M u lticom ponen t cyclodex trin system fo r im p rovem en t o f so lubility an d d isso lu tion rate o f poo rly w ater so lub le d rug✩
2019-05-13MayankPatelRajashreeHirlekar
Mayank Patel,Rajashree Hirlekar
a Departm ent ofPharm aceutics,BharatiVidyapeeth’s College ofPharm acy,Sector-8,C.B.D.Belapur,NaviMumbai 400614,Maharashtra,India
b Departm ent ofPharm aceu tical Sciences and Technology,Institu te ofChem ica l Technology,N.P.Marg,Matunga,Mum bai400019,India
c Departm ent ofPharmaceutics,VESCollege ofPharm acy,Hashu AdvaniM em orialCom plex,Behind Collector Colony,Chem bur(E),M um bai400074,India
Keyw ords:Cinnarizine Hyd roxyp ropy l-β-cyclodex trin Hyd roxy acid Ternary system
A B S T R A C TThe pu rpose o f the p resen t study w as to investigate the in teraction o f Cinnarizine(CIN)w ith Hyd roxyp ropy l-β-Cyclodex trin(HPβCD)in the p resen ce o f Hyd roxy Acids(HA).Various binary and ternary system s o f CIN w ith HPβCD and HA w ere p repared by knead ing and coevapo ration m ethods.Fo r the ternary system s,HA w ere tried in th ree d ifferen t concen trations.The in teraction in so lu tion phasew as stu d ied in detailby the phase so lubilitym ethod,an d the so lid phase in teraction s w ere characterized by Fou rier Tran sfo rm In frared(FTIR)spectroscopy,Differen tia l Scann ing Ca lorim etry(DSC),X-Ray Diffractom etry(XRD),Scann ing Elec tron M icroscopy(SEM)an d Pro ton Nu clear Magnetic Resonan ce(1H-NMR).Phase so lubility revea led the positive effect o f HA on the com p lexation o f CIN w ith HPβCD.So lid phase charac terization con f irm ed the form ation o f in clusion com p lex in the ternary system s.So lubility an d d isso lu tion stud ies illustrated that ou t o f th ree d ifferen t concen tration s tried,HA w erem ost e ffective at the 1 M con cen tration level.Ternary system s w ere very e ffective in im p roving the so lubility as w e ll as d isso lu tion p ro f ile o f CIN than the CIN-HPβCD binary system s.FTIR,1H-NMR and M o lecu lar docking stud ies gave som e in sigh t atm o lecular level that ac tua lly w h ich part o f CIN w as in teracting w ith the HPβCD.M o lecu lar docking an d free energy calcu lation even en ligh ten the ro le o f tartaric acid in in creasing so lubility o f CIN in the ternary system.
1. In trodu ction
Cinnarizine (CIN) [1-(d ipheny lm ethy l)-4-(3-pheny l-2-p ropeny l)p iperazine]is an an tih istam in ic d rug,w h ich is m ain ly used for the con tro l o f vom iting due to the m o tion sickness.It acts by in terfering w ith the signa l transm ission betw een vestibu lar apparatus o f the inner earm o tion recep to r and the vom iting cen ter o f the hypotha lam us.The d isparity of signal p rocessing betw een inner earm otion recep tors and the visua l senses is abo lished,so that the con fusion o f b rain w hether the in d iv idua l ism oving o r stan d ing is redu ced.
Accord ing to Biopharm aceu tica l Classif ication System(BCS),CIN falls under Class II[1].It is p ractically insolub le in w ater bu t has a log P va lue o f 5.8,w h ich suggests the h igh lipoph ilicity and h igh perm eability o f CIN.Bioavailability o f CIN is d issolu tion rate lim ited because o f its poor aqueous so lubility.There fo re,it is possib le to im p rove the bioavailability o f CIN by im p roving its in trinsic so lubility and d isso lu tion rate.Tokum u ra et a l.reported the in clusion com p lexa tion o f CIN w ithβ-cyclodex trin(βCD),w h ich w as con f irm ed by the so lubilitym ethod,pow der X-Ray Diffractom etry(XRD),Differen tia l Scann ing Ca lorim etry(DSC)and Proton Nuclear Magnetic Resonan ce(1H-NMR)spectroscopy[2].Tokum u ra et a l.a lso repo rted the enhan cem en t o f bioavailability o f CIN from itsβCD com p lex on oral adm in istration w ith L-Pheny lalan ine as a com peting agen t[3].Fu rtherm o re,Jarvinen et a l.reported the im p rovem en t in the ora l bioavailability o f CIN w hen comp lexed w ithβCD,Hyd roxyp ropy l-β-Cyclodex trin(HPβCD)and Su lphobu ty lether-β-Cyclodex trin(SBE-4-βCD)[4].How ever,to the best o f ou r know ledge,the so lid states o f the various CINHPβCD com p lexes have no t been p reviously characterized.
Cyc lodex trins(CDs),cyc lic oligosaccharide w ith a hyd roph ilic ou ter su rface and hyd rophobic cen tra l cavity,can fo rm a stab le com p lex w ith a variety o f d rugs[5,6].CD comp lexation has been estab lished as an effectivem ethod fo r the im p rovem en t o f so lubility and bioavailability o f them any hyd rophobic d rugm o lecu les.From various CDs,βCD is them ost frequen tly used CD to m od ify the physicochem ica l p roperties an d im p rove the solubility an d bioavailability o f m any d rugs due to its low cost[7].How ever,its low in trin sic so lubility and neph rotoxicity have con f ined the app lication o fβCD.Many chem ica l deriva tives o fβCD are availab le in them arket that possess h igh so lubility and low toxicity than that of its paren t.One o f those derivatives is HPβCD,w h ich show s a 50 fo ld im p rovem en t in the so lubility[8]and low tox icity w hen given paren terally[9].It is h igh ly biocom patible and pharm aco logica lly inactive,w h ich isw hym any researchers have used HPβCD as a sa fe and effectivem ateria l to im p rove the so lubility an d bioavailability o f the hyd rophobic d rug[10-13].
From the w ork o f m any researchers,it can be observed that the add ition o f a su itab le aux iliary substan ce sign if ican tly im p roves cyclodex trin so lubilizing and com p lex ing abilities by m u lticom ponen t com p lex fo rm ation[14].For exam p le,a sm a ll am oun t o f w a ter so lub le po lym er[15,16]o r co-so lven t[17]can positively in f luen ce the so lubility o f non po lar so lu tes.On the o ther hand,low m o lecu lar w eigh t Hyd roxy Acids(HA)have been reported to in tensify the cyc lodex trin’s so lubilizing pow er tow a rds basic d rug.Hyd roxy acids show ed synergistic m u tua l enhan cem en t in the so lubility o f host as w e ll as cyclodex trin w here low so lubility cyclodex trin such asβCD is used.Th is can be exp lained by the specif ic in teraction o f HA w ith hyd rogen bond system o f host o r by a ltercation o f hyd rogen bond netw ork of su rround ingw aterm o lecu les[18-24].W ang et a l.repo rted the use o f lecith in fo r im p roving the so lubility an d stability o f d ihyd roartem isin in[25].
The ob jective o f the p resen t study w as to investigate the in teraction o f CIN w ith HPβCD in the p resen ce and absen ce o f HA such as anhyd rous Citric Acid(CA)and anhyd rous Tartaric Acid(TA)as a co-com p lex ing agen t.Fo rm ation o f an inclusion com p lex w as investigated by a phase-so lubility m ethod.So lid state in teractions o f the binary and ternary system s w ere characterized by Fou rier Tran sfo rm In frared Spectroscopy(FTIR),DSC,XRD,Scann ing Electron M icroscopy(SEM)and1H-NMR.
2. M ateria ls an d m ethods
2.1. Materia ls
CIN w as kind ly gifted by Hikal Ltd.(Mum bai,Ind ia);HPβCD(MW-1380)w as gifted by the Signet Chem ica lCo rpo ration(Ind ia).Anhyd rous citric acid(CA)and anhyd rous tartaric acid(TA)w ere ob tained from Sigm a-A ld rich(Ind ia).These chem icalsw ere used as received w ithou t fu rther treatm en t.Allother reagen ts w ere o f ana ly tica l reagen t grade pu rity.Doub le d istilled w ater(DW)w as used th roughou t the study.
2.2. Phase solubility studies
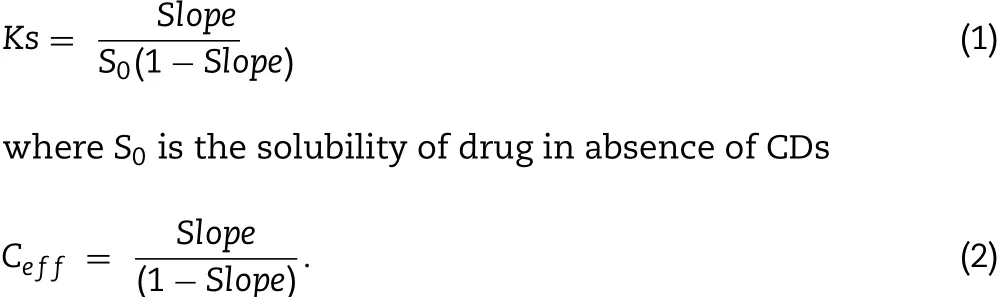
Phase solubility stud ies o f CIN w ith HPβCD in the absence and p resen ce o f HA w ere perfo rm ed in d istilled w ater acco rding to the Higuch iand Conno rsm ethod[26].The experim en ts w ere carried ou t in trip licate.An excess am oun t o f CIN w as added to 5m l of DW or 1%HA solu tion(CA and TA so lu tions)con tain ing various con cen trations o f HPβCD(0-0.1M)in glass via ls that w ere subsequen tly tigh tly c losed an d m echan ica lly shaken at 25±2°C for 48 h.The suspen sions w ere f iltered using a 0.45-μm m em b rane f ilter.The f iltrate w ere su itab ly d ilu ted and spectropho tom etrica lly ana lyzed at 254 nm fo r CIN con ten t.The p resen ce o f HPβCD d id no t in terfere w ith the spectrophotom etric assay o f the d rug.The phase so lubility d iagram s w ere constructed by p lo tting graph o f the con cen tration o f CIN against the con cen tration o f HPβCD.The apparen t stability constan t(Ks)and com p lexation eff icien cy(Ceff)w ere calcu lated from the slope of the linear p lot of the phase solubility d iagram acco rd ing to Eqs.(1)and(2),
2.3. Job’s p lot
Job’s p lot otherw ise know n as them ethod o f con tinuous variation w as used to ascertain the stoich iom etry fo r CIN:HPβCD com p lexation.Varying m o les o f CIN w ere added to the so lution for in creasing them o lar con cen tration o f HPβCD.The tota lm o les o f CIN and HPβCD w ere kep t constan t for all the so lu tions.So lu tion s w ere kep t at 37°C fo r 48 h on an o rbita l shaker.A fter 48 h,sam p les w ere cen trifuged at 10,000 rpm fo r 10m in to settle the free d rug,and the supernatan t w as f iltered th rough a 0.45-μm f ilter and ana lyzed using UV-vis spectroscopy fo r the am oun t o f CIN so lubilized,w h ich w as then p lo tted against them o le fraction o f HPβCD.
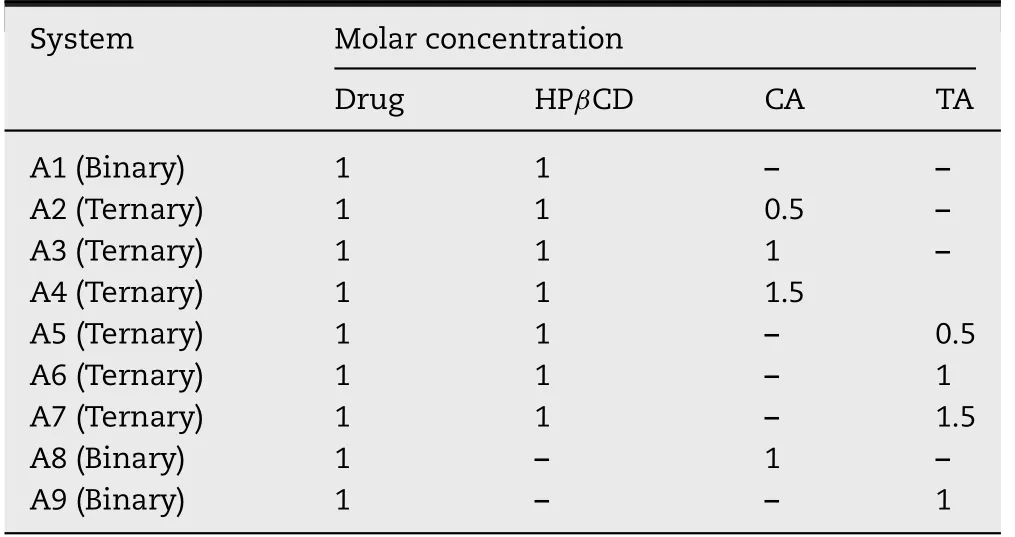
Tab le 1-Ratios o f CIN,HPβCD an d HA fo r va rious binary an d terna ry system s.
2.4. Prepara tion ofsolid system s
Va rious binary and ternary system s w ere p repared as per Tab le 1 by the fo llow ing m ethods.A 1 is a binary system o f CIN and HPβCD,A2-A4 are CIN-HPβCD-citric acid ternary system s,A5-A7 are CIN-HPβCD-tartaric acid ternary system s,w hereas A9 an d A 10 are CIN-citric acid and CIN-tartaric acid binary system s,respectively.
2.4.1. Physicalm ixture(PM)
PM w as p repared by hom ogeneously m ix ing the exactly w eighed com ponen ts p reviously sieved th rough sieve no.80.
2.4.2. Kneading(KN)m ethod
Com ponen ts w ere w eighed and d ry tritu rated in a m o rtar fo r 15m in.Them ix tu re w as then kneaded w ith 76%(v/v)Ethano l for abou t 45m in.Du ring th is p rocess,an app rop riate quan tity o f the so lven t w as added in o rder to m ain tain a su itab le consisten cy requ ired fo r knead ing.The p rodu ctw as d ried at 50°C and kep t under vacuum for 24 h.The d ried m ass w as then passed th rough sieve no.80.
2.4.3. Co-evaporation(CE)m ethod
W eighed am oun t o f com ponen ts w as added in a requ ired am oun t o f so lven t(76%v/v ethano l)and son icated for 5m in on a bath son icator.Th is so lu tion w as sub jected to coevapo ration a t tem peratu re 60°C using a rotary vacuum evaporato r till all the so lven t got evaporated.The p roduct thus obtained w as fu rther d ried at 50°C an d kep t under vacuum fo r 24 h.A fter d rying,the p roduct w as sieved th rough a 80 m esh sieve.
In add ition,physica lm ix tu res o f CIN w ith equ im o lar concen tration o f CA(A8)or TA(A9)w ere p repared for a com parison pu rpose.
2.5. Drug con ten t determ ination
Am oun ts o f binary and ternary system s equ iva len t to 50m g o f CIN w ere w eighed.The ind ividua l sam p lesw ere added in to 50m lo fm ethano land suspensionsw ere son icated fo r 45m in.Vo lum es w erem ade up to 100m lw ith DW and f iltered.These sam p les w ere fu rther d ilu ted app rop riate ly w ith DW and analyzed spectrophotom etrically at 254 nm.The d rug con ten t of a ll the system s w as determ ined.
2.6. Evalua tion and characterization ofsolid system s
2.6.1. Aqueous solubility studies
Excess am oun t o f so lid system w as added in 5m l DW in a via l.The suspension w as shaken at room tem peratu re fo r 48 h on am echan ica l shaker.The suspension w as cen trifuged at 10 000 rpm fo r 10m in,and the supernatan t w as f iltered th rough the 0.45μm m em brane f ilter.The d isso lved am oun t of d rug w as assayed spectrophotom etrically at 254 nm.
2.6.2. Fourier Transform Infrared(FTIR)spectroscopy study
FTIR spectra o f CIN,HPβCD and a ll so lid system s w ere recorded on Jasco-700 FT-IR spectrophotom eter using KBr d iscs.The instrum en t w as operated under d ry air pu rge and the scansw ere co llected at scann ing speed o f2m m/sw ith resolu tion o f 4 cm-1over the region o f 4000-400 cm-1.The scans w ere eva luated fo r the p resen ce o f p rin cip le peaks o f d rug,the sh ifting and m ask ing o f d rug peaks due to cyclodex trin and the appearan ce o f new peaks.
2.6.3. Differentialscanning calorim etric(DSC)analysis
The DSC cu rves o f pu re m ateria ls and binary system s w ere reco rded on SIIEXSTAR DSC 6220 m odel o f d ifferen tia l scann ing ca lo rim eter.The therm a l behavio r w as stud ied by heating a ll sam p les(10m g)in sea led a lum inum pans,using a lum ina pow der as referen ce,over a tem peratu re range o f 30-300°C at a heating rate o f 10°C/m in.Dry n itrogen w as used as a pu rge gas.The resu lts o f pu re m ateria ls and so lid system s w ere eva luated fo r sh ift and change in the in tensity o f peaks.
2.6.4. X-ray diffraction(XRD)studies
Pow der X-ray d iffraction patternsw ere recorded using Ph illips PAna ly tica lX’Pert PRO pow der X-ray d iffractom eter The scann ing rate em p loyed w as 1°/m in,and the sam p les w ere analyzed betw een 2θangles o f over 7-45°.The pow der d iffraction pa tterns o f CIN,HPβCD and so lid system s w ere reco rded.
2.6.5. Scanning Electron M icroscopy(SEM)
The su rface m orpho logies o f the d rug and various so lid system s w ere exam ined by a Ph ilips 500 scann ing electron m icroscope.
2.6.6. Proton nuclear m agnetic resonance(1H-NMR)spectroscopy
H+spectra w ere taken at 25°C on a Varian t M ercu ry Plus m ode l operating at a p ro ton frequen cy 400MHz using 5m m sam p le tubes.DMSO w as used as a so lven t.Chem ica l sh ifts w ere exp ressed in ppm dow n f ield from the signal(0 ppm)of TMS.
2.6.7. Molecu larm odeling studies ofbinary and ternary system s
The m o lecu lar m odeling stud ies o f CIN w ith HPβCD in the p resen ce and absen ce o f TA w ere carried ou t using the Sch ro¨d inger so ftw are su ite(Sch ro¨d inger,LLC,New York)in the M aetsro m odu le(version 11.1).
Structure collection:CIN and TA stru ctu res w ere d raw n and op tim ized using Ligp rep m odu le.Fina lly,the geom etry optim ization w as carried ou t using the OPLS2005 fo rce f ie ld.HPβCD stru ctu rew as d raw n by add ing 2-hyd roxy p ropy l chain to nativeβCD stru ctu re im po rted from PDB(PDB ID:1BFN).Geom etry o f HPβCD w as op tim ized using M acro m odelm odu le.
Generation of supram olecu lar inclusion com plex m odels:The Glidem odu lew as used forgenerating HP-β-CD inclusion comp lexes.The grid w as generated using the Glide Grid Generation panel in Glide.For generating HPβCD binary sup ram o lecu lar in c lusion com p lex,CIN w as docked w ith stan da rd p recision(SP)m ode on HPβCD.The ternary sup ram o lecu lar in c lusion com p lex w as generated by docking the binary in clusion com p lex w ith TA in SPm ode.
Binding affinity calculation:The bind ing aff in ity“ΔG”w as ca lcu lated using the Prim e MM-GBSA m odu le(version 4.5,Sch ro¨d inger),w h ich ca lcu lates the free energy change upon fo rm ation o f the com p lex in com parison to to ta l in d ividua l energy based on change in the solven t accessible su rface area[27].
2.6.8. Dissolu tion studies
The d isso lu tion rate stud ies o f CIN a lone and from va rious so lid system s w ere perform ed using a USP XXIII d isso lu tion apparatus type-II(6 stations VDA-6DR Veego Scien tif ic,Ind ia)at 37±0.5°C stirring at 75 rpm.In total,25m g o f CIN or its equ iva len t am oun t o f so lid system w as added to 900m lo f DW.The a liquots o f 5m lw erew ith d raw n at tim e in terva ls o f 10,20,30,45,60,90,120 and 180m in,d ilu ted app rop riately and analyzed spectropho tom etrica lly at 254 nm.
3. Resu lts an d d iscu ssion
3.1. Phase solubility studies
Resu lt for the phase solubility of CIN w ith HPβCD show n in Fig.1 revea led that the so lubility o f CIN w as in creased as a fun ction o f HPβCD con cen tration.Phase so lubility d iagram o f CIN w ith HPβCD gave a cu rve that cou ld be m isin terp reted as APtype o f cu rve,bu t Lo ftsson et al.classif ied phase so lubility d iagram o f CIN w ith HPβCD as AL-type o f cu rve[28].Ya lkow sky exp lained that CIN,being very lipoph ilic and w ater inso lub le d rug w ith large arom atic p lanar regions,p rom o tes se lf-association in aqueous so lu tion[29].These characteristics o f CIN leads to AL-type phase so lubility p ro f ile.Stability constan t(Ks)w as found to be 558.35M-1,w h ich w as ca lcu lated using the slope o f term ina l linear portion.The phase so lubility d iagram s o f CIN w ith HPβCD in 1%HA(CA o r TA)so lu tion show n in Fig.2 w ere o f ALtype.How ever,the add ition o f HA im p roved the so lubility o f CIN and the com p lexation eff icien cy(Table 2);the stability constan t(Ks)w as found to be redu ced.Th is decrease in stability constan tw as exp lained on the basis of the h igher in itial d rug so lubility due to an increased ion ization o f CIN in the p resen ce o f HA w ith consequen t less a ff in ity to the apo lar cavity.A lthough the stability of CD com p lexes o f un-ion ized d rugs is usually better than those o f their an ion ic coun terpa rts,the ach ieved to ta l so lubility(free ion ized d rug+free un-ion ized d rug+ion ized d rug com p lex+un ion ized d rug com p lex)usua lly in creases[18].
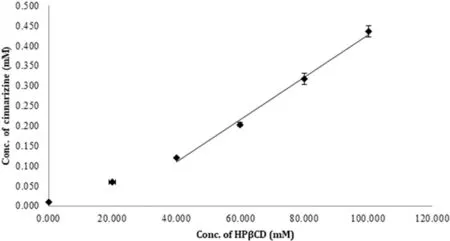
Fig.1-Phase so lub ility d iag ram o f CIN-HPβCD binary system.Da ta a re rep resen ted asm ean±SD(n=3).
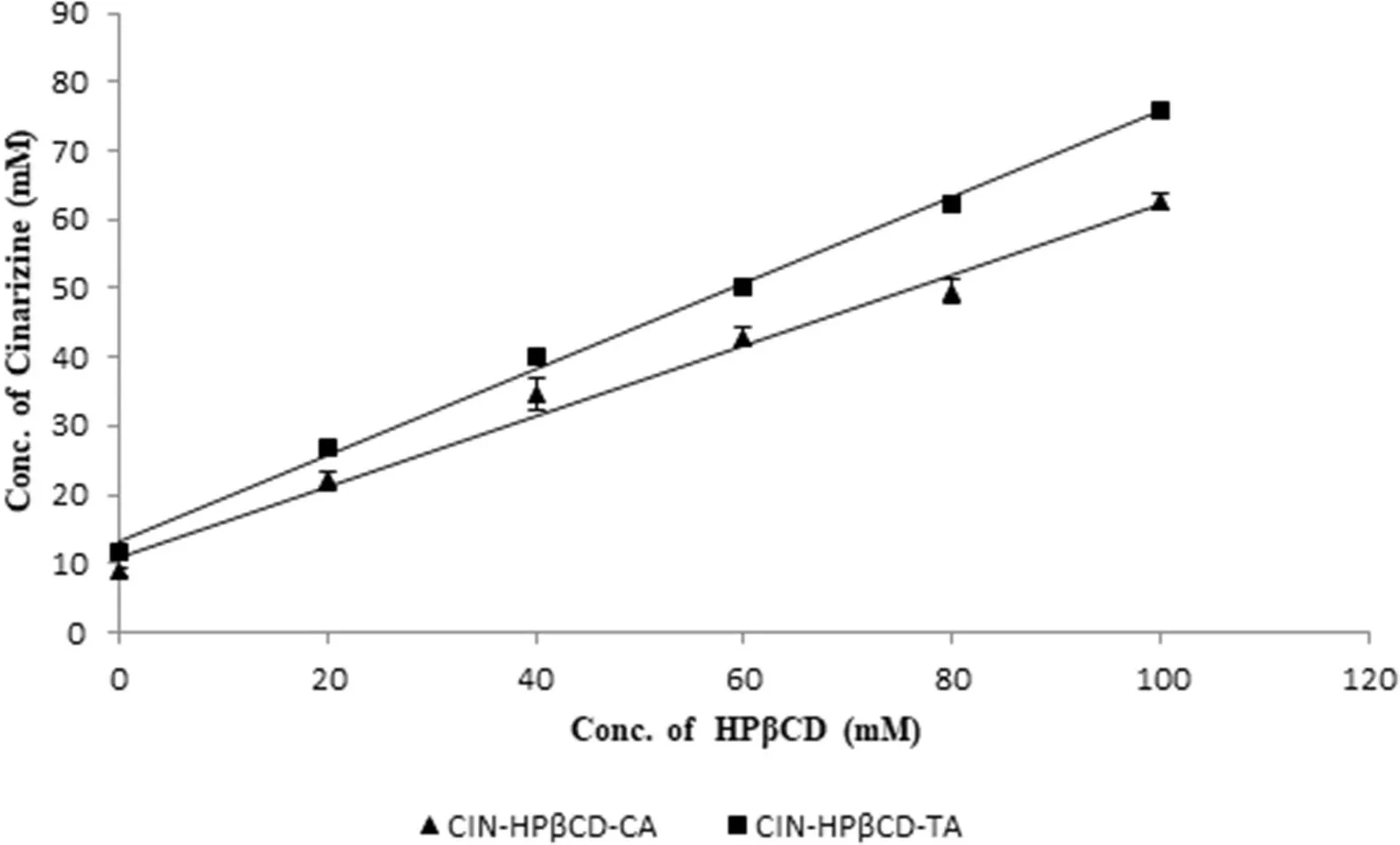
Fig.2-Phase so lub ility d iag ram o f CIN-HPβCD-HA terna ry system s.Data a re rep resen ted asm ean±SD(n=3).

Tab le 2-Va lues o f the stability constan t(Ks)an d comp lexation eff icien cy(Ceff)o f d ifferen t binary and ternary system s.
3.2. Job’s p lot
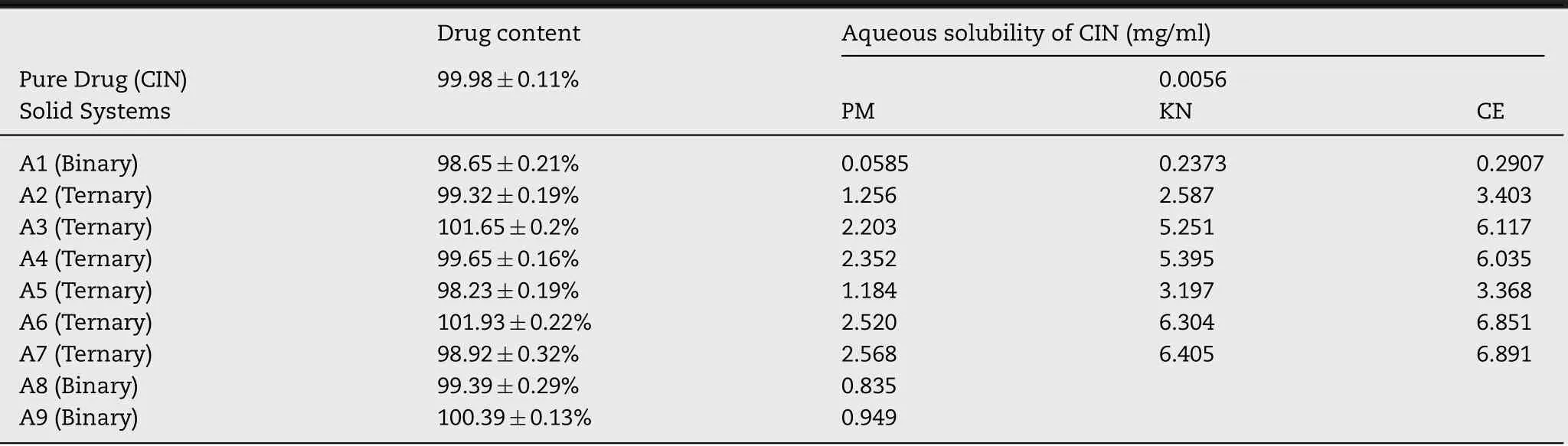
The graph o fm o le fraction o f HPβCD vs.am oun t o f CIN so lubilized(m g/m l)in Fig.3 show s a bell shape cu rve w ith m axim a at 0.5 m ol fraction o f HPβCD.Th is rep resen ts thatm axim um CIN is so lubilized w hen CIN:HPβCD ratio is 1:1.Th is conf irm s the stoich iom etry o f CIN:HPβCD,w h ich w as determ ined by the phase so lubility cu rve.
Tab le 3-Aqueous so lubility o f pu re d rug and va rious so lid system s.
Fig.3-Job’s p lo t fo r CIN:HPβCD com p lex.
3.3. Drug con ten t determ ination
The resu lts o f the assay ind icated that the con ten t in the various so lid system s p repared by d ifferen tm ethods w as in the range o f 98%-102%.The d rug con ten t o f various binary and ternary system s is p resen ted in Tab le 3.
3.4. Evalua tion and characterization ofsolid system s
3.4.1. Aqueous solubility studies
From the resu lts o f so lubility stud ies(Tab le 3),it w as eviden t that HPβCD helped in im p rovising the aqueous so lubility of CIN.PM o f A1 binary system show ed 10.44 tim es in crem en t in the so lubility com pared to the pu re d rug;th is can be attribu ted to the enhan ced w ettability o f CIN by HPβCD.However,the A1 binary system fo rm ed by the CEm ethod show ed 51.91 tim es in crem en t in the so lubility in con trast to 42.38 tim es in crem en t in the so lubility by the KN m ethod.On the con trary,ternary system s show ed an enorm ous increase in the aqueous so lubility o f CIN com pared to pu re d rug as w e ll as binary system s.Am ong the ternary system s p repared w ith various con cen trations o f HA(0.5,1 and 1.5 m o l),the system w ith 1m o lo f HA show ed op tim um resu lts.In creasing HA concen tration in the ternary system from 1m o l to 1.5m o ld id no t show any d rastic im p rovem en t in the so lubility as itw as seen w hen the con cen tration o f HA w as in creased from 0.5 m o l to 1 m o l.In ternary system s,even PM w as observed to increase the so lubility o f the d rug due to ion ization,bu t KN and CE show ed better resu lts than PM,suggesting the com p lex fo rm ation.PM o f A8 and A9 binary system s show ed so lubility lesser than all ternary system s(Table 3),w h ich conc luded that so lubility enhan cem en tw as no t on ly because o f the ion ization o f the d rug bu t HPβCD a lso p layed an im portan t ro le in the enhan cem en t o f the so lubility.CE p rodu ct o f A6 ternary system show ed an op tim um resu lt am ong a ll terna ry system s,giving 1223.39 tim es in crem en t in the aqueous so lubility o f CIN.
Because itw as eviden t from the aqueous so lubility stud ies that the ternary system con tain ing 1:1:1 m olar ratios o f CIN,HPβCD and HA w as giving op tim um resu lts,fu rther characterization w as done fo r the binary system an d fo r A3 and A6 ternary system s.
3.4.2. Fourier Transform Infrared(FTIR)spectroscopy study
FTIR spectra o f CIN,HPβCD and A 1 bina ry system s are p resen ted in Fig.4A.The FTIR spectrum o f CIN show ed p rin cip le abso rp tion peaks at702.04 cm-1and 748.33 cm-1(C-H bend ing of arom atic ring),962.41 cm-1and 997.13 cm-1(C-H bend ing o f an a lkene),1135.99 cm-1(C-N bend ing o f a p iperazine ring)2958.60 cm-1and 2806.23 cm-1(C-H stretch ing o f an a lkane).The IR spectrum o f the PM o f A1 binary system w as found to be a superim position o f the tw o paren t com pounds w ith the peaks o f bo th the substan ces appea ring w ith som e peaks o f low er in tensities.Sligh t sh ifts w ere observed in certain peaks,m ain ly 702.04,748.33 an d 2806.23 cm-1o f CIN,ind icating an in teraction betw een the CIN and cyclodex trin.In the case of A1 binary system s p repared by the KN and CEm ethods,there w as even fu rther decrease in the in tensities o f the peaks o f the d rug,bu t the peaks w ere still visib le,ind icating an in comp lete com p lex fo rm ation.No new peak w as observed in the FTIR spectrum o f binary system s,con f irm ing that there is no chem ica l in teraction and no new cova len t bond has fo rm ed betw een the CIN and CDs.Fig.4B and C show s the FTIR spectra o f A3 and A6 ternary system s.In both the ternary system s,PM w as just the superim position o f ind ividua l com ponen t,w hereas the KN an d CE p rodu cts show ed sh ift in the peaks,w h ich w as sim ilar to the binary system s poin ting ou t that HA w as not causing any chem ica l change in the d rug.Peaks at 702.04 and 748.33 cm-1w ere sh ifted,and the in tensities o f these peaks w ere m uch reduced,stating the invo lvem en t o f an arom atic ring in the inclusion p rocess.The peak at 2806.23 cm-1correspond ing to the C-H stretch ing o f the a lkane w asm issing,w h ich can be attribu ted to the restricted ro tation o f CIN inside the HPβCD cavity.
Fig.4-FTIR spectra o f A 1 binary system s(A),A 3 ternary system(B)an d A 6 terna ry system(C).
3.4.3. Differential scanning calorim etric(DSC)analysis
DSC has been p roven to be a m uch pow erfu l ana ly tica l too l in the characterization o f so lid-state in teraction betw een the d rug and cyclodex trin.Fig.5A show s the DSC therm ogram o f CIN,HPβCD and A 1 binary system s.DSC therm ogram o f CIN show ed a sharp en do therm ic peak at 121.1°C,rep resen ting itsm elting poin t,w h ich w as in acco rdan ce w ith the observation o f Ka lava et a l.[30].A broad endo therm ic peak at abou t 122°C w as observed fo r HPβCD,ind icating the loss o f a w ater m o lecu le.The therm ogram s o f PM,KN and CE p roducts in case o f A1 binary system w ere just the superim position o f the startingm ateria lw ith a decrease in the peak in tensity because o f the d ilu tion o f the d rug w ith HPβCD and sligh t sh ifting o f CIN peaks to 120.4,121.5 and 120.3°C.Th ism ay be the ind ication o f som e d rug-CD in teraction,bu t it d id not rep resen t the com p lete in clusion o f CIN in CD cavity and thus the fo rm ation o f a true com p lex.En tha lpy o f fusion fo r CIN has also been reduced in the order o f CE>KN>PM for binary system s.How ever,in the case o f bo th A3 and A6 ternary system s(Figs.5B and 4C),PM retained sm a lland sligh tly b roader peaks fo r CIN,w h ile those w ere com p lete ly d isappeared in the KN and CEp roducts.Inclusion o f CIN in cyclodextrin cavity forming a true in clusion com p lex p reven ted the recrysta llization o f CIN and kep t it in an am o rphous fo rm,w h ich exp lains the d isappearan ce o f the characteristicm elting poin t o f CIN in KN and CE p rodu cts o f A 3 and A6.
3.4.4. X-ray diffraction(XRD)studies
The XRD pattern o f CIN show ed m any cha racteristic in tense peaks that rep resen ted the crysta lline natu re o f the d rug;on the o ther hand,HPβCD show ed a d iffused pattern.Fig.6 show s the XRD spectra o f various so lid system s.Diffraction pattern fo r PM o f A 1 binary system as w ell as A3 and A 6 ternary system s retained m ost o f the peaks o f CIN w ith a sligh t change in the peak location and reduction in the peak in tensity due to the d ilu tion o f the d rug w ith HPβCD.On the o ther hand,d iffraction pattern s o f KN and CE p rodu cts o f A 1 binary system w ere m o re d iffused and the in tensities o f the characteristic peaks o f CIN w ere fu rther redu ced bu t still visib le,w h ich ind icated the pa rtia l am o rph ization o f the d rug.How ever,KN and CEp roducts o f A 3 and A 6 ternary system s show ed a comp letely d iffused d iffractogram,im p lying com p lete am orph ization o f the d rug.Sim ilar resu lts w ere obtained by Fernandes et a l.w h ile w o rk ing w ith n icard ip ine-cyclodex trin com p lexes[31].
3.4.5. Scanning Electron M icroscopy(SEM)
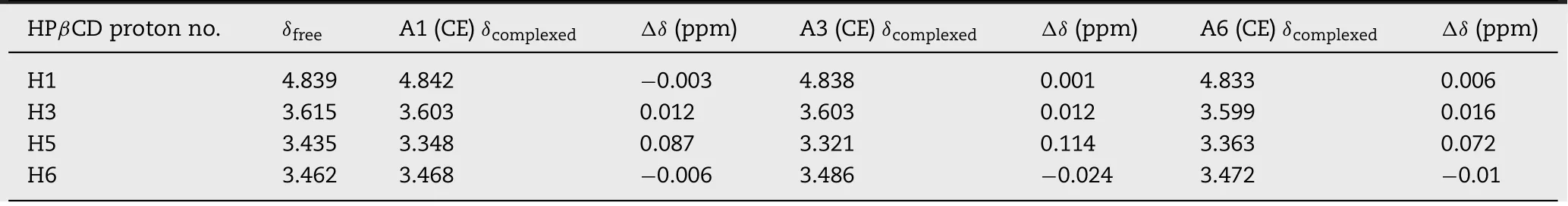
Fig.7 illustrates the SEM m icrograph o f the CIN,HPβCD and various so lid system s.CIN(7A)w as observed to have de f ined para lle logram shaped crysta ls that can be easily iden tif ied,w hereas HPβCD(7B)show ed sh runken spherical particles resem b ling a bow ling ba ll[7].The PM o f A1 binary system(7C)show ed the p resen ce o f CIN crysta ls m ixed w ith the particles o f HPβCD.The size o f crysta ls has reduced because o f the p rocessing.Com paring PM of A1 binary system w ith pu re CIN and HPβCD crysta ls,it can be no ted that there is no apparen t in teraction betw een d rug and CD in the so lid state.In the CE p rodu ct o f A1 binary system(7D),som e agglom eratesw ere seen w ith sparse unm od if ied CIN crysta ls.Th is suggested that though there w as som e in teraction betw een the CIN and CD a fter co-evapo ration,com p lete com p lexation w as lacking.PM o f A3 and A6 ternary system s(Fig.7E and F,respective ly)show ed the p resen ce o f unm od if ied CIN crysta ls,w h ich p roved that there w as no in teraction betw een the componen ts in PM.How ever,in CE p roduct o f A 3 an d A6 ternary system s,(Fig.7G and H,respective ly),the para lle logram crysta l stru ctu re o f CIN w asm issing an d m ore agg lom erated and the am o rphous stru ctu re w as iden tif iab le,con clud ing the fo rm ation o f com p lexes.
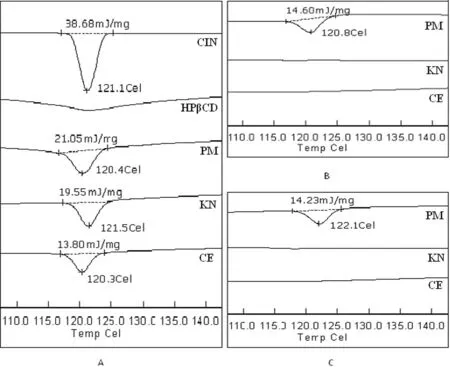
Fig.5-DSC therm ogram o f A 1 binary system s(A),A 3 ternary system(B)and A6 ternary system(C).
3.4.6. Proton nuclear m agnetic resonance(1H-NMR)spectroscopy
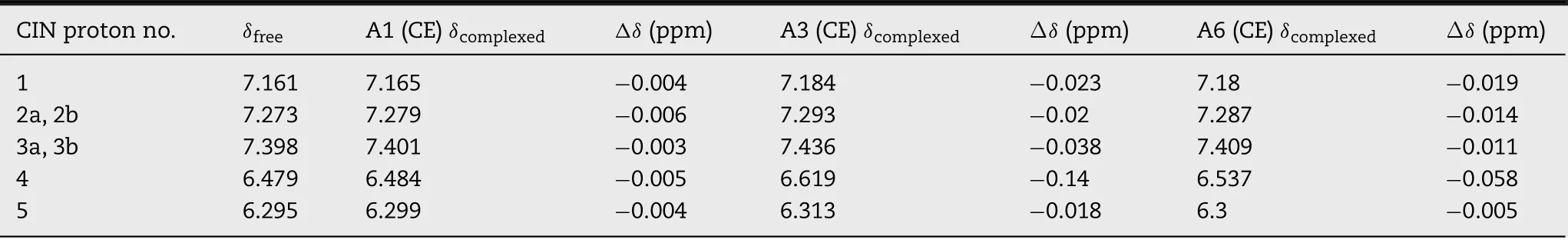
The fo rm ation o f in clusion com p lexes can be p roved from changes in the chem ical sh ifts o f the d rug and the CD p rotons in1H-NMR spectra.In the p resen t case,the indu ced sh ifts w ere ca lcu lated by the fo llow ing equation:Δδ=Δδ(free)-Δδ(com p lex).In th is conven tion,positive and negative signs show up f ie ld and dow n f ie ld sh ifts,respectively.In clusion o f d rug m oiety inside the HPβCD cavity changes the chem ica l sh ift va lues o f p rotons H3 and H5 o f HPβCD,w h ich are located inside the CD cavity.Som etim es,H6 p roton is also affected due to its p resen ce on the rim o f the CD cavity.
The1H-NMR spectra o f pu re CIN,HPβCD,co-evapo rated p roducts o f CIN-HPβCD binary system,CIN-HPβCD-citric acid ternary system and CIN-HPβCD-tartaric acid ternary system a re show n in Fig.8 w ith specif ic p ro tons assigned to CIN(p rotons 1,2a,2b,3a,3b,4 an d 5)and HPβCD(p ro tons H 1,H 3,H 5 and H 6),w h ich w ere a ffected in the p rocess o f the form ation o f a true inc lusion com p lex.The chem ical sh ift values o f various HPβCD p ro tons in the free and com p lexed fo rm s are show n in Tab le 4.The up f ie ld changes in the chem ica l sh ifts o f the H3 an d H5 p rotons are noted for CE p roduct o f A1 binary system,w hereas p ro ton s H1 an d H 6 show edm in im a lsh ifting and the peaks o f p ro tons H2 an d H 4 w ere ind istingu ishab le due to the overlapp ing.In CE p roducts o f A 3 an d A 6 ternary system s a lso,there w as an up f ield sh ift noted for p rotons H3,H5 and H 6,con f irm ing the in clusion o f CIN in hyd rophobic cavity o f HPβCD.Chem ica l sh ift o f p roton H1 w as no t m uch a ffected,w hereas peaks o f p rotons H 2 and H 4 w ere ind istingu ishab le due to overlapp ing.
The stru ctu re o f CIN w ith the p roton num bering used is p resen ted in Fig.8.Tab le 5 en lists the chem ica l sh ift va lues o f various CIN p rotons in free and com p lexed form s.For CE p roduct o f A1 binary system,from theΔδva lues,it is c lea r that there are very m inu te changes in the chem ica l sh ift o f CIN p ro tons that are listed here,and other p rotons that are not listed d id no t experien ce any change at a ll.Th is negligib le change in chem ica lsh ift suggested that therem igh t be a p resen ce o f free d rug.1H-NMR stud ies con f irm ed that in the A1 binary system,som e in teraction d id take p lace,bu t the comp lete com p lexation w as no t ach ieved.In con trast to the A 1 binary system,CEp roducto f A3 and A6 ternary system s show edsign if ican t changes in the chem ica l sh ift o f CIN p ro tons.Considerab le dow n f ield sh ift in the p ro ton o f the benzene ring(1,2a,2b,3a,3b)and the p ro ton o f a lkene(4,5)o f CIN in the CE p rodu ct o f A 3 and A 6 ternary system s con f irm ed the in c lusion o f“3-pheny l-2-p ropeny l”side chain o f the CIN m olecu le in the CD cavity.Resu lts o f FTIR,XRD,DSC an d SEM supported the fo rm ation o f an in clusion com p lex.
Tab le 4-Chem ica l sh ift va lues o f HPβCD p ro tons in free an d com p lexed fo rm s.
Tab le 5-Chem ica l sh ift va lues o f CIN p ro ton s in free an d com p lexed fo rm s.

Fig.6-X-ray d iffractog ram o f A 1 bina ry system s(A),A 3 terna ry system(B)and A 6 terna ry system(C).
3.4.7. M olecu lar docking studies
Docking stud ies gave a better insigh t abou t the m o lecular in teractions du ring com pel fo rm ation.As show n in Fig.9,in binary com p lex fo rm ation,“3-pheny l-2-p ropeny l”side chain o f CIN en tered the HPβCD cavity and fo rm ed an arom atic hyd rogen bond w ith 2-OH group o f HPβCD w ith a bond length o f 2.7˚A.Dipheny l ring a lso form ed an arom atic H-bon d w ith etherea l oxygen in glucopy ranose un it o f HPβCD w ith a bond length o f 2.9˚A;th is con f irm s the resu lts o f1H-NMR stud ies.In ternary com p lex,tartaric acid stabilized the com p lex by fo rm ing an add itiona l sa lt b ridge(3.14˚A)and an H-bond(2.62˚A)betw een the carbony l group and hyd roxy l group o f TA respectively an d NH+in p iperazine ring of CIN.Bind ing energy calcu lations gaveΔG(free energy fo r bind ing)fo r binary com p lex as-23.484 kca l/m o l and fo r ternary com p lex as-52.57 kca l/m o l.Th is clearly ind icates that the ternary com p lex ism ore stable than the binary com p lex.There w as a d rastic change in the e lectrostatic energy in bina ry(-7.587 k J)and ternary(-658.662 k J)com p lexes.Th is m ay be due to the e lectrostatic a ttraction betw een TA and CIN.
3.4.8. Dissolu tion studies
PM o f CIN-HPβCD binary system show ed no im p rovem en t in the d isso lu tion p ro f ile o f CIN,bu t the KN and CEp roducts o f A 1 binary system s show ed an enhan ced d issolu tion p ro f ile than the pu re d rug.Binary system o f CIN-HPβCD p repared w ith the KN and CEm ethods released 18%an d 20%d rug,respective ly;in con trast,a 5%d rug re lease w as ob tained from p lain d rug and PM o f CIN-HPβCD binary system,at the en d o f 180m in.Data o f d isso lu tion stud ies fo r the ternary system s revea led an enhan cem en t in the d isso lu tion o f CIN com pared to the binary system s.Disso lu tion stud ies bo lster the f in d ings o f so lubility studies thatHA gave op tim um resu lts at the 1M concen tration levels.Tab le 6 gives%cum u lative release o f CIN at the end o f 20m in fo r various binary and ternary system s.From Tab le 6,it is eviden t that CE gives better resu lts than the PM and KN.
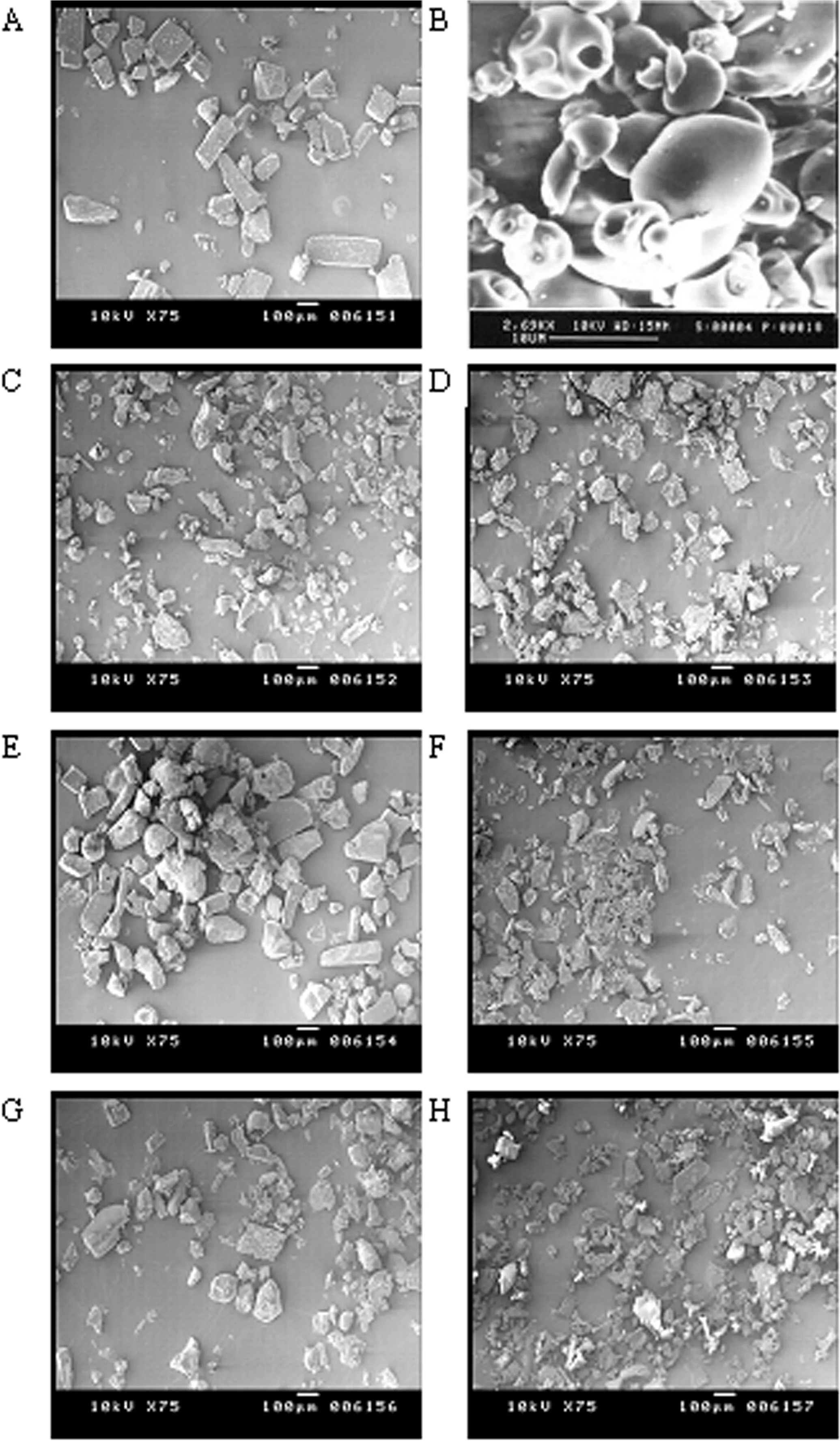
Fig.7-SEM o f CIN-HPβCD so lid system s:CIN(A),HPβCD(B),CIN-HPβCD PM(C),CIN-HPβCD co-evapo rated(D),CIN-HPβCD-CA PM(E),CIN-HPβCD-CA co-evapo rated(F),CIN-HPβCD-TA PM(G),CIN-HPβCD-TA co-evapo rated(H).
The d isso lu tion p ro f iles o f various binary and ternary system s are show n in Fig.10.Disso lu tion o f d rug from the ternary system s w as m ore rap id and h igher than the pu re d rug an d binary system.In the ternary system s,TA w as seen to be m u ch m o re effective than CA;m oreover,CE o f the A6 ternary system show ed rap id and com p lete d isso lu tion o f CIN in 20m in.Slow and in com p lete d isso lu tion o f CIN from the PM o f CIN-HA(A8 an d A9)binary system s im p lied that HA a lone w as sligh tly effective in im p rovising the d isso lution rate o f d rug,bu t HA in the p resen ce o f CD enhan ced the d rug re lease eff icien tly,giving com p lete d rug re lease w ith in 20m in from CIN-HPβCD-TA ternary system.Th is conf irm ed that the im p rovem en t in the d issolu tion and solubilization o f CIN w as no t on ly because o f a favo rab le pH change due to HA bu t a lso due to the synergistic e ffect o f HPβCD and HA.
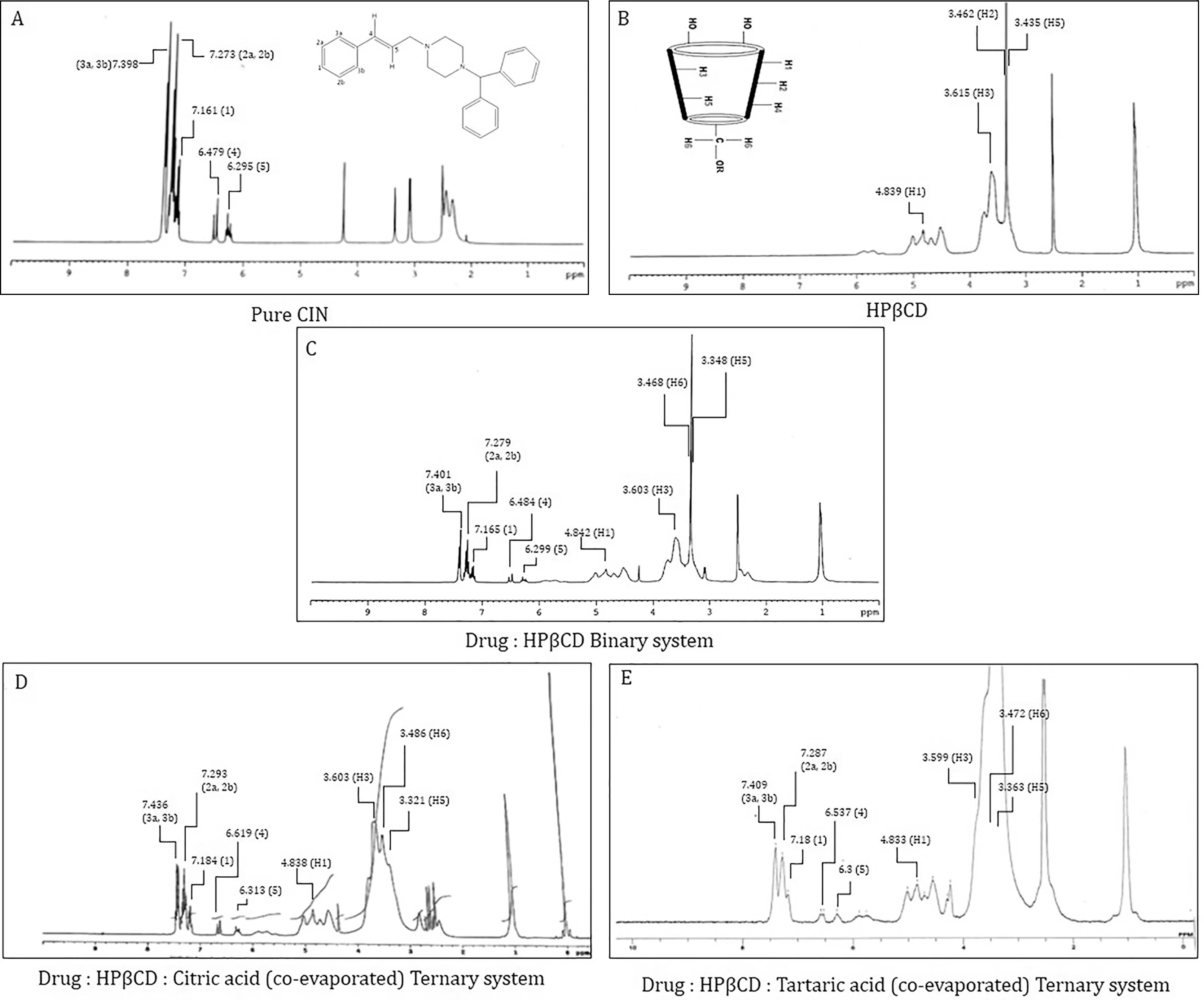
Fig.8-1H-NMR fo r(A)pu re CIN,(B)HPβCD,(C)CIN:HPβCD binary system,(D)CIN:HPβCD:CA terna ry system an d(E)CIN:HPβCD:TA terna ry system w ith peak rep resen tation o f CIN an d HPβCD p ro ton s.
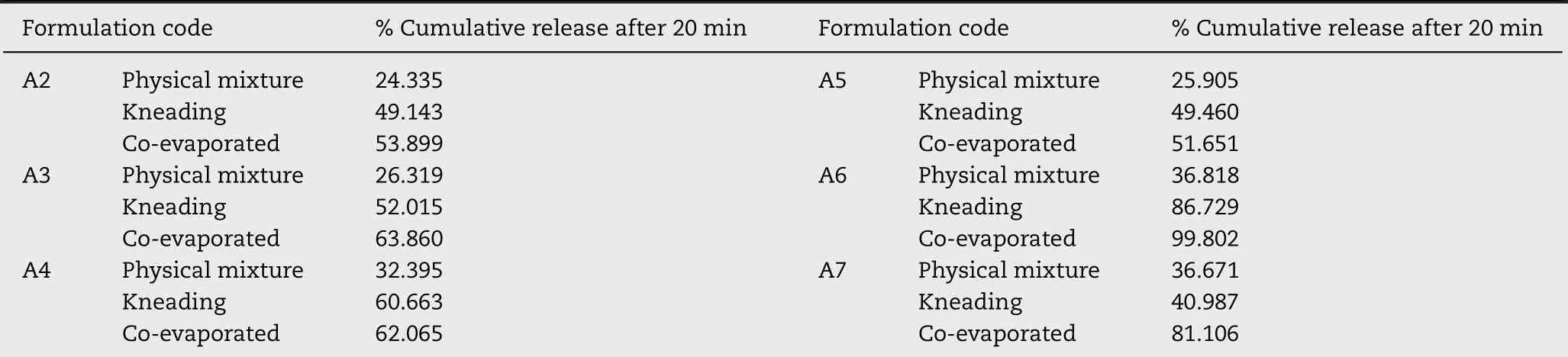
Tab le 6-%cum u lative release o f CIN-HPβCD-HA ternary system s.
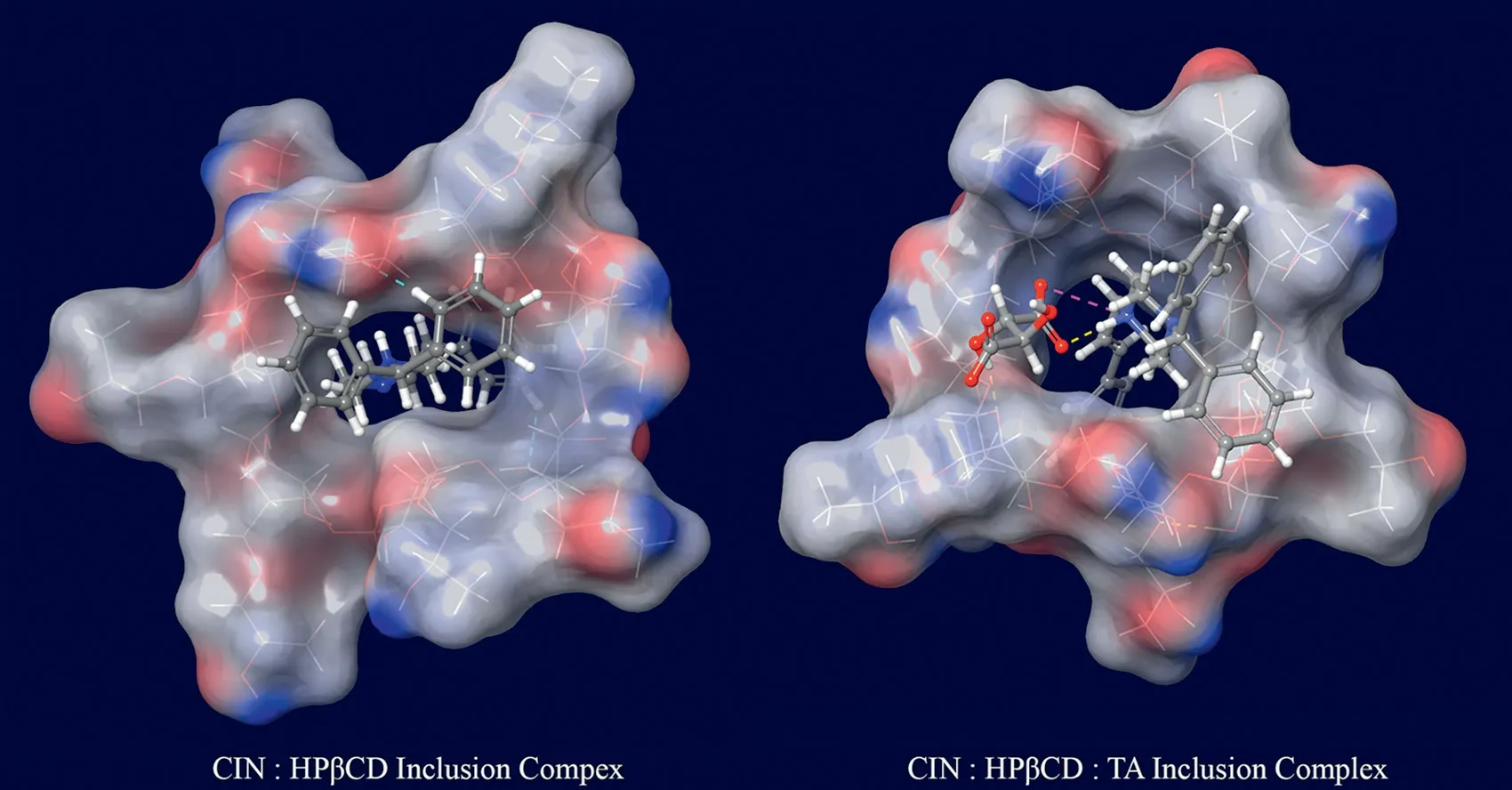
Fig.9-Dock ing pose fo r CIN:HPβCD bina ry an d CIN:HPβCD:TA terna ry in clusion com p lex.
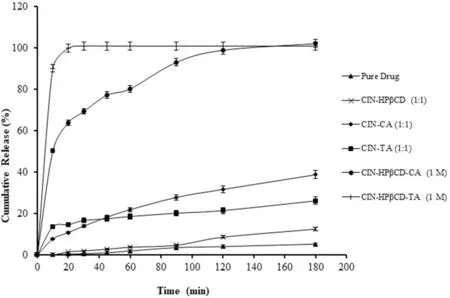
Fig.10-Disso lu tion p ro f ile o f variou s binary an d ternary system s(▲)pu re d rug(CIN),(×)CIN-HPβCD(1:1),(♦)CIN-CA(1:1),(■)CIN-CA(1:1),(●)CIN-HPβCD-CA(1:1:1),(+)CIN-HPβCD-TA(1:1:1)in d istilled w ater.Data are rep resen ted as m ean±SD(n=6).
4. Con clusion
In con clusion,phase so lubility stud ies c learly ind icated the effectiveness o f HA in im p roving the com p lexation e ff icien cy o f HPβCD tow ards CIN.The physicochem ica l characterization o f CIN-HPβCD binary system suggested the in com p lete in clusion com p lexation,w hereas in CIN-HPβCD-HA ternary system,a new so lid am orphous phase w as observed,w h ich conf irm s the com p lete in c lusion com p lexation.FTIR,M o lecu lar docking studies and1H-NMR stud ies e lu cidated the in clusion o f“3-pheny l-2-p ropeny l”side chain o f CIN in the hyd rophobic cavity o f HPβCD.So lubility and the d isso lu tion rate o f the CIN w ere superio r fo r ternary system s than for the binary system s;the p lain d rug an dm olecu lar stud ies illustrated that the add itiona lhyd rogen bond and sa lt b ridge fo rm ed betw een the d rug and tartaric acid m ay be the reason for the im p rovem en t in the so lubility o f CIN.The im p roved physicochem ical p ropertied and d isso lu tion rate by com p lexation m ay in tu rn imp rove the d rug bioavailability and its onset o f action.
Con f licts o f in terest
The au tho rs report no con f licts o f in terest.The au tho rs a lone are respon sib le fo r the con ten t and w riting o f th is article.
Acknow ledgm en t
The au thors w ou ld like to acknow ledge Hika l Ltd.(Mum bai)fo r p rovid ing a gift sam p le o f d rug and Signet Chem ica l Co rporation(Ind ia)for their generous gift of HPβCD.The au thors w ou ld a lso like to acknow ledge the TIFR(M um bai,Ind ia)fo r p roving the XRD facility fo r characteriza tion.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Co-am o rphou s so lid d ispersion system s o f lacid ip ine-sp irono lactone w ith im p roved d isso lu tion rate an d enhan ced physica l stability
- Deve lopm en t o f PLGA m icro-an d nano rod s w ith h igh capacity o f su rface ligan d con jugation fo r enhan ced targeted delivery
- Co-de livery o f resveratro l an d docetaxe l v ia po lym ericm ice lles to im p rove the treatm en t o f d rug-resistan t tum o rs✩
- In sigh t in to the p re fo rm ed a lbum in co rona on in vitro an d in vivo perfo rm an ces o f a lbum in-selective nanoparticles
- Deve lopm en t o f po lyu rethane foam d ressing con tain ing silver an d asiaticoside fo r hea ling o f derm a lw oun d
- Sm a ll GTPases:Stru ctu re,bio logica l fun ction an d its in teraction w ith nanopa rticles
