it an effective way against esophageal cancer[14]. Our results demonstrated that BJOE can induce cell apoptosis in EC109 and JAR cells and improve the effect of radiotherapy. Tumor hypoxia has an inhibitory effect on radiotherapy. By western blot analysis, we found that the expression of Bax increased,while Bcl-2 remained stable. A previous study had shown that Bax and Bcl-2 are coregulators of cell apoptosis[32]. It has been reported that hypoxia-inducible factor 1α could regulate the expression of Bax and Bcl-2 by inhibiting p53, leading to inhibition of apoptosis. Pan et al[33]found that BJOE could increase apoptosis of ESCC cells by inhibiting the expression of hypoxia-inducible factor 1α α. Hence, up-regulation of Bax expression may be caused by the suppression of BJOE in esophageal cancer.
Meanwhile, the expression of p21 dramatically increased, which suggested that the apoptosis induced by BJOE might be involved with cycle-related proteins as well.Cyclin D1 and CDK4/6 regulate cell cycle progression from G1 to S phase[34], which is an important target of anti-proliferation in the cancer treatment[35,36]. Our results showed that the expression of cyclin D1 and CDK4/6 was significantly downregulated, suggesting that JOE-mediated apoptosis might be induced by blocking the G0/G1 transition. Thus, it was concluded that BJOE enhanced the effect of radiotherapy via the cyclin D1-CDK4/6 axis.

Figure 3 The promotion of the apoptotic rate of esophageal carcinoma cells during radiotherapy in combination with Brucea javanica oil emulsion. A: The viability of EC109 and JAR treaded with BJOE and radiotherapy; B: The values of EC50 and LC50 of EC109 and JAR; C: Clonogenic assays using radiotherapy in combination with BJOE (C) and cell apoptotic detection (D). In all the above, at least three repeats were carried out, and the mean ± SD is presented, aP < 0.05; bP <0.01. BJOE: Brucea javanica oil emulsion; SD: Standard deviation; EC50: Half-maximal effective concentration; LC50: Half-maximal lethal concentration.
In conclusion, BJOE, as a traditional Chinese medicine, not only inhibits the growth and capability of migration and invasion of esophageal cancerous cells but also improves the effect of radiotherapy on esophageal cancer by promoting cell apoptosis.These findings may provide a novel way for the therapy of esophageal cancer.
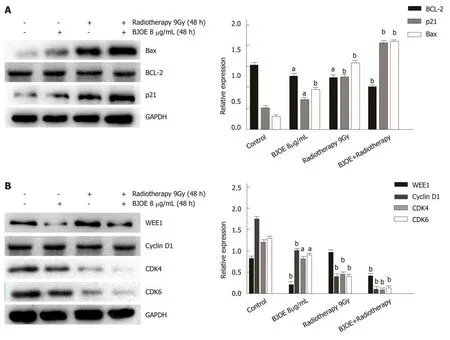
Figure 4 Brucea javanica oil emulsion radiosensitizes esophageal carcinoma cells via cyclin D1-CDK4/6 axis. A: Western blot analysis and the relative expression of BCL-2, BAX, and p21; B: Western blot analysis and the relative expression of WEE1, Cyclin D1, CDK4, and CDK6. Above will be decapitalized as mean± SD, aP < 0.05; bP < 0.01. BJOE: Brucea javanica oil emulsion; CDK: Cyclin-dependent kinase.
ARTICLE HIGHLIGHTS
Research background
Esophageal cancer is one of the most common cancers around the world. Brucea javanica oil emulsion (BJOE) is widely used against various cancers, but its anti-cancer effect and molecular mechanism on esophageal cancer is not clear.
Research motivation
To develop new anti-cancer drugs or radiosensitizer to improve the efficacy of radiotherapy against esophageal cancer.
Research objectives
To explore BJOE’s anti-cancer effect and its potential as a new radiosensitizer on esophageal cancer and to investigate its molecular pathways.
Research methods
The inhibitory effect of BJOE on esophageal carcinoma cells was examined by cell viability assays. Cell migration and invasion of EC109 and JAR cells treated with BJOE were measured by wound-healing assays and transwell assay. The apoptosis rate of EC109 treated with BJOE combined with radiotherapy was measured by Hoechst staining. The expression of apoptosisand cycle-related proteins was detected by western blotting.
Research results
Our results suggested that BJOE strongly inhibits the growth of esophageal cancer cell lines.BJOE significantly reduced the migration and invasion abilities of EC109 and JAR. More importantly, BJOE promoted apoptosis of esophageal carcinoma cells to enhance the effect of radiotherapy. The expression of Bax and p21 were increased, while the expression of Bcl-2 remained stable. The expression of cyclin D1 and cyclin-dependent kinase 4 (CDK4)/6 was significantly decreased.
Research conclusions
BJOE could effectively suppress cell growth and promote cell apoptosis of esophageal cancerous cells during radiotherapy through cyclin D1-CDK4/6 axis.
Research perspectives
As a potential anti-cancer drug or radiosensitizer, BJOE may be a viable for esophageal cancer in the future, although more research is needed to verify its effect and safety. Besides, the cyclin D1-CDK4/6 axis that mediated BJOE’s anti-cancer effect may provide important information and therapeutic targets against esophageal cancer. Additional pathways about the cell cycle may be worthy to investigate and discuss in the future.
杂志排行
World Journal of Gastroenterology的其它文章
- From 2-dimensional to 3-dimensional: Overcoming dilemmas in intestinal mucosal interpretation
- Optimizing radiotherapy with immune checkpoint blockade in hepatocellular carcinoma
- Contribution of pancreatic enzyme replacement therapy to survival and quality of life in patients with pancreatic exocrine insufficiency
- Hepatocellular adenoma: An unsolved diagnostic enigma
- Trimethylamine N-oxide attenuates high-fat high-cholesterol dietinduced steatohepatitis by reducing hepatic cholesterol overload in rats
- Interleukin-22 receptor 1 is expressed in multinucleated giant cells:A study on intestinal tuberculosis and Crohn's disease
