Contribution of pancreatic enzyme replacement therapy to survival and quality of life in patients with pancreatic exocrine insufficiency
2019-06-13PeterLayerNataliyaKashirskayaNatalyaGubergrits
Peter Layer, Nataliya Kashirskaya, Natalya Gubergrits
Abstract The objective of this study was to analyze the current evidence for the use of pancreatic enzyme replacement therapy (PERT) in affecting survival and quality of life in patients with pancreatic exocrine insufficiency (PEI). Systematic searches of the literature were performed using the PubMed database. Articles were selected for inclusion if they reported findings from trials assessing the effects of PERT on quality of life, survival, malabsorption, growth parameters (such as height, body weight and body mass index), or gastrointestinal symptoms (such as abdominal pain, stool consistency and flatulence). PERT improved PEI-related malabsorption and weight maintenance in patients with cystic fibrosis, chronic pancreatitis, pancreatic cancer, and post-surgical states. In patients with chronic pancreatitis, PERT improved PEI-related symptoms and quality of life measures.Several small retrospective studies have also suggested that PERT may have a positive impact on survival, but long-term studies assessing this effect were not identified. PERT is effective for treating malnutrition and supporting weight maintenance, and it is associated with improved quality of life and possibly with enhanced survival in patients with PEI. However, there is evidence that not all patients with PEI receive adequate PERT. Future work should aim to assess the long-term effects of PERT on the survival of patients with PEI.
Key words: Pancreatic exocrine insufficiency; Pancreatic enzyme replacement therapy;Survival; Quality of life; Malabsorption; Cystic fibrosis; Chronic pancreatitis; Pancreatic cancer; Post-surgical states
INTRODUCTION
Effectively understanding the nutritional implications of pancreatic exocrine insufficiency (PEI) is fundamental if physicians are willing to improve the survival and quality of life in patients with compromised pancreatic function[1]. In patients with PEI, secretion of pancreatic enzymes and bicarbonate is insufficient to maintain a normal digestion[2,3]. Impaired food digestion can result in nutrient malabsorption and malnutrition, as well as disturbed regulation of gastrointestinal (GI) motor and secretory functions[2,3]. Thus, patients with PEI frequently complain of steatorrhea,diarrhea, and/or other usually unspecific GI symptoms associated with fat maldigestion[4-8]. PEI may be undiagnosed in some patients who may be suspected to suffer from other GI disorders, such as irritable bowel syndrome[8]. Furthermore,diagnosis may be difficult to establish, particularly with mild or moderate disease[9].Awareness and education in the management of PEI may be needed to ensure that patients affected by the severe and chronic diseases with which it is associated are managed appropriately, in order to increase their survival and improve long-term quality of life[2,7,10].
Often, patients develop PEI as a result of changes to the pancreas caused by lifelimiting or terminal diseases[1]. Conditions such as cystic fibrosis (CF) or chronic pancreatitis (CP) lead to loss of pancreatic parenchyma, while pancreatic cancer may cause obstruction of the pancreatic duct, particularly if the tumor is located in the head of the pancreas. Celiac disease results in decreased pancreatic stimulation, and Zollinger-Ellison syndrome inactivates pancreatic enzymes. Surgical resections, such as gastrectomy or duodenopancreatectomy, may also lead to decreased and delayed pancreatic stimulation and loss of pancreatic parenchyma[11,12]. Overall, the most common causes of PEI are CF, CP, and pancreatic cancer[2].
Malnutrition resulting from PEI is the main clinical manifestation of the disease[12].It can be associated with serious consequences, leading to increased morbidity and reduced survival[1,7], and it may be associated with a reduced quality of life[13].Malnutrition resulting from PEI in CF can lead to stunted growth in childhood,weight loss in adults, and deterioration of pulmonary function; PEI can also lead to decreased length of survival compared with pancreatic-sufficient patients[2,14]. In non-CF conditions, e.g., CP and pancreatic cancer, PEI-related malnutrition is associated with weight loss that can lead to the development of comorbidities, and negatively impacts on patient prognosis[15-17].
Pancreatic enzyme replacement therapy (PERT) involves supplementation of pancreatic enzymes in patients with PEI and is effective in reducing the severity of GI symptoms; it also improves GI dysregulation and nutrient absorption, thus preventing malnutrition[7,15]. PERT has the potential to improve the nutritional status of patients suffering from PEI and, therefore, may increase survival and improve quality of life of these patients[1,2,7,16]. Treatment is usually well tolerated, with mild GI symptoms (e.g., stomach pain, nausea, and bloating) reported as major adverse events[7]. Very high doses of PERT have been associated with fibrosing colonopathy in patients with CF; however, such high doses are not recommended by current treatment guidelines[7,17,18].
This review aims to analyze the current evidence for the effectiveness of PERT in increasing survival and improving quality of life in patients with PEI, particularly those with severe and chronic underlying diseases such as CF, CP, or pancreatic cancer (see Supplementary material for literature search details).
EFFECTS OF PERT ON MALNUTRITION, QUALITY OF LIFE,AND SURVIVAL OF PATIENTS WITH PEI
CF
No studies directly assessing the link between PERT and increased survival of patients with PEI and CF were identified in the literature, and only one study assessed quality of life. However, a number of randomized, placebo-controlled studies as well as longer-term, open-label studies evaluating various PERT formulations, e.g., pancrelipase (pancreatin) or liprotamase, were identified and are discussed here, as these provide insights on the effects of PERT on nutritional status,growth parameters, or GI symptoms.
In the seven short-term, randomized, double-blind, placebo-controlled studies evaluated, administration of PERT was shown to significantly increase fat and protein absorption compared with placebo in patients ≥ 7 years of age with CF (P < 0.001;Supplementary Table 1)[19-25]. A typical coefficient of fat absorption (CFA) of 40-50% is observed in patients with PEI and CF without treatment[26]. In the short-term studies included here, the CFA ranged from 72.7% to 88.6% in patients treated with PERT vs 47.4% to 62.8% in patients who received placebo, and the coefficient of nitrogen absorption (CNA) from 80.3% to 87.2% in patients treated with PERT vs 45.0% to 65.7% in patients who received placebo (Supplementary Table 1). Greater improvements in CFA and CNA were noted in severe disease, which is characterized by a placebo CFA < 40%[19,20]or ≤ 50%[21,22]. Stool consistency, frequency, and mean stool fat were improved in patients receiving PERT compared with placebo, as well as other malnutrition-related symptoms, e.g., bloating, abdominal pain, and flatulence.
The positive effects of PERT in patients with CF are further supported by findings from open-label studies in different age groups, including an 8-wk study in children< 2 years of age[27], a 12-wk study in children 1 mo to < 4 years of age[28], a 12-mo study in children ≥ 7 years of age[29], and a 14-d study in children 6-36 mo of age[30](Supplementary Table 2). In addition to improvements in fat and protein absorption, PERT was associated with age-appropriate increases in growth parameters. In children < 2 years of age, significant increases in the weight-for-age z-score were observed after 8 wk of PERT, as well as increases in the length-for-age z-score (Figure 1A)[27]. Similar results were observed over 12 wk of PERT in children 1 mo to < 4 years of age, with marked increases particularly observed in children < 2 years of age (Figure 1B)[28]. In older children ≥ 7 years of age, 12 mo of PERT was shown to lead to and maintain age-appropriate growth and weight gain[29]. During the study in older children ≥ 7 years of age none of the subjects lost > 5% body weight; 67.5% and 62.8% of children 7 to < 12 years of age and 12 to < 17 years of age gained > 5% body weight,respectively[29].
CP
In four short-term, randomized, double-blind, placebo-controlled studies conducted in patients with CP, PERT improved on-treatment CFA and CNA values (Supplementary Table 3)[31-34]. In a trial in 27 patients, fat absorption significantly improved in patients receiving PERT compared with placebo (P = 0.0185)[31]. PERT also led to a significant reduction in fat excretion (P = 0.0181) and stool frequency (P = 0.0015) and improved stool consistency (P = 0.0102). These results are consistent with those obtained in a dose-response, cross-over study evaluating two doses of PERT[32].Patients were randomized to a low-high or high-low dosing sequence. Following 18-22 d of PERT, fat and protein absorption significantly increased from baseline (P <0.001). Furthermore, PERT was associated with significant increases in body weight compared with the placebo run-in period (P ≤ 0.021) and in body mass index (BMI)(P≤ 0.020). PERT also significantly increased high-density lipoprotein cholesterol levels(P < 0.001) compared with the placebo run-in period, while low-density lipoprotein cholesterol and fat-soluble vitamin levels (A, E, and K) remained unchanged over the study duration[32](Supplementary Table 3).
The effects of long-term PERT on nutritional status and body weight of patients with CP were assessed in two open-label studies conducted for up to 1 year (Supplementary Table 4)[35,36]. These were both preceded by a short, randomized phase where the effects of PERT vs placebo on fat and protein absorption were measured. One of these studies was conducted in 54 patients randomized to receive PERT or placebo for 7 d[34], followed by an open-label phase for 6 mo, where all patients in the analysis (n =48) received PERT[35]. At the end of the randomized phase, fat and protein absorption were greater in patients receiving PERT compared with placebo[34]. A clinically relevant and statistically significant improvement in body weight (P < 0.0001; Figure 2A) was observed at the end of the 24-wk, open-label treatment phase[35]. Stool frequency was significantly reduced (P < 0.001), and improvements in clinical symptoms and the Clinical Global Impression scale of clinical symptoms were reported; the proportion of patients with no symptoms or mild symptoms overall increased (49.1% at baseline to 83.0% at study end)[35]. However, no clinically meaningful changes in quality of life [assessed by the 36-item Short Form (SF-36)Health Survey] were observed at the end of the 24-wk treatment period[35].
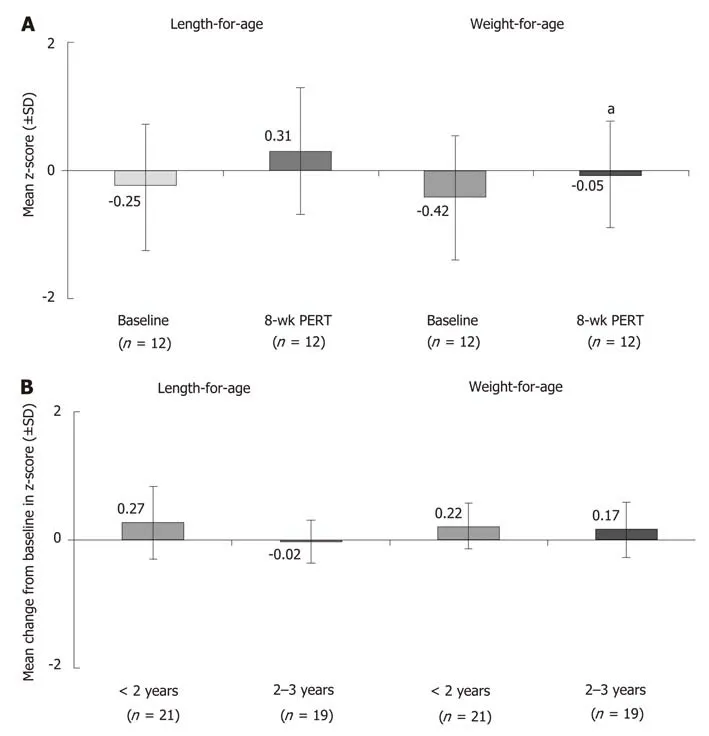
Figure 1 Effects of pancreatic enzyme replacement therapy on body weight in children with cystic fibrosis and pancreatic exocrine insufficiency. A: Age-adjusted z-scores1 for length and weight at baseline and after 8 wk of pancreatic enzyme replacement therapy (PERT) in children < 2 years of age[27]; B: mean change from baseline after 12 wk of PERT in age-adjusted z-scores1 for length and weight in children 1 mo to < 4 years of age[28]. 1Zscores are commonly used to assess growth percentiles and compare patients of different ages and at different rates of growth. It indicates the number of standard deviation away from the mean. aP < 0.05. PERT: Pancreatic enzyme replacement therapy; SD: Standard deviation.
In India, a 1-wk, randomized, double-blind, placebo-controlled study was conducted[33]followed by a 51-wk, open-label extension study[36]. PERT was associated with significant improvements in fat and protein absorption (P = 0.001 and P = 0.005,respectively), together with reductions in mean stool frequency, stool weight, stool fat, and stool nitrogen[33,36]. Moreover, significant improvements in body weight (P =0.001; Figure 2A) and BMI (P = 0.001) were observed after 1 year of PERT[36]. An increase in body weight of ≥ 7% occurred in 61.7% of patients. Overall, the median BMI increased from 19.2 kg/m2at baseline to 20.9 kg/m2at the end of the open-label phase[36].
In both the open-label extension studies discussed above, PERT led to improvements in PEI-related symptoms, such as abdominal pain, stool consistency, and flatulence (Figure 2B)[35,36]. In the 24-wk study, no clinically meaningful changes in quality of life assessed using the SF-36 were observed at the end of the treatment period[35]. However, in the 51-wk study, quality of life was enhanced with PERT;seven of the eight components of the SF-36 and the two summary scores improved(including significant improvements in bodily pain, general health, vitality, roleemotional, mental health, and the mental component summary)[36].
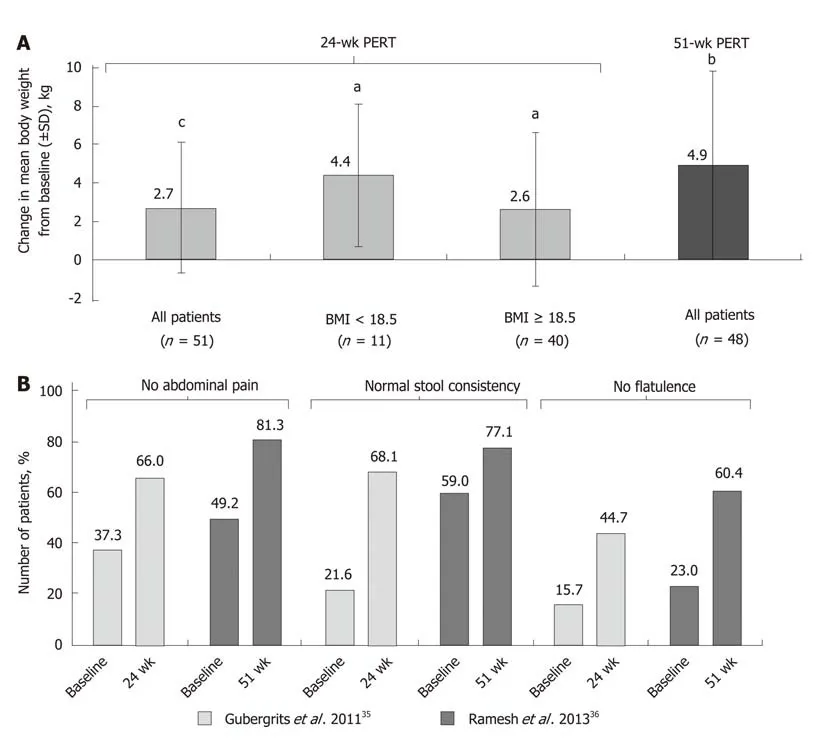
Figure 2 Mean change from baseline in body weight[35,36] (A); improvement in clinical symptoms of pancreatic exocrine insufficiency in patients with cystic fibrosis following long-term pancreatic enzyme replacement therapy[35,36] (B).aP < 0.05; bP < 0.001; cP < 0.0001 vs baseline. BMI: Body mass index; PERT:Pancreatic enzyme replacement therapy; SD: Standard deviation.
Pancreatic cancer
Two studies were identified that described the effects of PERT in patients with unresectable pancreatic cancer. One study in patients with advanced, unresectable cancer of the pancreatic head region and suspected pancreatic duct obstruction reported that PERT prevented weight loss in an 8-wk, randomized, double-blind,placebo-controlled study[37](Supplementary Table 5). Patients randomized to PERT gained weight, while patients on placebo lost weight over the study duration (Figure 3). Furthermore, fat absorption increased by 12% with PERT, whereas it decreased by 8% in the placebo group[37]. A more recent randomized, double-blind, placebocontrolled study suggested that PERT had no significant effect on weight loss in patients with unresectable pancreatic cancer at 8 wk (P = 0.614; Supplementary Table 5)[38]. There was also no improvement in quality of life with PERT, and no significant difference in overall survival (5.84 mo with PERT vs 8.13 mo with placebo; P = 0.744;Supplementary Table 5 and Table 6)[38].
Pancreatic surgery
Two studies were identified that described the effects of PERT in patients that had undergone pancreatic surgery; indications for surgery were CP or pancreatic cancer[39,40]. PERT in patients with partial/total pancreatic resection resulted in significant improvements in fat and protein absorption in a 1-wk, randomized,placebo-controlled study (P < 0.001; Supplementary Table 7)[39]. Patients from this study then received PERT for 51 wk in an open-label extension, where improvements in fat and protein digestion were achieved and maintained to the end of follow-up(Supplementary Table 8)[39]. At this time point, body weight had also significantly increased compared with baseline (P < 0.05) (Figure 4)[39]. In a further small,uncontrolled study, PERT was administered for 2.5 years to patients following pancreatic surgery. Significant weight gain and decreased fecal fat excretion were observed during follow-up[40].
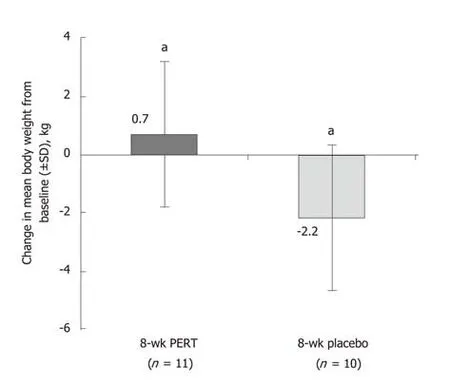
Figure 3 Effects of 8 wk of pancreatic enzyme replacement therapy on body weight of patients with unresectable pancreatic cancer[37].aP < 0.02. PERT: Pancreatic enzyme replacement therapy; SD: Standard deviation.
DISCUSSION
The aim of this review was to analyze the current evidence for the effectiveness of PERT in increasing survival and improving quality of life in patients with PEI,particularly those with chronic and severe underlying diseases such as CF, CP, or pancreatic cancer. Evidence from clinical trials supports the efficacy of PERT in treating PEI-related malabsorption and maintenance of a healthy weight in patients with PEI due to these underlying diseases.
CF is caused by mutations in the gene encoding CF transmembrane conductance regulator (CFTR): a protein highly expressed in pancreatic ductal epithelia,responsible for allowing the flow of anions and fluid into the ductal lumen[41]. As a result of these mutations, patients with CF have a decreased flow of anions and fluid into the pancreatic ductal lumen, leading to precipitation of proteins in the ductal lumen and, therefore, obstruction and damage to the pancreas[41]. Consequently,severe PEI is very common in newborns with CF, and is seen in approximately 85% of cases[18,26,42]. Although we identified a lack of studies in the literature explicitly measuring the link between PERT and prolonged survival of patients with CF,randomized, placebo-controlled studies show that PERT increases fat and protein absorption[19-25], while longer, open-label studies show that PERT improves the relevant clinical outcome of age-appropriate growth and weight gain or maintenance[27-29]. A single retrospective study in Moscow identified a potential link between PERT and life expectancy of patients with CF[43]. This study showed that,between 1993 and 2013, improvements in CF management, which included the introduction of PERT, led to an increase in life expectancy from 16 to 39 years[43]. These data suggest a potential link between PERT and life expectancy of patients with CF,although future long-term studies are needed to investigate this further.
The positive effects of PERT on PEI-associated malabsorption in children < 2 years of age with CF is particularly important as the first 2 years of life represent a period of intense growth, with high energy needs[27]. Therefore, addressing PEI-related malabsorption with PERT in those patients may be crucial to maintaining ageappropriate growth. Furthermore, wasting, which is linked with malnutrition, has been shown to be an independent prognosis predictor for survival in patients with CF[16,26,44,45]. The 5-year prognosis is better in patients with > 85% ideal body weight than in patients below that target (survival: 83.8% vs 53.4%, respectively; P <0.0001)[45]. PEI-related malnutrition and impaired growth correlate with reduced lung function[46], which can ultimately increase mortality. An analysis of the European CF Patient Registry demonstrated that PEI was associated with a statistically significant(P < 0.0001) decrease in the forced expiratory volume (FEV1) vs patients without PEI[47]. Furthermore, patients with PEI were twice as likely to experience severe lung disease (defined as FEV1< 40%) vs patients without PEI[47]. This indicates that the lack of pancreatic exocrine function is associated with worsening prognosis in CF.Collectively, the evidence suggests that preventing malnutrition and maintaining body weight with PERT may improve the prognosis of patients with PEI due to underlying CF.
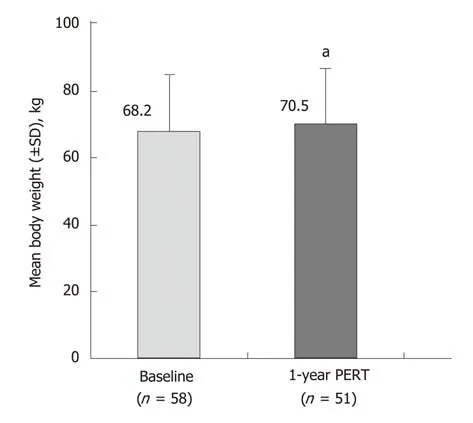
Figure 4 Effects of 51 wk of pancreatic enzyme replacement therapy on body weight of patients following total/partial resection[39].aP < 0.05 vs Baseline. PERT: Pancreatic enzyme replacement therapy; SD: Standard deviation.
CP is characterized by inflammation of the pancreas, leading to fibrosis, atrophy,and irreversible damage to pancreatic tissue[48]; PEI usually develops within 5 to 10 years of disease duration[49]. The presence of PEI in patients with CP is associated with a higher prevalence of diabetes, and lower levels of nutritional markers, e.g.,magnesium, hemoglobin, albumin, prealbumin, and retinol binding protein[50]. The resulting malnutrition can lead to weight loss and can increase the morbidity and mortality risk in untreated patients[12,50]. Clinical trials have demonstrated that PERT improves PEI-related malabsorption and importantly increases body weight in patients with CP[31-36]. However, the impact of PERT on patient survival has only been assessed within the context of pancreatic surgery for CP[51]. The absence of PERT following surgery was a significant and independent risk factor for long-term mortality following hospital discharge, according to a retrospective observational study[51].
Relief of PEI-related symptoms, such as abdominal pain, flatulence, or steatorrhea,can improve quality of life in patients with CP and PEI[13,52]. Furthermore, in a 51-wk study PERT has been shown to lead to an improvement in seven of the eight components of the SF-36 and the two summary scores (including improvements in bodily pain, general health, vitality, role-emotional, mental health, and the mental component summary)[36]. The current evidence base, therefore, suggests that PERT may be effective in enhancing quality of life measures as well as addressing PEIrelated symptoms, malnutrition, weight loss, and increased mortality risk in patients with CP. However, further long-term studies are needed to establish if any link exists between PERT and survival in these patients.
Diagnosis of pancreatic cancer is typically associated with a poor prognosis, as patients usually present at a late stage of disease progression; the 5-year survival rate was estimated at 7% during the 2004-2010 period[53]. Around 20% of patients are eligible for surgical resection of the tumor[54], and median survival ranges from 23.7-39.5 mo for these patients[55]to 8.5-11.1 mo for those with unresectable cancer and metastatic disease[56,57]. One of the predictive factors of survival in patients with advanced cancer is malnutrition[58]. Weight loss of > 10% of pre-illness stable weight is associated with increased metastases and significantly reduced survival compared with patients without dramatic weight loss[59]. Furthermore, weight stabilization in patients with unresectable pancreatic cancer was found to be associated with improved survival and quality of life[60].
PEI is highly prevalent in patients with resectable or unresectable pancreatic cancer,with an estimated prevalence of 50%-100% and 46%-100% in these patients,respectively[54]. This can be attributable to tumor growth, which may result in a combination of obstruction of the pancreatic duct, destruction of pancreatic parenchyma, and duodenal infiltration[3]. Moreover, relevant postoperative anatomical changes may occur after tumor resection, resulting in profound disturbances of coordinated intraluminal mixing of chyme with biliopancreatic secretions[3,11,61,62]. Of note, in patients with unresectable cancer, PEI may not always manifest with symptoms such as steatorrhea[54]. Severe PEI, i.e., fecal elastase-1 levels≤ 20 µg/g, is an independent factor associated with poor prognosis in those patients and should, therefore, be addressed[63].
Although limited data on the effects of PERT in patients with pancreatic cancer are available (as most of the evidence comes from evaluation of patients with CP), clinical evidence suggests that PERT can prevent weight loss both in patients with unresectable pancreatic cancer[37]and patients recovering from pancreatic resection[61].Furthermore, a retrospective analysis conducted in patients with unresectable pancreatic carcinoma found that PERT and nutritional advice were associated with increased survival (301 d [95% confidence interval (CI): 151-451] vs 89 d [95%CI: 30-148]; P = 0.002)[64]. Another retrospective study in patients with unresectable pancreatic cancer similarly reported that PERT resulted in longer survival (189 d[95%CI: 167.0-211.0] vs 95 d [95%CI: 75.4-114.6]; P < 0.001), especially in patients with significant weight loss at diagnosis[65]. The authors concluded that PEI and its treatment should be taken into consideration for optimal patient care, to increase survival and quality of life[65]. Similarly, a recent population-based study in patients with pancreatic cancer found that PERT was associated with an increased medial survival time (274 d vs 140 d; P < 0.001), suggesting a significant benefit for patients[66].Finally, a review of patients undergoing pancreatoduodenectomy for cancer also found that PERT was independently associated with improved survival on multivariate analysis (33.1 mo vs 26.7 mo; P = 0.046)[67]. These findings support the fact that weight loss in patients with terminal pancreatic cancer is often one of the main factors affecting survival and quality of life. Weight loss is often observed postsurgery, but is also observed in non-surgical patients who suffer from fat and protein malabsorption due to PEI[68].
In contrast to the studies summarized above, a recent open-label, randomized trial evaluating the benefits of PERT in 88 patients with unresectable pancreatic cancer revealed no significant differences in BMI or nutritional status between the PERT and non-PERT groups at 8 wk. Median overall survival was 19.0 mo in the PERT group compared with 12.0 mo in the non-PERT group, but this difference was not statistically significant (P = 0.07)[69].
Despite the suggested beneficial effects of PERT in patients with pancreatic cancer,a recent retrospective analysis conducted between 2010 and 2012 showed that PERT is underused in patients with metastatic pancreatic cancer[68]. Over 70% of the 129 patients with pancreatic cancer referred to specialist palliative care had malabsorption-related symptoms, e.g., abdominal pain, bloating, wind, and steatorrhea, but only 21% were prescribed PERT[68]. Similarly, a recent populationbased study reported that only 21.7% of 4554 patients with pancreatic cancer were prescribed PERT[66]. A review of patients undergoing pancreatoduodenectomy for cancer reported that only 43.1% of patients received PERT[67]. Furthermore, a survey conducted in patients following pancreatic surgery (due to CP or pancreatic cancer)and receiving PERT showed that these patients were under-dosed in daily practice[70].Hence, addressing the symptoms of PEI and the associated malnutrition in patients with pancreatic cancer could be key in reducing the significant burden of this disease.Recent clinical opinion is that PERT should be considered for the management of PEI in patients with pancreatic cancer[65]; however, despite PERT being a relatively simple and inexpensive treatment, it has yet to gain recognition from oncologists[71].
CONCLUSION
This review of the current literature supports the effectiveness of PERT in addressing PEI-related malabsorption in patients with CF, CP, or pancreatic cancer. However,there is a lack of long-term trials assessing the effect of PERT on patient survival(especially in patients with CF or CP). Despite these limitations, there is substantial evidence to support the hypothesis that PERT allows patients with PEI to improve body weight by preventing malnutrition, a known risk factor for decreased survival[7].Furthermore, adequate PERT reduces the occurrence of GI symptoms and abdominal pain commonly associated with PEI, thereby improving patients’ quality of life[13,52].
In clinical practice, the absence of PERT, or undertreatment with PERT, may not be uncommon during management of patients with PEI[40,70]. Likely explanations include the variability of clinical PEI manifestations, which are not always suggestive of maldigestion (particularly in the absence of overt steatorrhea), and difficulties in the routine diagnosis of non-severe PEI[9]. Hence, efforts should be made to raise clinical awareness about PEI, both in terms of the correct diagnostic procedures and the optimal management strategies. It can be surmised from the (albeit indirect) evidence presented in this review that in patients with undertreated PEI, adequate PERT may be associated with improved quality of life. The question of whether PERT also affects the long-term survival of patients with PEI should be the subject of future studies.
ACKNOWLEDGEMENTS
Editorial assistance was provided to authors by Alpharmaxim Healthcare Communications.
杂志排行
World Journal of Gastroenterology的其它文章
- From 2-dimensional to 3-dimensional: Overcoming dilemmas in intestinal mucosal interpretation
- Optimizing radiotherapy with immune checkpoint blockade in hepatocellular carcinoma
- Hepatocellular adenoma: An unsolved diagnostic enigma
- Trimethylamine N-oxide attenuates high-fat high-cholesterol dietinduced steatohepatitis by reducing hepatic cholesterol overload in rats
- Brucea javanica oil emulsion improves the effect of radiotherapy on esophageal cancer cells by inhibiting cyclin D1-CDK4/6 axis
- Interleukin-22 receptor 1 is expressed in multinucleated giant cells:A study on intestinal tuberculosis and Crohn's disease
