Diet and medical foods in Parkinson’s disease
2019-05-26KlusLngYukikoNkmurNingChnJinjunGuoShighikoKnyKthrinLngShimingLi
Klus W.Lng,Yukiko Nkmur,Ning Chn,Jinjun Guo,Shighiko Kny,Kthrin M.Lng,Shiming Li
a Department of Experimental Psychology,University of Regensburg,Regensburg,Germany
b Tianjiu Research and Development Center for Exercise Nutrition and Foods,Hubei Key Laboratory of Exercise Training and Monitoring,College of Health Science,Wuhan Sports University,Wuhan,China
c China Institute of Sport Science,Beijing,China
d Graduate School of Information Science,Nara Institute of Science and Technology,Ikoma,Japan
e Department of Psychology,University of Winchester,Winchester,United Kingdom
f Department of Food Science,Rutgers University,New Brunswick,NJ,USA
Keywords:
ABSTRACT
1. Introduction
Parkinson’s disease (PD) is the most common neurodegenerative disease with the exception of Alzheimer’s disease [1]. It is characterized by a slow and progressive degeneration of pigmented, dopamine-containing neurons in the substantia nigra pars compacta. Degeneration of other dopaminergic neurons also occurs, although to a lesser extent [2]. The resulting dopamine deficiency in the basal ganglia causes a movement disorder with the classical parkinsonian motor signs of bradykinesia, muscular rigidity, rest tremor, and impairment of posture and gait [3,4].Individuals with PD also present with various non-motor symptoms, such as cognitive deficits, olfactory dysfunction, psychiatric changes, autonomic dysfunction, and sleep disturbances; some of these symptoms may precede motor dysfunction by many years[5–11].The neuropathological hallmark of PD is the aggregation of a misfolded protein, α-synuclein, in Lewy bodies and Lewy neurites.These toxic inclusions are associated with neurodegenerative changes primarily in the dopaminergic nigrostriatal system, but also in the locus coeruleus as well as in cortical and limbic areas[12].
Ageing is the major risk factor of PD, which affects approximately 1% of adults older than 60 years [13]. The mean age of onset of PD is thought to be in the early-to-mid 60s [14]. In people with young-onset or juvenile-onset PD, initial symptoms can arise between the ages of 21–40 years or before the age of 20 years,respectively[15].Young-onset PD affects 5%–10%of patients[16].
Pathophysiological mechanisms involved in PD include oxidative stress, mitochondrial dysfunction, and abnormal aggregation of α-synuclein[17].The brain is particularly susceptible to oxidative damage due to its high utilization of oxygen, high content of iron, and the presence of unsaturated fatty acids, which are targets for lipid peroxidation [18–20]. Inflammation may play a role in both the initiation and progression of α-synuclein accumulation[21].
While the etiology of PD is unknown, it appears to involve a complex interaction of genetic and environmental factors[22].The genes known to be associated with PD can be causally linked to the disease in only a small minority of cases [23]. In regard to environmental factors,organochlorines and other pesticides appear to play a role in the development of PD [24–28]. Although the cause of the neuronal loss in the brain in PD remains poorly understood,recent findings support the hypothesis that pathological changes in PD may spread from the intestinal tract to the brain via the vagus nerve [12,29]. The demonstration of α-synuclein aggregations in the salivary glands and intestinal mucosa at least a decade before the onset of PD motor symptoms [30,31], and a reduction in the risk for PD by almost 50%observed in people who had undergone vagotomy as a therapeutic intervention for peptic ulcer [32] lend further support to this hypothesis.
The mainstay of the treatment of PD is the administration of drugs [33] that increase dopamine levels, such as levodopa [34]or selective monoamine oxidase-B inhibitors [35], or that directly stimulate dopamine receptors[36–38].However,PD also involves neurotransmitters in addition to dopamine and affects brain areas outside the basal ganglia [39–44]. The available treatments are symptomatic; disease-modifying treatments that slow or halt the neurodegenerative process in PD remain elusive. The onset of PD precedes the cardinal signs of bradykinesia,rigidity and rest tremor by many years. The motor signs of PD become evident following damage to approximately 50% of dopaminergic substantia nigra neurons and a reduction of dopamine levels in the striatum by around 80% [45]. Non-motor symptoms, such as rapid eye movement sleep disorders,constipation,or hyposmia have been shown to be present up to 20 years prior to the occurrence of motor signs[10]. In light of the long preclinical (premotor) period [46], it is possible that lifestyle-related factors may advance the development of PD.A number of modifiable lifestyle risk factors have been identified. The most consistent associations found were between smoking and coffee drinking and a decreased PD risk[23,47].A further possible factor is nutrition,since regular intake of specific food ingredients interacts with cell metabolism.Biological mechanisms underlying a role of diet in the development of PD may include,for example, protection of neurons from oxidative injury by antioxidants and neuroprotection by polyunsaturated fatty acids(PUFAs)[48].
2. Dietary factors in PD
Specific macronutrients and micronutrients may be involved in the etiology of PD. An example illustrating the influence of food intake on the occurrence of parkinsonism is the amyotrophic lateral sclerosis-parkinsonism/dementia complex found mainly in Guam,the cause of which is the consumption of neurotoxic cycad seeds[49,50]. Various foods and nutrients have been proposed as possible risk factors for PD. It should be emphasized, however, that all attempts to establish links between nutrition and PD require a consideration of the causality of such associations, since reverse causation cannot be excluded,i.e.PD could cause a preference for certain foods.
A meta-analysis summarizing findings on macronutrients and the risk of PD reported that the dietary intake of protein,carbohydrate,cholesterol and energy may not be independently associated with PD risk [51]. Studies on the influence of individual nutrients and foods on the risk of PD have produced inconsistent findings[48,52].In a systematic review,foods identified as potential nutritional risk factors were reported,by at least one case-control study,to have been consumed significantly more often by individuals with PD compared to controls;these foods included vegetables,xanthophylls, xanthins, lutein, carbohydrates, monosaccharides, refined sugar, junk food (high in fat and sugar), lactose, animal fat, nuts,and seeds [52], while a significantly reduced intake of fish, eggs,bread, potatoes, alcohol, coffee, tea, niacin, pantothenic acid and folate was observed in the same group[52].
A prediction of the influence of consumption of food groups and quality of diet on PD was undertaken in a population comprising 4524 people(2388 men and 2136 women)aged 40–79 years with no PD diagnosis[53].Eighty-five cases of incident PD were observed during a follow-up period of 41 years.Statistically significant associations could not be found between most food groups assessed and the incidence of PD [53]. A small number of exceptions included positive associations for fruits and berries in men and milk intake in women and an inverse association for the consumption of meat products in women. The diet quality index used did not predict PD [53]. Most of the combined results for men and women were statistically non-significant; exceptions were an increased risk of PD for milk intake, and a decreased risk for the consumption of processed meat and sausages[53].Meat is a source of the vitamin niacin,which has been reported to be inversely associated with PD[54]. However, a large cohort study found no associations for the intake of total meat,red meat,poultry,or fish[55].
A cross-sectional study attempted to discover whether nutrition-related aspects of lifestyle were associated with PD symptom severity in 1053 people with self-reported idiopathic PD[56].Regression analysis was performed on baseline data; the analyses were adjusted for age, gender, and years since diagnosis.Foods identified to be associated with reduced PD progression rate included fresh vegetables,fresh fruit,nuts and seeds,non-fried fish,olive oil,wine,coconut oil,fresh herbs,and spices[56].Foods associated with more rapid progression of PD included canned fruits and vegetables,diet and non-diet soda,fried foods,beef,ice cream,yogurt, and cheese. Nutritional supplementation with fish oil and coenzyme Q10 was associated with a decrease in the rate of PD progression, while iron supplements were associated with a quicker progression[56].
2.1. Milk and dairy products
An increased consumption of milk and dairy products has been reported in several prospective cohort studies to be associated with an elevated risk of PD[57–59].For example,such an association was examined using data from two large prospective cohort studies,with a total of over 125,000 participants and a follow-up period of at least 24 years [60]. The total intake of dairy products was not significantly associated with PD risk,while the consumption of low-fat dairy foods(skim and low-fat milk)showed an association with the risk in both men and women[60].The findings of a metaanalysis of prospective cohort studies also suggest that dairy foods may be positively associated with an elevated risk for PD[61].The linear dose–response relationship showed an increase in PD risk by 17%for every 200 g/day increment in milk consumption and 13%for every 10 g/day increment in the intake of cheese[61].
Explanations of an increased PD risk following dairy food consumption include the exposure to neurotoxins and the reduction in serum uric acid concentrations.Dairy products may be contaminated with neurotoxins through their exposure to pesticides,which have been suggested to increase the risk of PD [58,62,63]. Substantial evidence suggests that urate is inversely associated with PD risk and may have protective effects [64–69]. Milk and dairy proteins have been shown to have antiuricemic effects, in that the proteins casein and lactalbumin reduce serum urate levels in healthy people[70,71].Furthermore,the intake of low-fat,but not high-fat,dairy produce has been linked to a decreased risk of gout[72].
In a study from Hawaii, the association of midlife milk intake with PD was investigated by measuring neuron density in the substantia nigra and examining exposure to organochlorine pesticides in relation to the amount of milk consumption [73]. Neuron density was found to be lowest in non-smoking decedents who had consumed large quantities of milk.When cases of PD and dementia with Lewy bodies were excluded from the sample,adjusted neuron density was significantly reduced,by 41.5%,for milk consumption>16 oz/d compared to an intake less than this amount.In individuals who had consumed the most milk,residues of heptachlor epoxide,an organochlorine pesticide present at excessively high concentrations in milk in Hawaii in the 1980s, were found in 9 of 10 brains(90%), in comparison to 26 of 41 brains (63.4%) in those who had consumed no milk [73]. The study concluded that milk consumption was associated with neuron loss in the substantia nigra in decedent brains unaffected by PD.
In summary, dairy foods may be associated with an elevated risk of PD.This needs to be confirmed in well-designed prospective cohort studies.
2.2. Mediterranean diet
Protective effects of a Mediterranean dietary pattern have been shown for many diseases,including neurodegenerative conditions such as Alzheimer’s disease [74,75]. Adherence to the Mediterranean diet has also been shown to be associated with a reduced risk for PD [76,77]. While following the diet was associated with a later age of PD onset in a case-control study [77] it tended to decrease the risk for fully developed PD in a prospective study[76].In addition, adherence to the Mediterranean diet was reported to be associated with a reduced probability of prodromal PD in older people in Greece [78]. This finding of a link between diet and the prodromal state of PD is particularly interesting, since preventive approaches may be more effective in this phase. However, other lifestyle factors,such as physical activity levels in a Mediterranean culture, may limit the generalizability of diet-related findings. To summarize, the evidence for a protective effect of the Mediterranean diet on PD risk is suggestive.However,it is,at present,not possible to“prescribe”the Mediterranean diet as a preventive measure in PD,since knowledge of the type and quantity of individual food components and of biologically active phytochemicals (with an established chemical structure, content and efficacy) required for effective neuroprotection is,as yet,lacking.
2.3. Nutrients
Numerous nutrients have been posited to target and attenuate the risk factors of PD and,thus,to potentially prevent or delay the progression of PD. These compounds include PUFAs, several vitamins, coenzyme Q10, sulfur-containing compounds, polyphenols,stilbenes,and phytoestrogens[79].
2.3.1. Omega-3 fatty acids
Omega-3 PUFAs serve as energy substrates and membrane components, and play an essential role in maintaining undisturbed neurobiological functions. Omega-3 PUFAs have been shown to have a wide spectrum of activities that may be relevant in the neurodegenerative process in PD. These include the promotion of actions mitigating inflammation,oxidative stress,neurotrophic factors,and apoptosis.
Docosanoids and elovanoids from omega-3 fatty acids have been shown to contribute to pro-homeostatic cellular modulation,inflammatory responses, and neuroprotection [80]. Preliminary evidence from translational and clinical studies supports antiinflammatory effects of omega-3 PUFAs in older age[81].This needs to be confirmed by high-quality intervention studies.Growing evidence suggests that neuroinflammation plays a central role in the pathogenesis of PD and may therefore provide a target for neuroprotection[82,83].At present,it is difficult to identify the molecular mechanism underlying neuroprotective effects of omega-3 PUFAs on the nigrostriatal pathway. Mechanisms that have been suggested to be involved include a decrease in the production of pro-inflammatory eicosanoids [84,85] and the anti-inflammatory properties of mediators originating from eicosapentaenoic acid(EPA)and docosahexaenoic acid(DHA)[86,87].
A reduction in the post-mortem cerebral levels of brain-derived neurotrophic factor has been found in individuals with PD[88,89],and the administration of omega-3 PUFAs could be a useful strategy to stimulate the production of brain-derived neurotrophic factor in the brain.
Omega-3 PUFAs are involved in the regulation of several genes associated with oxidative stress and apoptosis [90]. Several studies have reported antioxidative properties of omega-3 PUFAs in animals [87] and their ability to modulate apoptotic processes found in the brains of patients with PD;mega-3 PUFAs have been demonstrated to upregulate anti-apoptotic proteins while downregulating pro-apoptotic proteins[91].
Post-mortem analyses of the brains of PD patients and of PD animal models indicate that PD per se as well as its pharmacological treatment may alter brain PUFA levels [92]. Animal studies have shown that oral administration of DHA can alter brain levels of this PUFA and modify brain functions.This provides an opportunity to use DHA as a nutraceutical in brain disorders,including PD[93].
A higher intake of PUFAs may be inversely associated with the risk for PD [51]. In a prospective population-based cohort study,more than 5000 individuals were evaluated for the risk of developing PD in relation to the dietary consumption of fatty acids, as assessed using a food intake questionnaire [94]. After a follow-up period of 6 years,high consumption of omega-3 PUFAs was shown to be associated with a reduced risk of PD [94]. These correlative findings need to be confirmed in intervention studies.
The effects of co-supplementation of omega-3 fatty acids and vitamin E on clinical signs and metabolic parameters in PD was examined in a randomized,double-blind,placebo-controlled clinical trial comprising 60 individuals with PD. Thirty participants received 1000 mg omega-3 fatty acids from flaxseed oil and 400 IU vitamin E for 12 weeks, while the other group (n=30) received placebo [95]. In comparison to placebo, omega-3 fatty acids and vitamin E led to a statistically significant improvement in clinical status, as assessed using the Unified PD Rating Scale after 12 weeks[95].Furthermore,a reduction in high-sensitivity C-reactive protein,an increase in total antioxidant capacity and elevated glutathione concentrations were found compared to placebo [95]. In summary, this study showed favorable effects of omega-3 fatty acids and vitamin E on both clinical and metabolic status in PD.
The available evidence from epidemiological, preclinical, and clinical research suggests that omega-3 PUFAs may be a therapeutic strategy for PD [96]. In order to determine whether omega-3 PUFAs can provide neuroprotective effects in PD,biological markers for an early identification of individuals at risk of PD need to be found.Furthermore,the demonstration of neuroprotective efficacy in clinical studies is a significant challenge [97]. In addition, the symptomatic effects of omega-3 PUFAs as an add-on treatment to dopamine agonistic medications should also be investigated [98].The therapeutic potential of omega-3 PUFAs in PD warrants further study.
2.3.2. Vitamins
2.3.2.1. Vitamin D. Vitamin D is an important neurosteroid that is required for brain development and function [99,100]. Vitamin D has been suggested to be associated with a wide variety of neurological diseases, such as multiple sclerosis, stroke, and neurodegenerative diseases[101].Vitamin D receptors and the enzyme 1-alpha-hydroxylase, which activates vitamin D, can be found in many regions of the human brain[102].Findings of animal studies suggest that vitamin D may protect against excitotoxicity,enhance antioxidant concentrations,and act as a neurotrophic factor[101].The question arises whether vitamin D could be capable of preventing neurological diseases or of slowing their progression.While neuroprotective effects of vitamin D are supported by clinical findings[101],the association of vitamin D with PD is less clear.
Serum vitamin D concentrations appear to be inversely associated with the risk and severity of PD [103,104]. Higher serum 25-hydroxyvitamin D levels and the vitamin D receptor FokI CC genotype have been shown to be associated with milder PD[105]. In a meta-analysis including seven studies with a total of 1008 PD patients and 4536 healthy controls, the patients had lower mean serum levels of 25-hydroxyvitamin D than controls[106]. In addition, PD patients with vitamin D insufficiency (25-hydroxyvitamin D levels <75 nmol/L) showed an increased risk of PD, with the risk being twofold in patients with vitamin D deficiency(25-hydroxyvitamin D <50 nmol/L)[106].Another metaanalysis comprising 2866 individuals with PD and 2734 controls found lower serum vitamin D levels in PD patients than in the control group[104].Moreover,serum vitamin D levels showed a strong negative correlation with the severity of PD [104]. However, it is unclear whether low concentrations of vitamin D are a cause,correlate,or consequence of PD,since this issue cannot be examined in cross-sectional studies.For example,low vitamin D in people with PD may be due to impaired mobility and consequent decreased access to sunlight or to a loss of appetite and low consumption of foods containing vitamin D and other bioactives.Prospective studies have reported conflicting results.While an association between PD incidence and low vitamin D levels as well as a significant dose–response relationship were found in one study conducted in Finland[107],another study from the United States could not find an association between vitamin D and the incidence of PD [108].Both studies were well-designed and the difference in findings may be related to different environmental exposures.For example,an important difference between the two studies is the markedly lower vitamin D concentrations in the Finnish study.An association between vitamin D and PD may be detectable only when the range of vitamin D levels is low.
In regard to the efficacy of vitamin D in the treatment of PD,the findings of a randomized, placebo-controlled, double-blind trial with 114 patients with PD suggest that vitamin D3 supplementation (1200 IU daily) may stabilize PD for a short period in participants with FokI TT or CT genotypes[109].
In summary, the available literature provides evidence supporting possible protective and symptomatic effects of vitamin D in PD [110]. How vitamin D deficiency influences the risk for PD is unknown. There is currently no robust evidence indicating anti-inflammatory effects of vitamin D in old age or PD [81]. The evaluation of the therapeutic efficacy of vitamin D requires further research.
2.3.2.2. B vitamins. B vitamin deficiency is a frequent cause of neurological disability and impairment globally [111–113]. The rationale for a role of B vitamins in PD is linked to homocysteine,which is a metabolite of the essential amino acid methionine cycle.Homocysteine shows multiple neurotoxic pathogenetic effects in neurodegenerative disorders, including PD [114,115]. Individuals with PD have elevated levels of homocysteine compared to agematched healthy people[116–118].Increased homocysteine levels may accelerate dopaminergic cell death in PD through neurotoxic effects [119–121], and a reduction of plasma homocysteine may decrease the risk of PD.The synthesis of methionine from homocysteine requires B vitamins as cofactors.Thus,high B vitamin intake reduces plasma homocysteine levels and could have protective effects regarding PD.
Vitamin B1 is an essential cofactor of some key enzymes required for oxidative metabolism in the brain, and high levels of vitamin B1 are found in the human substantia nigra [122].Decreased concentrations of striatal dopamine are found when vitamin B1 is deficient, and intrastriatal vitamin B1 administration has been demonstrated to increase dopamine release [123].A possible relationship between PD and a dysfunction in vitamin B1 metabolism has been suggested by recent studies[124].
A prospective, population-based cohort study of 5289 participants aged >55 years, suffering from neither dementia nor parkinsonism, examined the association between the risk of incident PD and dietary intake of folate, vitamin B12, and vitamin B6[125]. After a mean follow-up period of 9.7 years, 72 participants with incident PD were identified. No association was found for dietary folate or vitamin B12,while higher dietary intake of vitamin B6 was associated with a significant reduction in PD risk[125].Vitamin B6 may decrease the risk through antioxidant effects unrelated to homocysteine metabolism and through its involvement in the synthesis of dopamine [125]. In addition, a meta-analytic assessment of the associations between PD and folate, vitamin B6, and vitamin B12 indicated that individuals with PD had reduced levels of vitamin B12 and similar levels of folate in comparison with controls[126].High dietary intake of vitamin B6 may be associated with a reduced risk of PD, while the intake of folate and vitamin B12 did not seem to reduce the risk[126].
A sustained improvement in motor and non-motor symptoms was found in 50 individuals with PD during the 3–27-month follow-up period in an open-label pilot study evaluating the effects of 100 mg intramuscular vitamin B1 administered twice weekly[127,128].
Many issues in the evaluation of the role of B vitamins in PD remain to be addressed. These include the investigation of B vitamin levels in people with PD not treated with levodopa,the dosages of B vitamins consumed,the use of self-administered diet questionnaires,and the assessment of other dietary components.
2.3.2.3. Antioxidant vitamins. Since oxidative stress appears to play a role in the neurodegeneration in PD[129,130]and dietary antioxidants,such as vitamins C,E,and carotenoids,can prevent oxidative damage [131], these food bioactives have been hypothesized to protect against PD [132]. However, a meta-analysis of epidemiological studies examining the association between PD and vitamin A and carotenoids reported that the published findings are insufficient to draw firm conclusions regarding the association between dietary intake or blood concentrations of vitamin A/carotenoids and the risk of PD[133].Furthermore,the prospective investigation of associations between PD risk and intake of vitamin C, vitamin E,and carotenoids did not support the hypothesis that antioxidant vitamins reduce the risk of PD[134].
2.3.3. Polyphenols
Polyphenols have been demonstrated to have potent antioxidant and anti-inflammatory properties [135–137]. In view of the role of inflammation and oxidative stress in the dopaminergic neurodegeneration in PD, dietary polyphenols, such as anthocyanins,catechins,curcumin,resveratrol,and theaflavins may have neuroprotective and therapeutic potential in PD [138]. Neuroprotective effects of various polyphenols have been demonstrated in animal models of PD[139].
A large prospective study comprising 130,000 participants showed that high habitual intake of flavonoid-rich food and beverages was associated with a reduced risk of PD[140].In particular,the antioxidant,anti-inflammatory,and anti-excitotoxic activities of tea polyphenols have been suggested to have protective effects in PD[141].A decrease in the risk of PD was reported for black tea drinking[142–145],while green tea drinking could not be shown to be associated with changes in PD risk[145].In addition,long-term consumption of tea has been reported to delay the onset of motor signs of PD by as much as over 7 years [146]. A meta-analysis of available findings showed that tea drinkers have a reduced risk of PD compared to non-drinkers[147].
In summary, the evaluation of a role of polyphenols in PD requires further research. An important factor limiting the use of dietary polyphenols is their relatively low bioavailability in vivo.
2.3.4. Coenzyme Q10
Coenzyme Q10 (ubiquinone) is a fat-soluble coenzyme found primarily in the mitochondria of eukaryotic cells.It plays a prominent role in the electron transport chain and in the generation of ATP. Coenzyme Q10 can prevent the loss of dopaminergic neurons in parkinsonism induced in animals by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine [148,149] and serve as a neuroprotective compound in the prevention and therapy of PD[150].
A case-control study comprising 22 individuals with PD and 88 controls matched for age and sex found a statistically significant difference in the percentage of people with a deficiency in coenzyme Q (34% of PD patients versus 8.5% of controls [151]). In an early randomized, placebo-controlled, double-blind study of 80 people with early-stage PD,participants in the treatment group showed a dose-dependent greater reduction in functional decline,as assessed with the Unified PD Rating Scale,than controls[152].It is unclear whether these effects of coenzyme Q10 are neuroprotective or merely symptomatic. However, recent meta-analyses of available studies concluded that the administration of coenzyme Q10 neither slows functional decline nor provides beneficial symptomatic effects in PD[153,154].
3. Developing medical foods for PD
Medical foods or foods for special medical purpose are produced according to a special processing formula in order to meet the specific dietary needs of people with metabolic disorders or other diseases.In the United States,a medical food is defined as“a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles,are established by medical evaluation”[155].All ingredients of medical foods must be recognized as safe.In contrast to approved drugs,however,medical foods are not subject to the rigorous review of the US Food and Drug Administration.In the European Union,products considered medical foods in the United States are regulated as dietary foods for special medical purposes[156]. The possibility of developing medical foods in PD is based on findings related to ketogenic diets and preliminary studies on foods possibly meeting the specific dietary needs of individuals with PD.
3.1. Ketogenic diets
Ketogenic diets have a fasting-like effect,which produces a state of ketosis, and have been proposed to have therapeutic potential in neurodegenerative diseases.The deterioration of glucose utilization in the brains of people with Alzheimer’s disease is a potential target of dietary manipulations involving the supplementation of the normal glucose supply with ketone bodies[157,158].Studies in animal models of PD have shown beneficial effects of ketone bodies on the course of experimental parkinsonism[159].Preliminary evidence suggests that dietary interventions may be able to influence motor and non-motor symptoms in PD. However, the ideal fat to carbohydrate ratio of such diets is unknown [160]. A lowfat and high-carbohydrate diet may ease the passage of tyrosine,which is the dopamine precursor,to cerebrospinal fluid and trigger an insulin-induced increase in dopamine in the brain [161,162].Conversely, the rationale for a high-fat/low-carbohydrate (ketogenic) diet is premised on the finding that defects in respiratory chain complex I activity have been found in the substantia nigra and frontal cortex of PD patients[163,164].It has been suggested that ketones produced by a high-fat, low-carbohydrate diet may have the potential to circumvent this defect via a complex II-dependent mechanism and may therefore improve mitochondrial oxidative phosphorylation in the brain[165].Moreover,a ketogenic diet may also improve the energy metabolism of both central and peripheral neurons through the stimulation of mitochondrial biogenesis[166].
A feasibility study has evaluated the effects of a ketogenic diet in five individuals with PD[167].The motor scores in these patients were shown to improve following the ketogenic diet,but the study was limited by the small sample size, the lack of a placebo group,and a short duration of 4 weeks. A pilot randomized, controlled 8-week trial compared the efficacy and safety of a ketogenic diet versus a low-fat,high-carbohydrate diet in 47 PD patients in a hospital clinic setting, with 38 patients completing the trial [168].The ketogenic diet group maintained physiological ketosis over the study period of 8 weeks. Both diet groups showed significant improvements in both motor and non-motor symptoms,with the ketogenic group showing greater improvements in the more disabling non-motor symptoms,which respond less well to levodopa[168].The most common adverse effects were intermittent exacerbation of the PD tremor and/or rigidity in the ketogenic group and excessive hunger in the low-fat group.
In summary,a ketogenic diet may offer complementary effects in addition to levodopa treatment. Further trials are required to evaluate whether therapeutic hyperketonemia leads to symptomatic improvement in the short term or delays the progression of PD in the long term.
3.2. Mannitol
Mannitol,6-carbon polyol,is a sweetener,which is approved as an osmotic diuretic agent by the U.S.Food and Drug Administration[169]. Mannitol is able to open the blood–brain barrier [170] and can therefore help overcome the challenge of delivering therapeutic compounds through this barrier,which is difficult to permeate with drugs [171,172]. More importantly, several osmolytes, such as polyols including mannitol, have been shown to exert a potent effect on the stabilization of protein structure (“chemical chaperones”) [173,174] and may reduce neurotoxic protein aggregation in amyloidogenic diseases[175].
The administration of mannitol to a drosophila fly model of PD [176] resulted in a significant decrease in the amount of α-synuclein aggregates in the brain and an improvement in behavioral deficits [177]. A reduction in α-synuclein accumulation was also found in several brain areas of the mThy1-human α-synuclein transgenic mouse model[178]after treatment with mannitol[177].These findings suggest that mannitol is an α-synuclein aggregation inhibitor promoting α-synuclein clearance in cell bodies and exerting neuroprotective effects in a transgenic mouse model of PD. However, a self-assessed symptom reduction in individuals with PD who were not blind to the administration of mannitol[179] does not allow any conclusions regarding the efficacy of mannitol in the treatment of PD. This needs to be evaluated in randomized controlled trials with a sufficient number of participants.
3.3. Palmitoylethanolamide
N-acylethanolamines, such as palmitoylethanolamide, are endogenous lipid signaling molecules which have been shown to promote the resolution of neuroinflammation and exert neuroprotective activities in animal models of neurodegenerative diseases and stroke[180].Chronic administration of palmitoylethanolamide has been demonstrated to protect tyrosine hydroxylase-positive neurons in the substantia nigra pars compacta in an animal model of PD, i.e. mice treated with the neurotoxin 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine[181].In addition,the decrease in astroglial and microglial activation observed following palmitoylethanolamide was associated with an improvement of motor deficits[181].In mice treated with 6-hydroxy-dopamine,repeated administration of palmitoylethanolamide led to elevated tyrosine hydroxylase expression in the striatum as well as improved motor functions and markers of inflammation,endoplasmic reticulum stress, and apoptosis in the striatum [182]. Furthermore,ultra-micronized palmitoylethanolamide was reported to improve spasticity, pain, cognitive abilities, and activities of daily living in individuals with stroke [183], to reduce circulating proinflammatory cytokines and to improve quality of living in people with multiple sclerosis[184].
Thequestionofwhetherultra-micronizedpalmitoylethanolamide(Normast®,a food for special medical purposes)can promote neuroinflammatory resolution and possibly a slowing of the progression of PD has been evaluated [185]. The effects of palmitoylethanolamide were investigated in a prospective observational study comprising 30 individuals (16 males and 14 females,mean age 73±8 years,mean duration of PD 10±5 years)receiving drug treatment for PD (levodopa 200–1150 mg) and, in some patients, other PD medications, such as dopamine agonists,monoamine oxidase inhibitors, or catechol-O-methyltransferase inhibitors. The study lasted 15 months, with ultra-micronized palmitoylethanolamide administered at a daily dose of 1200 mg for three months,as add-on to levodopa and other PD medications,and subsequently at a reduced dose of 600 mg daily for up to 12 months [185]. Pharmacotherapy with levodopa or other drugs was maintained constant during the treatment period. Clinical assessment of motor and non-motor symptoms,using the revised Movement Disorder Society/Unified Parkinson’s Disease Rating Scale,was performed before the addition of palmitoylethanolamide and at months 1,3,6,and 12 after addition[185].Add-on administration of ultra-micronized palmitoylethanolamide resulted in a significant and progressive reduction in the total MDS-UPDRS score[185].The mean score difference between baseline and end of treatment was significantly reduced in most motor and non-motor symptoms. Furthermore, the number of participants presenting with symptoms at baseline was reduced following 12 months of treatment with ultra-micronized palmitoylethanolamide. Side effects attributable to palmitoylethanolamide were not reported by the patients included[185].In summary,the results of the observational study suggest that ultra-micronized palmitoylethanolamide as an adjuvant treatment slows disease progression and reduces disability in individuals with PD and may,therefore,be a diseasemodifying compound [186]. However, a randomized controlled trial including a control group and comprising a larger sample should be conducted in order to confirm the findings.
3.4. Gut–brain axis and microbiome in PD
The investigation of the interaction between gut microbiota and PD may lead to novel approaches to early intervention in PD. The gut microbiome appears to be an environmental factor modulating the physiological functions of the host,and appears to play an important role in the development and maintenance of the central and enteric nervous systems.Several mental,neurobehavioral,and neurodegenerative disorders have been linked to gut microbiota [187–191]. Numerous effects of the gut microbiota on brain function seem to depend on vagal activation [192–194]. A role of gut microbiota and microbial metabolites has also received increasing attention in regard to the pathogenesis of PD[195,196].It has been proposed that intestinal inflammation links changes in altered gut microbiota composition to neurodegeneration in individuals with PD. In this regard, it has been shown that both the number and composition of gut microbiota and microbial metabolites are altered in people with PD[188].Gastrointestinal dysfunction is an early and common non-motor symptom in PD and is usually associated with inflammation and an accumulation of α-synuclein in the enteric nervous system. An overlap between PD and inflammatory bowel disease has been suggested by epidemiological and genetic findings [197]. Furthermore, epidemiological, experimental,and clinical findings suggest that intestinal inflammation may contribute to the pathogenesis of PD [189,198,199], and increasing evidence supports the proposed role of a pro-inflammatory gut microbiome in PD [187–202]. The hypothesis of low-grade inflammation of the gut is supported by findings of elevated mRNA expression of pro-inflammatory cytokines in colonic biopsies of PD patients[200].Chronic low-grade inflammation could lead to leakiness of the blood–brain barrier, activation of immune cells and neuro-inflammation in the brain. The deposition of α-synuclein in PD might be initiated by a toxin or pathogen in the enteric nervous system and be propagated to the brain by transsynaptic cell-to-cell transmission.Communication between gastrointestinal system and brain is mediated by a bidirectional system(‘gut–brain axis’)[203].A better understanding of gut–brain interactions could provide new insights into the pathogenesis of PD and potentially lead to new treatments.Case studies of the transplantation of fecal microbiota,for example,have reported an improvement of symptoms in PD[204].
In summary,further research is needed in order to better understand how alterations of microbially produced metabolites and bacterial products contribute to the pathogenesis of diseases. The deriving of beneficial effects through the modulation of gut microbiota by the administration of,for example,prebiotics or probiotics may be a novel approach to the prevention or treatment of PD.
3.5. Probiotics
Probiotics are live microorganisms claimed to provide health benefits by improving or restoring the balance of gut microbiota when consumed in adequate amounts [205]. Numerous animal and preclinical studies have shown potential benefits of probiotics in the prevention and therapy of gastrointestinal and central nervous system diseases[206–209].Gastrointestinal dysfunction,such as constipation, is common in people with PD and contributes to the pathological process of the disease. In this context, the intake of fermented milk, containing multiple probiotic strains and prebiotic fiber, for four weeks improved constipation in individuals with PD significantly better than placebo(pasteurized,fermented,fiber-free milk)[210].
Compelling pre-clinical evidence provides a strong rationale to conduct high quality randomized controlled trials evaluating the efficacy of dietary supplements,including microbiota-directed treatment, in PD [211]. Probiotics or prebiotics may affect gut microbiota composition in regard to type, variety, and ratio and may possibly reduce pro-inflammatory responses, thereby modulating initiation and/or progression of the neurodegeneration in PD.
The effects of supplementation with probiotics on movement and metabolic parameters in 60 patients with PD were evaluated in a randomized,double-blind,placebo-controlled clinical trial,with participants receiving either 8×109CFU/g daily probiotic containing Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus reuteri,and Lactobacillus fermentum(n=30)or placebo(n=30)for 12 weeks [212]. In comparison with placebo, intake of probiotics led to a statistically significant reduction in MDS-UPDRS scores.In addition, the administration of probiotics also decreased highsensitivity C-reactive protein and malondialdehyde,and increased glutathione levels.Furthermore,probiotic intake resulted in a significant decrease in insulin levels and insulin resistance and a significant rise in insulin sensitivity [212]. The supplementation with probiotics resulted in no reported side effects. A limitation of this study was that fecal bacteria loads were not assessed following the intake of probiotics.In summary,the supplementation of probiotics appears to have positive effects on symptoms and metabolic profiles and may considerably modulate gut microbiota in PD.This approach may open new avenues in the management of PD.
Since oxidative damage and inflammatory responses play a role in nigro-striatal neurodegeneration in PD [213], antioxidants and bioactive molecules produced by probiotics may reduce free radicals and oxidative stress [214]. Furthermore, probiotics can influence brain neurochemistry and behavior through the production of neurotransmitters, such as γ-aminobutyric acid,noradrenaline, dopamine, serotonin, and acetylcholine [215,216].The consumption of probiotics in people with PD may improve clinical signs via the inhibiting of indoleamine 2,3-dioxygenase and inflammatory factors such as interferon-γ and interleukin-6(IL-6)[217].Moreover,probiotics may improve high-sensitivity Creactive protein and oxidative stress through an increase in the production of gut short chain fatty acids[218],which may reduce high-sensitivity C-reactive protein by suppressing the synthesis of hepatic C-reactive protein[219].Altered parameters of glucose homeostasis may correlate with loss of dopaminergic function in PD and may lead to an increase in mortality[220].The finding of a normalization of insulin metabolism following probiotics intake[212]may indicate that treatment of PD with probiotics could reduce the risk of diabetes and of associated complications in PD.
In summary, gut microbial dysbiosis can be observed in individuals with PD. Microbial products and metabolites appear to contribute to PD pathology and the microbiota–gut–brain axis plays an important role in the disease. Probiotics and prebiotics may therefore be useful as novel treatments for PD.
4. Conclusions and future directions
Despite considerable improvements in the symptomatic therapy of PD,no progress in the development of treatments to halt or delay the progression of the disease has been made. Most of the drugs currently used in the treatment of PD replenish dopamine in the brain and provide symptomatic relief in early stages of the disease.However,these drugs do not slow or prevent the progression of PD.
Modifiable lifestyle variables associated with a reduced rate of PD progression may include dietary factors. Various foods and nutrients have been proposed as possible risk factors for PD with plausible biological hypotheses.Since most single food groups were not predictive of the occurrence of PD [53], the role of diet in the pathogenesis of PD may be modest. However, large prospective studies need to be conducted in order to clarify this issue.The finding of a reduced risk for the development of PD following a 6-year high intake of omega-3 PUFAs, as assessed using a food intake questionnaire,in a prospective study[94]suggests that further investigations of the role of omega-3 PUFAs in PD should be performed.Based on the findings described above,a nutritional supplement with potential promise in the future development of a medical food for PD could contain omega-3 fatty acids, vitamin D,B vitamins,and coenzyme Q,the dosages of which would need to be determined.Whether any beneficial effects of such a supplement would be merely symptomatic or,in fact,neuroprotective and capable of delaying the progression of PD would need to be evaluated. Foods commonly consumed consist of a variety of nutrients that may exert interactive or synergistic actions[221].The analysis of the effects of dietary patterns may therefore provide an alternative approach in the understanding of the role of diet in chronic diseases such as PD[77,76,222].
There are, at present, few approaches to the development of medical foods for the management of PD (see Table 1). Of these,none come close to fulfilling the criteria as defined by the U.S.Food and Drug Administration [155]. The development of a medical food for PD is hampered by the fact that the condition is an etiologically heterogeneous disease with multifactorial causes.Mechanisms suggested to play a role in PD include mitochondrial dysfunction, oxidative stress, inflammation, and abnormal aggregation of α-synuclein.In addition,gut microbial dysbiosis is found in people with PD and microbial metabolites and products seem to contribute to the pathophysiology of PD.
The heterogeneity of motor features in individuals with PD has produced a classification of subtypes of the disease [223]. While a consensus on the classification of PD subtypes has, as yet, not been reached, two main subtypes have been suggested on the basis of empirical observations;these are tremor-dominant PD and non-tremor-dominant PD (akinetic-rigid syndrome and postural instability gait disorder).Clinical observations have suggested differences between subtypes in regard to the course and prognosis of the disease,with a slower rate of progression and less pronounced functional disability in tremor-dominant PD than in non-tremordominant PD [224]. The underlying etiologies and pathogenetic mechanisms may differ between the subtypes [223]. In addition,Parkinson-plus syndromes,including dementia with Lewy bodies,multiple system atrophy, corticobasal degeneration, and progressive supranuclear palsy can be distinguished[225–227].
The pathogenetic alterations causing PD occur early in the premotor phase and involve areas in both peripheral and centralnervous system, in addition to the dopaminergic neurons of the substantia nigra. In the prodromal phase of PD, neurodegeneration can begin as early as the third decade of life [228]. This early phase of PD provides a potential temporal window during which disease-modifying treatments could be administered in order to prevent or delay the development and progression of the disease[229]. A major challenge for the evaluation of disease-modifying strategies in early phases of PD pathogenesis is the identification of individuals at risk. The gold standard for the diagnosis of PD is neuropathological post-mortem evidence of loss of pigmented neurons in the substantia nigra and of presence of Lewy bodies consisting of α-synuclein aggregation. Since PD is diagnosed when neurodegeneration in the substantia nigra pars compacta has progressed to neuronal loss of more than 50% [45,230], the identification of biological markers that allow a diagnosis of PD early in preclinical stages is a priority. Diagnostic tools for a definitive diagnosis early in the course of the disease do not exist.Individuals at risk for PD,possibly without marked nigral degeneration,could potentially be identified by transcranial sonography[231,232]or by polysomnography in REM sleep behavior disorder [233,234]. This calls for further research.
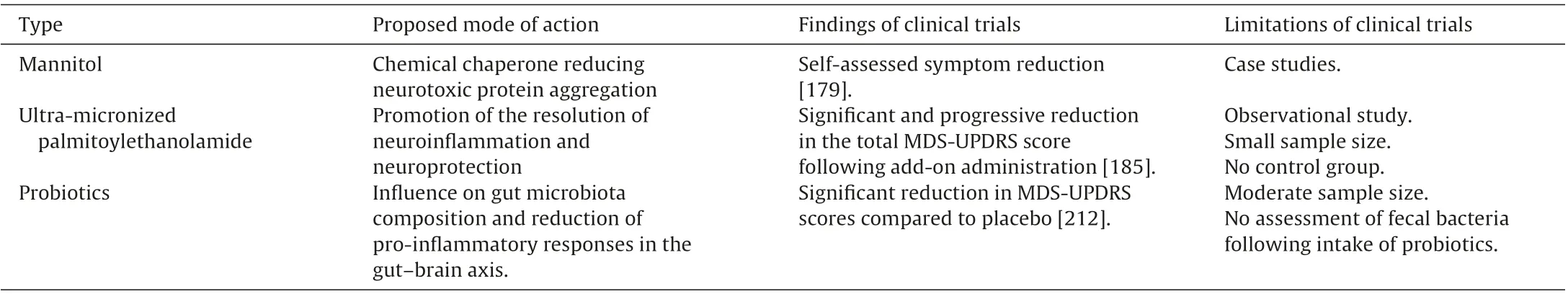
Table 1 Potential medical foods in PD.
When assessing possible effects of nutrients on PD progression,several factors should be considered.These include the duration of nutrient deficiency and PD risk,the genetic determinants of nutrient deficiency and susceptibility for PD, and the pharmacological consequences of nutrient administration on dopaminergic function and disease progression.The time frame used for the investigation of risk factors of diseases that occur late in life is critical.Risk factors assessed early in life may change with time,while those measured later may not have had sufficient opportunity to influence disease outcome.Since PD is a slowly progressing disease,trials evaluating disease-modifying strategies require extended follow-up periods.The heterogeneity of PD requires enrolment of large populations.Since motor symptoms occur late in the course of PD, they may not be ideal as outcome measures for neuroprotective interventions in early disease stages.PD may provide a model allowing the investigation of the link between intestinal inflammation and early stages of neurodegeneration. A prospective follow-up study over extended periods of time of patients with inflammatory bowel disease who are at risk for neurodegeneration, as determined using early premotor features or substantia nigra sonography, might to be a promising strategy. Possible disease-modifying properties of anti-inflammatory treatments could be evaluated using such an approach.
Several avenues may have potential in furthering the development of medical foods in PD. For example, ketogenic diets may have effects complementary to levodopa treatment. High quality trials should examine whether therapeutic hyperketonemia has symptomatic efficacy or the potential to delay the progression of PD. Preliminary findings suggest that ultra-micronized palmitoylethanolamide could provide meaningful improvements in motor and non-motor aspects of daily living and might be a useful adjuvant treatment in people with advanced PD [185].Randomized controlled trials need to confirm these findings.Furthermore, gut microbial dysbiosis and changes in microbial metabolites have been reported in PD [235]. Disturbances in the microbiota–gut–brain axis appear to be linked to microbial products and may lead to inflammatory processes in gut and brain.Interventions targeting gut microbiota, such as the supplementation of prebiotics and probiotics,have been demonstrated to have favorable effects on host health and may provide novel approaches to PD in regard to both symptomatic treatment and disease modification.Future research should focus on the microbiota–gut–brain axis and investigate how microbial populations exert pathogenetic or beneficial effects on brain function and contribute to the development of PD. Further studies should also investigate the interaction of food and diet with other factors, such as gene–diet interactions[236].
Conflict of interest
All authors declare no conflict of interest in regard to this paper.
杂志排行
食品科学与人类健康(英文)的其它文章
- Functional food products in Japan:A review
- Mathematical rules for synergistic,additive,and antagonistic effects of multi-drug combinations and their application in research and development of combinatorial drugs and special medical food combinations
- Biting force and tongue muscle strength as useful indicators for eating and swallowing capability assessment among elderly patients
- Influence of luteolin on the apoptosis of esophageal cancer Eca109 cells and its mechanism of action
- A value-added cooking process to improve the quality of soybean:Protecting its isoflavones and antioxidant activity
- Chemical constituents,biological functions and pharmacological effects for comprehensive utilization of Eucommia ulmoides Oliver
