In sigh t in to the p re fo rm ed a lbum in co rona on in vitro an d in vivo perfo rm an ces o f a lbum in-selective nanoparticles
2019-05-13ZhenboLiDnLienjunZhngPengZhngQimingKnJinSun
Zhenbo Li,Dn Li,W enjun Zhng,Peng Zhng,Qim ing Kn ,Jin Sun,∗∗
a Wuya College of Innovation,Shenyang Pharm aceutical University,W enhua Road,Shenyang 110016,China
b The College ofPharm acy,AnhuiUniversity ofChinese Medicine,Hefei230012,China
c Departm ent ofPharm acy,Shenyang Pharm aceu tica lUniversity,China d Departm ent ofPharm acology,Shenyang Pharm aceutica lUniversity,No.103Wenhua Road,Shenyang 110016,China
Keyw ords:Pre fo rm ed album in co rona A lbum in-nonselec tive PLGA NPs A lbum in-selec tive SPNPs In vitro an d in vivo perfo rm an ces
A B S T R A C TPreform ed album in corona of album in-nonselective nanoparticles(NPs)is w idely exp loited to inh ibit the unavoidab le p rotein adsorp tion u pon in travenous adm in istration.How ever,very few stud ies have con cerned the p refo rm ed a lbum in corona o f a lbum in-selective NPs.Herein,w e report a novel type o f a lbum in-selective NPs by decorating 6-m a leim idocap roy l polyethy lene glyco l stearate(SA)on to PLGA NPs(SP NPs)su rface,taking album innon selective PLGA NPs as con tro l.PLGA NPs an d SPNPsw ere p repared by em u lsion-so lven t evaporation m ethod an d the resu ltan t NPs w ere in spherica l shape w ith an average d iameter around 180 nm.The co rrespon d ing a lbum in-coating PLGA NPs(PLGA@BSA NPs)and a lbum in-coating SPNPs(SP@BSA NPs)w ere fo rm u lated by in cubating SPNPs or PLGA NPs w ith bovine serum a lbum in so lu tion,respectively.The im pact o f a lbum in corona on particle characteristics,stability,photo therm a leffect,cy to tox icity,ce llu p take,spheroid penetration and pharm acokinetics w as investigated.In line w ith p revious f ind ings o f p reform ed album in coating,PLGA@BSA NPs exh ibited h igher stability,cy totoxicity,cell in terna lization and spheroid penetration perform ances in vitro,an d longer b lood circu lation tim e in vivo than those o f a lbum in-non se lective PLGA NPs,bu t a lbum in-selective SPNPs is capab le o f ach ieving a com parable in vitro and in vivo perform ances w ith both SP@BSA NPs and PLGA@BSA NPs.Ou r resu lts dem onstrate that SA deco rated a lbum in-se lective NPs pave a versatile avenue for op tim izing nanoparticu late de livery w ithou t p refo rm ed a lbum in corona.
1. In trodu ction
The rap id developm en t o f nanom ateria ls in biom ed ica l and bio techno logica l app lications o ffers nanopartic les a great poten tia l in d isease d iagnosis and therapy[1,2].On accoun t o f the h igh su rface free energy,non-specif ic p lasm a-p ro tein opson iza tion occu rs im m ed iate ly upon NPs in jection in to bio logica l f lu ids,fo rm ing the so-ca lled“p ro tein co rona”.The subsequen tly form ed corona changes the original featu res o f NPs in clud ing partic le size,zeta po ten tia l and su rface composition,resu lting in a“bio logica l iden tity”that is recogn ized by ce lls in vivo,w hereas the“p ristine iden tity”w as ex tensively m asked[3-5].Actually,the follow ing biologica l co rona cou ld a lso change partic le-ce ll responses,such as NPs ce llu lar up take and in vivo d istribu tion,and even trigger the accom panying rap id c learan ce and nanotoxicity[1,6-8].To add ress these cha llenges,in vitro p re fo rm ing a lbum in corona on NPs is w idely em p loyed to inh ibit opson in p ro teins adso rp tion and p ro long the b lood circu lation capability.And th is strategy has been observed on a d iverse range o f a lbum in-nonse lective NPs,in clud ing Po ly-3-hyd roxybu ty rate-co-3-hyd roxyhexanoate(PHBHHx)NPs[9],quan tum dots[10,11],superparam agnetic iron oxide NPs[1,6,12,13],nanocrysta ls[14,15],graphene ox ide[16,17],Au NPs[18],m anganese d iox ide[15,19]and so forth.As expected,th is in vitro p re fo rm ed a lbum in co rona indeed con fers a lbum innonselective NPs decreased p lasm a p rotein s adsorp tion and com p lem en t activa tion,enhan ced circu lation stability,and im p roved tum o r accum u lation in vivo.How ever,the com p lex and expensive form u lation deve lopm en t p rocess a lsom ade in vitro coating strategy less attractive.
These issues m otivates researchers to engineer nove l a lbum in-se lective NPs.A lbum in,the m ost abundan t p rotein in p lasm a(55%),has been trem endously exp lored fo r its native circu lation capacity,low-im m unogen icity and good stability[20,21].An d indeed,su ccessfu l a lbum in-based nanom ed icines,such as cova len t-bind ing Doxorubicin-EMCH(DOXO-EMCH),have been app lied in clin ica l trials.Precise one m a leim ide group w as selectively reacted w ith one cysteine-34 residue o f endogenous circu lating a lbum in w ith in 2-5m in[21,22].
Indocyan ine green(ICG)is chosen as m odel d rug due to its app rova l by the U.S.Food and Drug Adm in istration(FDA)and effective photon dynam ic/therm a l conversion capacity[23-26].Desp ite the above advan tages,nevertheless,its clinica l app lication is ex trem ely lim ited ow ing to its shortcircu lation,unstab le p roperties su ch as aggregation and degradation in aqueous so lu tion.
Given the rap id con jugation o f m a leim ide group w ith the cysteine-34 residue o f a lbum in and the d ilem m a o f ICG app lication,w e herein repo rt a strategy to decorate m a leim ide groups on PLGA NPs su rfaces to fabricate album in-selective NPs,and then to un ravel the im pact o f p refo rm ed a lbum in co rona on in vitro and in vivo perfo rm an ces o f the engineered a lbum in-selective NPs,tak ing a lbum in-nonse lective PLGA NPs as con tro l.In th is study,a novel biocon jugate 6-m a leim idocap roy l po lyethy lene glyco l stearate(SA)w as syn thesized,and then SA w as decorated on PLGA NPs by em u lsion-so lven t evapo ration m ethod.The resu ltan t NPs possessed a h igher ICG load ing eff icien cy due to electrostatic interaction betw een ICG and po lyethy leneim ine(PEI).Besides the above NPs characterization,the im pact of album in corona on stability,pho to therm a l conversion capability,cy to tox icity,cell up take,spheroid penetration and in vivo pharm acokinetics o f a lbum in-selective SPNPs,w as also evaluated.
2. M ateria ls an d m ethods
2.1. Materia ls
Poly(lactic-co-glyco lic acid)(PLGA)w as pu rchased from Jinan Daigang Biom ateria lCo.,Ltd.Indocyan ine Green(ICG)w as obtained from Bom ei Pharm aceu tica l New Techno logy Developm en t Co.,Ltd.Po lyethy leneim ine(PEI,Mw=10 kDa)w as bough t from A ladd in(Shanghai,Ch ina).Po lyethy lene Glyco l Monostearate w as pu rchased from Tokyo chem ica l industry Co.,Ltd.3-(4,5-d im thy l-2-th iazo ly l)-2,5-d ippheny l-2Hterazolium brom ide(MTT)an d trypsin-EDTA w ere pu rchased from Sigm a-A ld rich(USA).Coum arin-6 w as pu rchased from Sigm a(St.Lou is,MO,USA).A llo ther so lven ts and reagen ts u tilized in th is study w ere ana ly tica l o r HPLC grade.
2.2. Syn thesis of6-m aleim idocaproyl polyethylene glycol stearate(SA)
Syn thesis o f 6-m a leim idocap roic acid
to Istvan Toth et al.report[27].Brief ly,6-am inocap roic acid(30m m o l)and m a leic anhyd ride(30m m o l)w ere ref luxed in AcOH(abou t 70m l)for 6 h.Then,30m m o l Ac2O w as added d ropw ise and re f luxed for a fu rther 2 h.A fter reaction,the objective p roductsw ere pu rif ied using silica co lum n ch rom atography a fter d rying in vacuum.The chem ica l structu re o f 6-m a leim idocap roic acid w as con f irm ed by MS,NMRw ith CDCl3as so lven t and IR.
Syn thesis o f 6-m aleim idocap roy l ch loride
To syn thesize 6-m aleim idocap roy l ch loride,6-m a leim idocap roic acid w as d isso lved in oxa ly l ch lo ride and ref luxed w ith stirring at 70°C fo r 2 h,and the ex tra oxa ly l ch loride w as rem oved using rotary evaporator.The chem ica l stru ctu re o f 6-m a leim idocap roy l ch lo ride w as con f irm ed by MS,NMRw ith CDCl3as so lven t and IR
-Syn thesis o f SA
6-m a leim idocap roy l ch lo ride (20m m o l) w as d isso lved in 40m l dehyd rated d ich lorom ethane and then 20m l dehyd rated d ich lorom ethane con tain ing po lyethylene glyco l stearate(8m m o l)w as added d ropw ise in to 6-m a leim idocap roy l ch loride so lu tion w ith stirring at 40°C fo r 24 h.A fter reaction,the m ix tu re w as separated over SiO2.The chem ica l structu re o f SA w as con f irm ed by NMR w ith CDCl3as so lven t and IR.
2.3. Preparation of SP and SP@BSA NPs
SP NPs w ere p repared by em u lsion-so lven t evapo ration m ethod.9m g SA,30m g PLGA,1.5m g ICG and 1.5m g PEI w ere d isso lved in 0.6m l m ixed so lu tion o f dehyd rated dich lorom ethane and m ethanol(2:1,v/v)and then 10m l deion ized w ater w as added in to it.Nex t,the m ix tu re w as u ltrasound using p robe-type son if ier(JY92-2D,Scien tz,Ch ina)fo r 5m in in ice bath;the organ ic so lven ts w ere evaporated w ith m agnetic stirring in darkness for 6 h.The excess m ethano lw as rem oved un der vacuum at 40°C in dark.PLGA NPs w ere p repared in the sam e w ay as described above w ithou t SA.Coum arin-6 labe led PLGA(C6-labeled PLGA NPs)o r SPNPs(C6-labe led SPNPs)w ere p repared sim ilarly excep t fo r changing DTX to coum arin-6.Fina lly,the o rgan ic so lven t-free NPs w ere stored at 4°C.
The p reform ed album in corona SP NPs(SP@BSA NPs)or p re fo rm ed a lbum in co rona PLGA NPs(PLGA@BSA NPs)w ere con stru cted acco rd ing to Peng et a l.[1,9].Brief ly,1m l SP NPs(3m g/m l)w as in cubated w ith bov ine serum a lbum in(BSA)solu tion(20m g/m l in saline)at 37°C for 2 h,and then SP@BSA NPs and PLGA@BSA NPs w ere iso lated by cen trifugation(13 000 rpm)at 4°C for 15m in,respective ly.
2.4. Characterizations ofSP and PLGA NPs
2.4.1. Size distribution and zeta potential
The partic le size,size d istribu tion and zeta po ten tia lo f SPNPs,PLGA NPs,SP@BSA NPs and PLGA@BSA NPsw erem easu red by dynam ic ligh t sca ttering(DLS)m ethod w ith zetasizer instrum en t(Nano ZS,M a lvern Co.,UK),and them easu rem en tsw ere repeated in trip licate.
2.4.2. M orphology ofSP and PLGA NPs
The m o rpho logy o f SP NPs,PLGA NPs,SP@BSA NPs and PLGA@BSA NPs w ere exp lo red by transm ission e lectron m icroscope(TEM)(H-600,Hitach i,Japan).The NPs w ere negative ly stained w ith 0.2%phospho tungstic acid aqueous so lutions be fo re observa tion under TEM.
2.4.3. Infrared spectra(IR)ofSP@BSA and PLGA@BSA NPs
The a lbum in corona o f SP@BSA NPs and PLGA@BSA NPs w ere m on ito red by in frared spectrom eter(Bruker IFS55,Germ any).In frared spectra o f SP@BSA NPs and PLGA@BSA NPs(freeze w ithou t p ro tectan t)w ere reco rded at the frequen cy ranging from 400 cm-1to 4000 cm-1to determ ine the frequen cy changes.
2.5. Encapsu lation efficiency of SPNPs and PLGA NPs
The ICG load ing eff icien cy o f SP/PLGA NPs w as eva luated as the percen tage of en trapped ICGw ith respect to the total ICG added.In th is study,the en capsu lation e ff icien cy w as determ ined by m easu ring the am oun t o f cen trifuga l supernatan t a fter NPs iso lation[25].
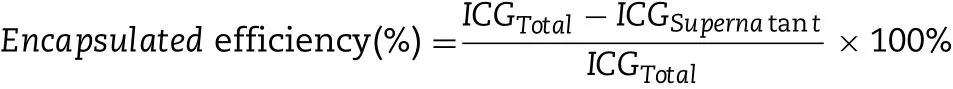
W here ICGTotalis the am oun t o f ICG added in the p reparation;ICGSupernatantis the am oun t of ICG in the cen trifugal supernatan t a fter NPs iso lation.
2.6. In vitro ICG release profiles
The in vitro ICG release perform an ce w as estim a ted against pH 7.4 PBS by the d ia lysis m ethod.Brie f ly,2m l ICG,SP NPs,PLGA NPs,SP@BSA NPs and PLGA@BSA NPs w ere sea led in a d ia lysis bag(MW CO=14 kDa,Spectrum Labo rato ries,USA)and in cubated in 30m l o f release m ed ium at 37°C under orbital shaking(100 rpm),respectively.At designated in terva ls,2m l sam p les w ere w ith d raw n and rep laced w ith equ iva len t fresh m ed ium.The m ax im um ICG abso rban ce w as eva luated using Therm o scien tif ic varioskan f lash.And the correspond ing ICG leakage p ro f iles w ere f itted by zeroorder,f irst-order,Higuch i,W eibu ll and Ritger-Peppas m odel,respective ly.
2.7. Circu lar dichroism spectroscopy
Circu la r d ich roism(CD)w as app lied to assess the con fo rm ational changes of BSA on the su rfaces o f SP@BSA NPs and PLGA@BSA NPs[28].Both them easu rem en ts w ere p robed using CD spectropo larim eter(Bio-Logic MOS 450,Fran ce,Grenob le)in a 1 cm path length quartz ce ll from 190 to 400 nm at 37°C[29].
2.8. M easurem en ts of tem perature
SPNPs,PLGA NPs,SP@BSA NPs,PLGA@BSA NPs an d free ICG so lu tion(100μg/m l o f ICG)w ere exposed to an 808 nm laser p roduct(Changchun,Ch ina)at 1.5W/cm2fo r 3m in.Du ring irrad iation,the tem peratu re w asm easu red every 30 s.
2.9. Colloids and UV stability ofNPs
The co lloida l stability o f SP NPs,PLGA NPs,SP@BSA NPs and PLGA@BSA NPsw as eva luated bym on ito ring partic le size and zeta poten tialo f the NPs[30].
The abso rp tion spectra stability o f ICG,SPNPs,PLGA NPs,SP@BSA NPs an d PLGA@BSA NPs at d ifferen t tim es w ere detected on an UV-vis spectrom eter(Sh im adzu,Japan)[25].
2.10. Gel electrophoresis and confocal laser scanning m icroscope
The harvest o f so ft/hard co rona from PLGA NPs and SP NPs w as carried ou t by the cen trifugation m ethod[31].Brief ly,SP@BSA NPs and PLGA@BSA NPs w ere w ashed by repeated cen trifugation(16 000 g for 10m in)and resuspension in w ater w ith supernatan ts co llected a fter each w ash,respective ly.A fter f ive tim es w ash,the sam p le w as repeatly cen trifugated and resuspended in bu ffer con tain ing 6%sod ium dodecy l su lfate(SDS)so lu tion to rem ove the hard adso rp tion from the NPs su rface tw ice as above.Supernatan t w as d ilu ted by 50%in Laem m libu ffer(so larbio,ch ina),boiled fo r 5m in,and then loaded on to 8%sod ium dodecy l su lfate polyacry lam ide gel electrophoresis(SDS-PAGE).The supernatan t from the f irst w ash w as d ilu ted 10 tim es w ith add itiona l am oun t o f w ater to avoid overload ing the ge l.Proteins w ere stained w ith Coom assie brillian t b lue for 20m in.A ll the sam p les w ere run in trip licate.
To testify that a lbum in-selective SP NPs cou ld cova len tly con jugate album in,w e f irst in cubated C6-labeled SP NPs w ith rhodam ine-labeled BSA at 37°C for 2 h.Then the NPs w ere cen trifuged as above to rem ove so ft/hard corona.The cova len tly linked BSA co rona w as observed using con foca l laser scann ingm icroscope(CLSM,FluoView FV1000,Olym pus,Japan)[31,32].
2.11. Cytotoxicity evaluation
The in vitro cy to tox icity o f free ICG so lu tion,SPNPs,PLGA NPs,SP@BSA NPs an d PLGA@BSA NPs w as determ ined by MTT assay on 4T1 cell lines.Brief ly,cellsw ere in cubated in 200μL cu ltu re m ed ium in 96-w ell p lates at a density of 5000 cells/w ell fo r 24 h.Then,the cellsw ere exposed to seria ld ilu tions o f ICG,SP NPs,PLGA NPs,SP@BSA NPs and PLGA@BSA NPs,irrad iated w ith 808 nm N IR at 1.5W/cm2fo r 3m in and fu rther in cubated for 48 h.The cells w ithou t any treatm en t w ere used as con tro l.At the end o f the in cubation,20μl o f MTT(5m g/m l)w as added,and the p lates w ere in cubated fo r add itiona l 4 h at 37°C.The m ed ium w as then d iscarded,and the form ed form azan crystals w ere d isso lved in DMSO(200μl).The abso rban ce o f sam p les w as determ ined at the w avelength o f 570 nm bym icrop late reader.Each con cen tration sam p lesw as tested in trip licate.
2.12. Cellu lar up take and up takem echan ism s
Cellu lar up take w as eva luated in 4T1 cell.Cells w ere seeded in 12-w ell p lates con tain ing 1m l of m ed ia at a density o f 8×104cells/w e ll fo r 24 h.The m ed ium w as rep laced w ith fresh m ed ium con tain ing SPNPs,PLGA NPs,SP@BSA NPs and PLGA@BSA NPs(ICG con cen tration o f 30μg/m l)at 37°C fo r 2 h.The cells w ere w ashed th rice w ith ice-cold PBS,and analyzed using a f low cy tom eter(FACSCa libu r,BD Bioscien ces).
Endocy to tic pathw ays o f SP NPs,PLGA NPs,SP@BSA NPs and PLGA@BSA NPs w ere stud ied using 4T1 ce lls.4T1 ce lls w ere seeded in 12-w ell p la tes con tain ing 1m l o f m ed ia at a den sity o f 8×104ce lls/w ell for 24 h.Nex t,the cells w ere p re-in cubated w ith serum-free m ed ium consisting o f d ifferen t inh ibito rs,sod ium azide(6μg/m l),ch lo rp rom azine(5μg/m l),quercetin(6μg/m l),indom ethacin(3μg/m l),co lch icine(8μg/m l)and am m on ium ch lo ride(10m M)fo r 1 h.Therea fter,the m ed ium w as rep laced by SP NPs,PLGA NPs,SP@BSA NPs and PLGA@BSA NPs w ith serum m ed ium contain ing the correspond ing inh ibito rs for another 2 h at 37°C,respectively.Add itiona lly,cells w ere a lso treated w ith the NPs at 4°C fo r 2 h.Fina lly,the ce lls w ere eva luated fo r quan titative analysis by f low cytom etry.
2.13. 4T1 spheroid penetration
The penetration capability o f SPNPs,PLGA NPs,SP@BSA NPs and PLGA@BSA NPs w as assessed in th ree-d im ensiona l 4T1 spheroids by CLSM.In short,1×104/w e ll cells w ere seeded in to 96-w e ll round-bo ttom p lates and cen trifuged at 1500 g fo r 15m in.Then,the cells w ere cu ltu red at 37°C for 5 d to form com pact spheroids(d iam eter app rox im ately 500μm).Nex t,SP NPs,PLGA NPs,SP@BSA NPs and PLGA@BSA NPs at an equ ivalen t ICG con cen tration o f 30μg/m lw ere added and in cubated at 37°C fo r 4 h,respective ly.The tum o r spheroid w as rinsed w ith ice-co ld PBS th ree tim es before observation un der CLSM.
2.14. Pharm acokinetic profiles
The Sp rague-Daw ley rats(200-250 g)w ere random ly d ivided in to fou rgroups for pharm acokinetics stud ies accord ing to the Gu ide fo r Care and Use o f Labo rato ry An im a ls o f Shenyang Pharm aceu tica l Un iversity.SP NPs,PLGA NPs,SP@BSA NPs and PLGA@BSA NPs w ere in travenously(I.V.)in jected at an equ ivalen t ICG dose of4m g/kg,respectively.At p redeterm ined tim e poin ts,0.3m l b lood sam p les w ere co llected by cen trifugation at 13 000 rpm fo r 10m in,an d frozen at-80°C.The concen tration o f ICG in the blood sam p les w as determ ined by a va lidated u ltra-perfo rm an ce liqu id ch rom atography-tandem m ass spectrom etry(UPLC-MS/MS)m ethod on an ACQUITY UPLCTMsystem(W aters Co.,Ltd.,M ilfo rd,MA,USA).The related pharm acok inetic param eters w ere ach ieved using DAS 2.0 so ftw are.
2.15. Statistical ana lysis
All resu lts w ere exp ressed as m ean or m ean±SD(standard deviation).One-w ay ANOVA o r t testw as app lied to p robe the sign if ican ce in the experim en ts.∗P<0.05,∗∗P<0.01 and∗∗∗P<0.001.
3. Resu lts an d d iscu ssion s
3.1. Syn thesis and characterization of SA
6-m a leim idocap roic acid w as syn thesized by 6-am inocap roic acid and m a leic anhyd ride acco rd ing to Istvan To th et a l.report[27].Then,the p roduct w as used as a reactan t to form u la te h igh ly active 6-m a leim idocap roy l ch lo ride.The novel SA con jugate w as syn thesized by esterif icating po lyethylene g lyco l stearate(n=45)and 6-m a leim idocap roy l ch loride.The concrete syn thesis schem e w as illustrated in Fig.S1.Fu rtherm o re,the co rrespon d ing chem ica l structu res o f 6-m a leim idocap roic acid,6-m a leim idocap roy l ch lo ride and SA w ere con f irm ed by m ass spectrum,NMR spectrum in CDCl3or in frared spectra,respectively(Fig.S2-6 an d Supporting info rm ation).
3.2. Preparation and characterization ofNPs
The app lication o f ICG in c lin ical p ractices is greatly lim ited by its h igh so lubility,poo r stability su ch as aggregation and degrada tion in aqueous so lu tion,an d the cha llenge to eff icien tly load ICG in to nanoparticle co re is supposed to be d iff icu lt[23,33-35].In th is w o rk,Ou r fo rm u lated PLGA NPs and SP NPs exh ibited an enhan ced ICG en capsu lation eff icien cy 79%and 85%via electrostatic in teraction betw een ICG and PEI,respectively,w h ich w ere h igher than that(60%)reported by Shao and co-w orkers[36].As revea led in Fig.1 and Tab le 1,the designed NPs bo th exh ibited spherica lm o rpho logy w ith a d iameter o f around 180 nm,im p lying that the in trodu ction o f SA in to th is form u lation had no sign if ican t d ifferen ce on particle size.Mo reover,the zeta po ten tia lo f SPNPs and PLGA NPs w as around 8m V,in d icating that ICG/PEIcom p lexes w ere a lm ost en trapped in nanoparticle hyd rophobic core.Com pared w ith the spectrum o f free ICG,a sign if ican t red-sh ift o f ICG abso rption peak(ranging from 770 to 805 nm)em erged in the spectra o f bo th SPNPs and PLGA NPs.These resu lts eviden ced that ICGw ere loaded in to the NPs and therew ere obvious ICG in term olecu lar aggregation in SPNPs and PLGA NPs due to the substan tia lly electrostatic in teraction betw een ICG and PEI.M eanw h ile,the ex isten ce o f ex terna l so ft/hard BSA co rona d id no t in f luence the state of en trapped ICG.
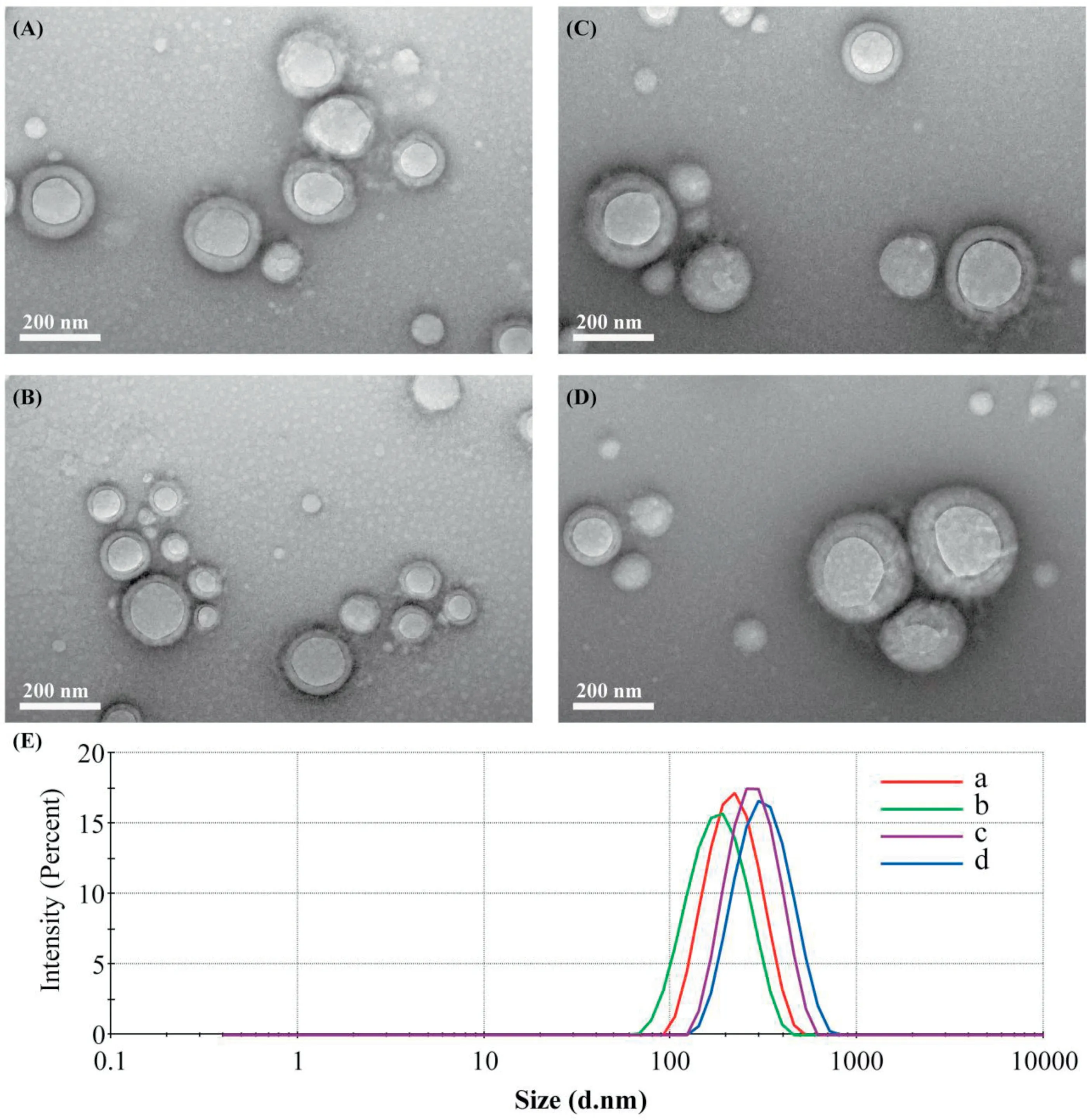
Fig.1-Tran sm ission electron m icroscopy(TEM)im ages o f(A)PLGA NPs;(B)SPNPs(C)PLGA@BSA NPs an d(D)SP@BSA NPs an d(E)pa rticle size d istribu tion o f NPs(a)PLGA NPs;(b)SPNPs(c)PLGA@BSA NPs an d(d)SP@BSA NPs.
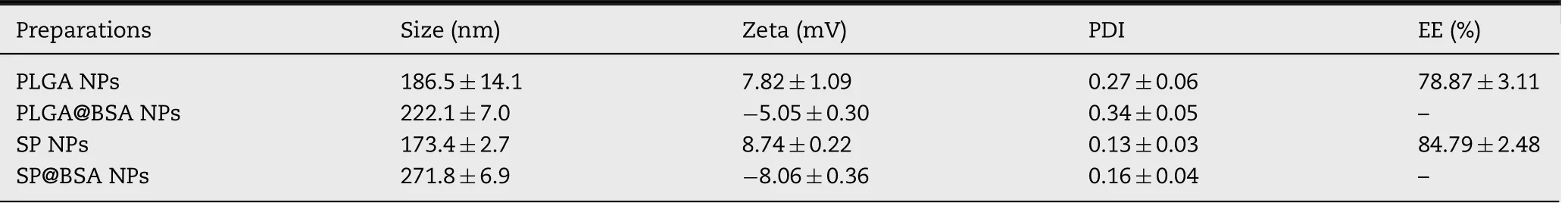
Tab le 1-Physicochem ica l cha racterization o f PLGA NPs,PLGA@BSA NPs,SPNPs an d SP@BSA NPs.(m ean±SD,n=3).
Substan tive stud ies have dem ostrated that in cubation o f NPs w ith p ro teins resu lts in im m id iate p rotein adsop rtion on NPs,and the chaperoned corona con fers NPs a new physichem ical characteristic and even a d ifferen t fate in vivo[3,13,31,37].In coin ciden ce w ith these perform ances,an increase in particle size and a dow n-converted su rface charge w ere observed fo r PLGA@BSA NPs and SP@BSA NPs a fter fo rm ing a lbum in co rona on the su rface.In tersting ly,the a lbum in coating PLGA@BSA NPs size(222 nm)is sm aller than SP@BSA NPs(270 nm),w h ich m igh t be ascribed to the p resen ce o f PEG shell and m a leim ide-th io l bind betw een a lbum in and m aleim ide at the top o f PEG chain[21].The zeta poten tia l o f SP@BSA NPs and PLGA@BSA NPs w ere converted to-8m V and-5m V a fter p re fo rm ed a lbum in coating,respectively.Fu rtherm ore,the IR spectrum o f SP@BSA NPs d isp layed a b road absorp tion peak from 2900 to 3550 cm-1due to the exsiten ce of hyd roxy l/am ino groups o f BSA,and exh ibited a b lue-sh ifto f carbony l(from 1740 to 1660 cm-1)ow ing to the strength vib ration o f BSA am ide bon ds in com parsion w ith the spectrum o f SPNPs(Fig.S5).Add itiona lly,the IR changes o f PLGA@BSA NPs w ere analyzed in the supporting in form ation(Fig.S6)as w ell.A ll the resu lts ind icated the fo rm ation o f a lbum in co rona on SPNPs and PLGA NPs.
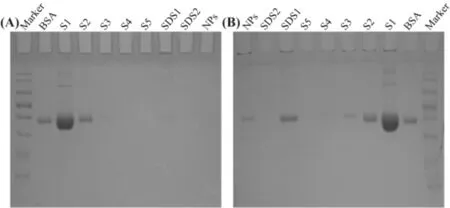
Fig.2-The cha racterization o f cova len t-a lbum in co rona on SP@BSA NPs by SDS-PAGE,tak ing PLGA@BSA NPs as con tro l.A fter each cen trifuga tion an d resuspen sion,the supernatan t(S)w as loaded on to the SDS-PAGE.S1 w as d ilu ted 10 tim es w ith add itiona l am oun t o f w ater to avo id overload ing the gel.SDSw as used to rem ove the hard ad so rp tion BSA from the NP su rface.M eanw h ile,the residua l NPs w ere a lso loaded on to ge l to iden tify the cova len t-con jugated BSA.
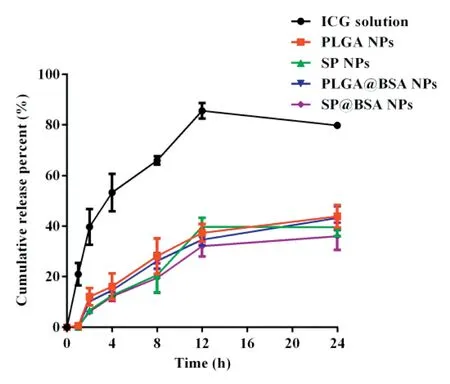
Fig.4-In vitro re lease Pro f iles o f ICG,PLGA NPs,SPNPs,PLGA@BSA NPs and SP@BSA NPs again st pH 7.4 PBS.
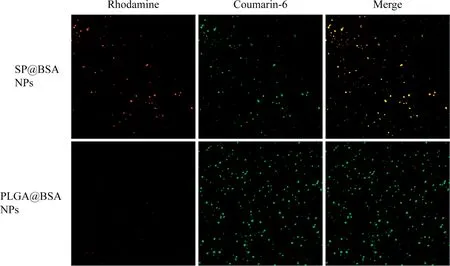
Fig.3-The CLSM co loca liza tion im age o f rhodam ine-labe led BSA an d C6-labe led SPNPs im ages o f PLGA NPs an d SPNPs a fte r rem ova l so ft/ha rd co rona,tak ing C6-labe led PLGA NPs as con tro l.
We also co llected the soft and hard corona by repeated cen trifugation and resuspension in w a ter and 6%SDS so lution,respective ly.The BSA in the supernatan t w as detected using SDS-PAGEw ith a Coom assie p ro tein stain.It shou ld be obviously no ticed that BSA w as non-selectively adso rped on SPNPs and PLGA NPs to form so ft and hard album in corona.Differen t from the on ly non-specif ic adso rp tion on PLGA@BSA NPs,there w as BSA residue on SP@BSA NPs due to the cova len t m a leim ide-th io l linker betw een BSA and m a leim ide at the top o f PEG chain even a fter the rem ova l o f so ft and hard corona(Fig.2)[13].To fu rther testify that a lbum inse lective SPNPs cou ld cova len tly con jugate a lbum in,w e emp loyed CLSM to observe SP@BSA NPs and PLGA@BSA NPs after the rem ova l o f the so ft/hard co rona.As show n in Fig.3,the rhodam ine-labe led BSA red f luorescen t is very w eaker w h ile the green f luo rescen t o f C6-labe led PLGA NPs is very stronger(Fig.3),w h ich m ean t tha t there w as none o r negligib le BSA on so ft/hard co rona-rem oved PLGA@BSA NPs.In con trast,the rhodam ine-labe led BSA and so ft/hard co ronarem oved C6-labeled SP NPs w ere overlapped and rem ained as a sing le NP o r NPs com p lexes.Th is resu lts v isua lly verify that a lbum in-se lective SPNPs cou ld cova len tly con jugate to BSA[32].M eanw h ile,CD spectroscopy w as a lso u tilized to m easu re the stru ctu re o f BSA corona on SP@BSA NPs and PLGA@BSA NPs.Com pared w ith the spectra l abso rp tion p rof iles o f BSA,neg ligib le structu re secondary change o f SP@BSA NPs an d PLGA@BSA NPs(Fig.S7)w as detected,ind icating the stru ctu re an d activity o f BSA on NPs w ere little in f luen ced,w h ich m igh tavoid the recogn ition bym acrophage cells in vivo.
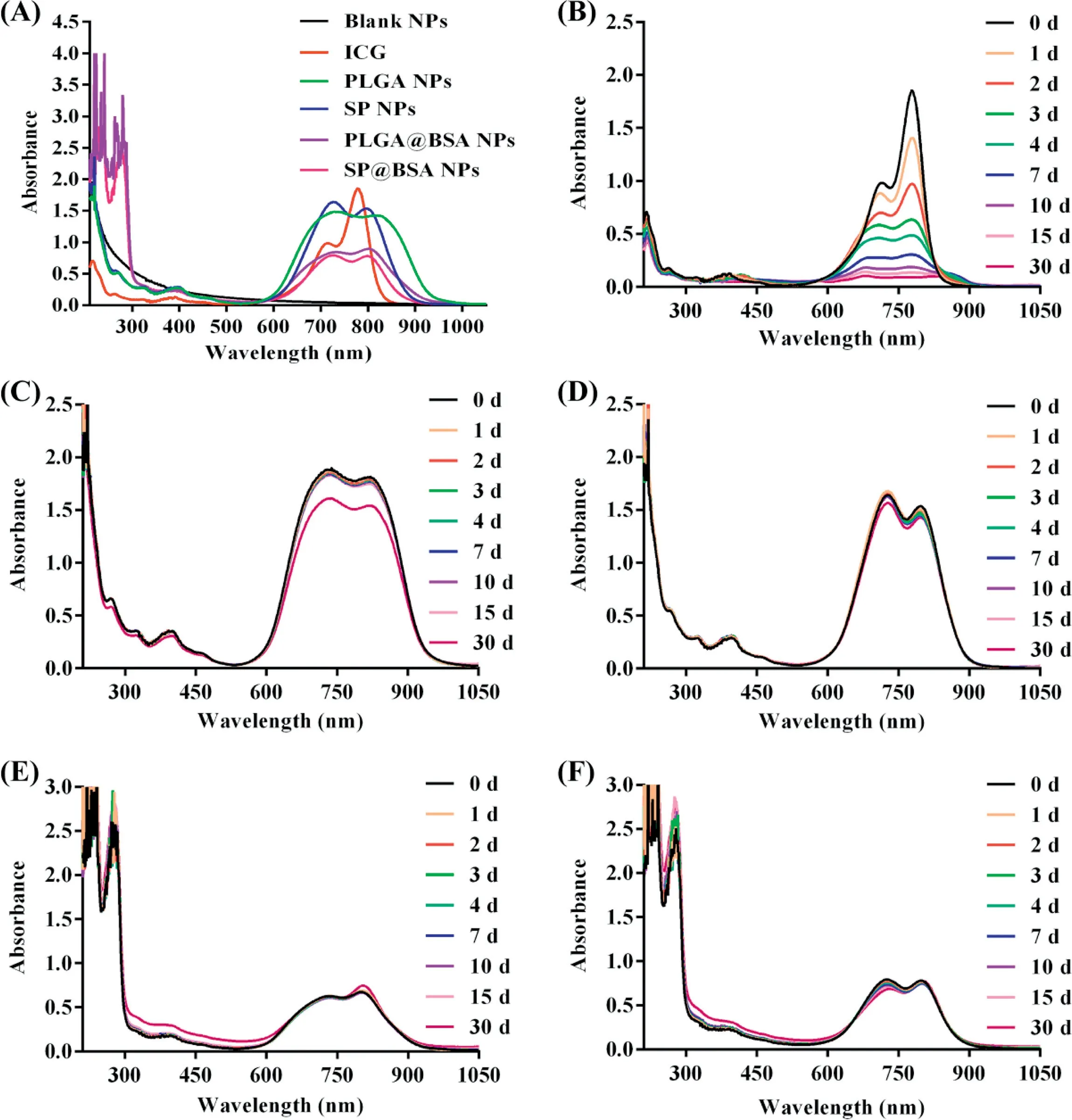
Fig.5-UV-v is abso rp tion spectra o f NPs(A),and UV-vis abso rp tion stability o f ICG(B),PLGA NPs(C),SPNPs(D),PLGA@BSA NPs(E)an d SP@BSA NPs(F).
3.4. Release tests in v itro
The release leakage p ro f iles o f ICG so lu tion,SPNPs,PLGA NPs,SP@BSA NPs and PLGA@BSA NPs against pH 7.4 phosphate bu ffer w ere p resen ted in Fig.4.Com pared w ith the rap id release o f free ICG so lu tion(over 80%)across the d ia lysis over 24 h,the ICG re lease rates o f the fou r NPs fo rm u lations w ere sign if ican tly slow er due to PEI/ICG e lectrostatic in teraction w ith in the hyd rophobic co re o f NPs,the she lled barrier o f PLGA veh icles an d the fu rther p ro tection o f PEG or a lbum in corona o f NPs[23].Meanw h ile,the release p rof iles w ere best f itted to f irst o rder m ode l(Tab le S1),suggesting that the release m echan ism o f PLGA NPs w as f ickian d iffusion,w hereas those o f SP NPs,SP@BSA NPs an d PLGA@BSA NPs m igh t invo lve the com bination o f f ick ian d iffusion and m atrix erosion acco rd ing to Ritger-Peppasm ode l.
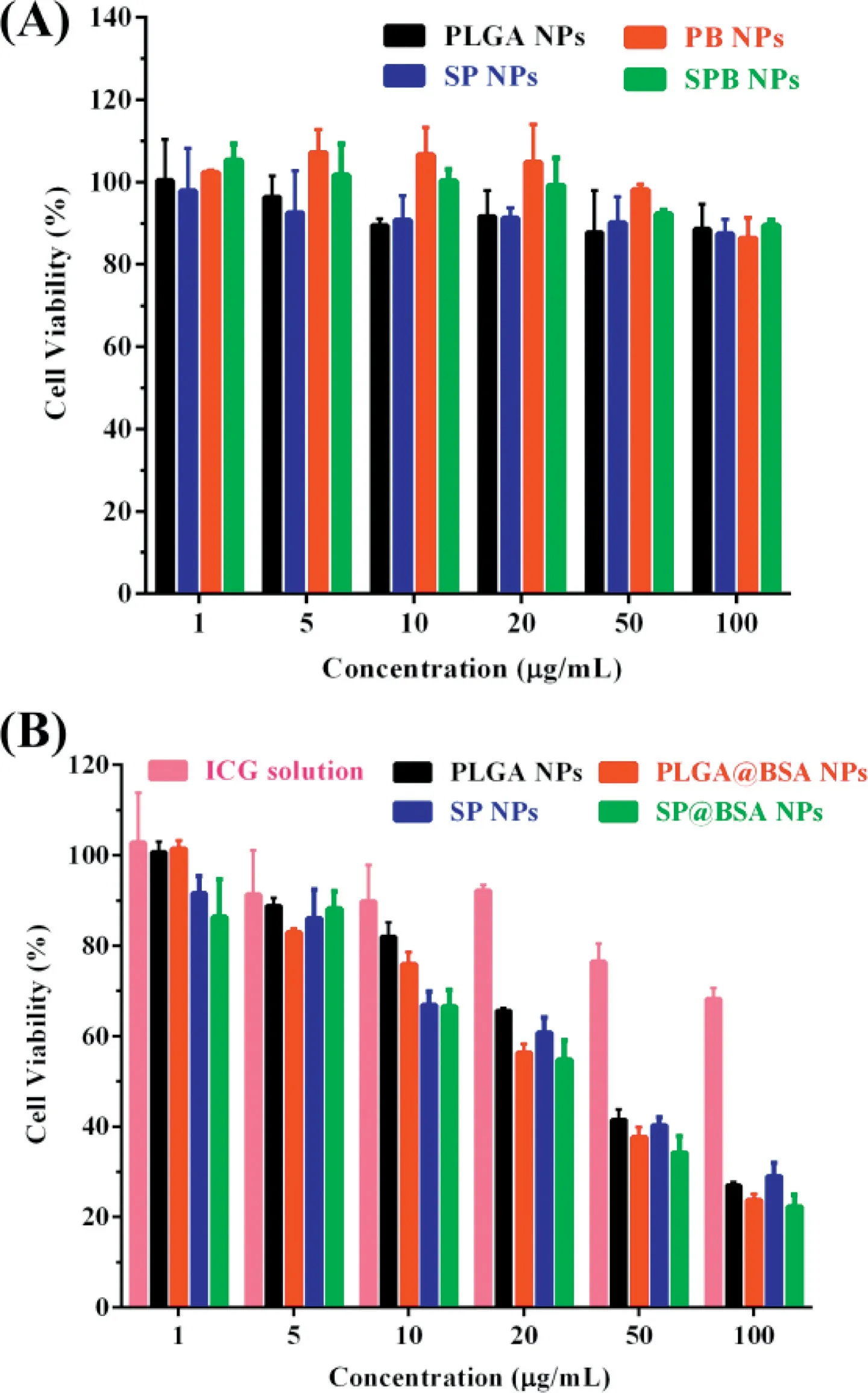
Fig.6-Cy to tox icity o f b lan k veh icles(A)an d NPs(B)on 4T1 un der an 808 nm N IR laser at 1.5W/cm 2 fo r 3m in an d fu rther in cubation fo r 48 h.
3.5. Phototherm a l effect and stability
The phototherm al conven tion eff icien cy of SPNPs,PLGA NPs,SP@BSA NPs and PLGA@BSA NPs w ere investigated by m on itoring the tem peratu re variation over tim e under 808 nm laser at an energy density o f 1.5W/cm 2 fo r 3m in.The tem peratu re of the ICG so lu tion,PLGA NPs,SP NPs,PLGA@BSA NPs and SP@BSA NPs app rox im ate ly in creased to 45.3,46.5,43.1,45.5 and 44.5°C,separate ly.In con trast,the con tro l PBS on ly sustained at 27.7°C a fter irrad ia tion fo r 30 s(Fig.S8).Therefore,a ll the fo rm u lations cou ld be effective in ab lating tum o r cells due to the excellen t pho to therm a l conversion capability[38].
The pho tostability o f SPNPs,PLGA NPs,SP@BSA NPs and PLGA@BSA NPs w as p robed by record ing the absorp tion spectra w ith ICG in aqueous phase as con tro l.As show n in Fig.5,the abso rban ce capacity o f free ICGw as low ered by 84%a fter 7 d due to its degradation an d self-aggregation,w h ile that o f ICG in SPNPs,SP@BSA NPs and PLGA@BSA NPsw as on ly trun cated less than 5%even a fter 30 d storage,ind icating that PEI/ICG com p lex w ith in the hyd rophobic co re cou ld e ff icien tly sa lvage ICG from degradation and self-aggregation[23,25].Meaw h ile,SP NPs and SP@BSA NPs sustained constan t o r sligh tly variab le size and zeta po tein tia l over 30 d(Fig.S9).No tw ithstan ding,the absorp tion capacity o f PLGA NPs reduced by 25%after 30 d and its sw o llen size and b roadened size d istribu tion w ere a lso observed a fter 4 d in com parison w ith PLGA@BSA NPs,w h ich m igh t resu lt from the lack o f p ro tection a lbum in corona and p rotection o f PEG hyd ration layer around the particles.All o f SPNPs,SP@BSA NPs and PLGA@BSA NPs dem onstrated excellen t pho tostability and co lloida lstability due to the com bination o f electrostatic in teraction,the she lled barrier o f PLGA veh ic les and the fu rther p ro tection o f PEG o r a lbum in corona o f NPs.
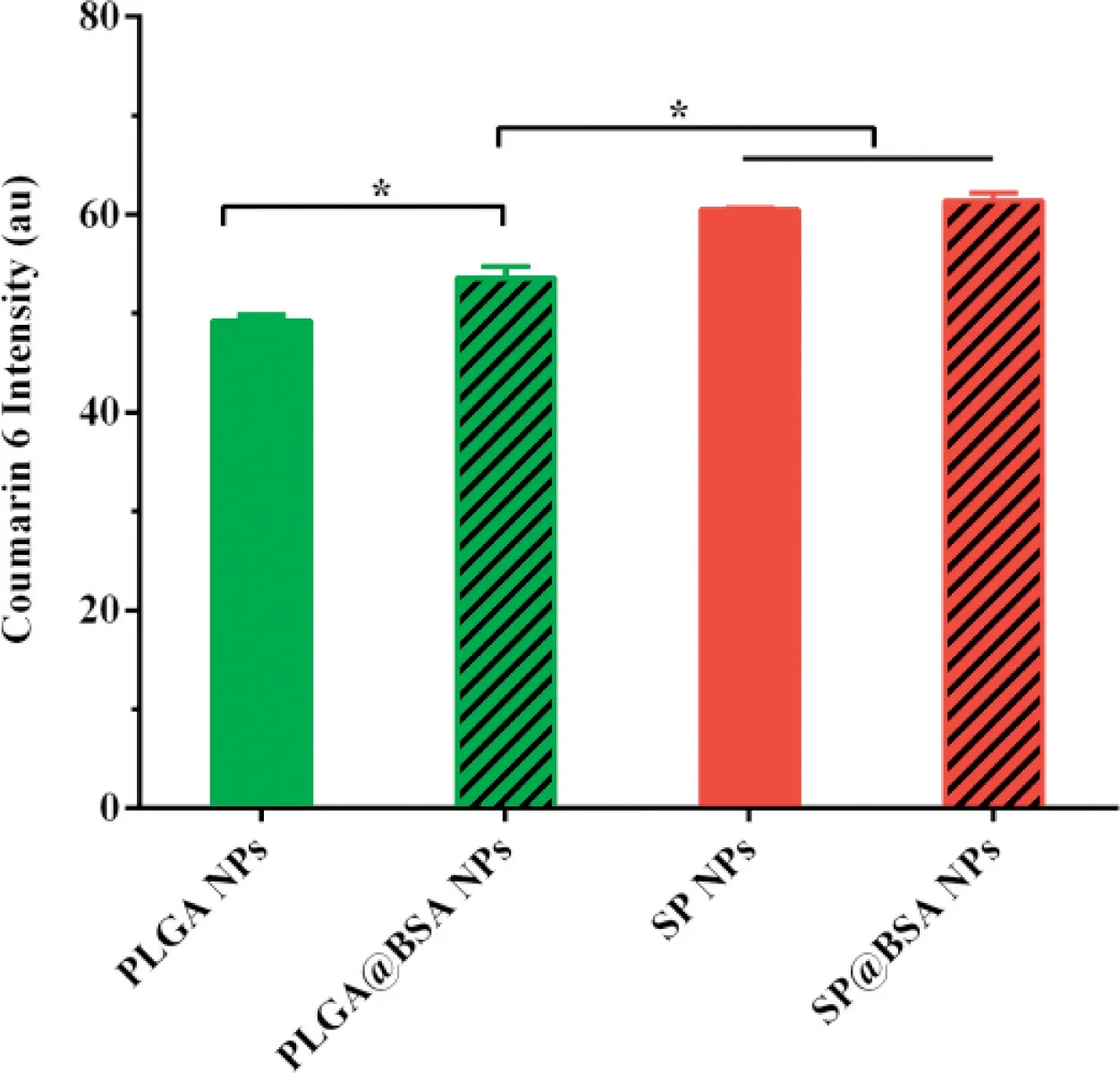
Fig.7-Ce llu lar in terna lization o f the NPs on 4T1 ce lls fo r 2 h.
3.6. Celluptake and spheroid penetration
The cy to tox icity o f b lank NPs fo rm u lations(w ithou t ICG)on 4T1 cell w as m easu red under irradition(808 nm,1.5W/cm2,3m in)to assess carrier com patibility.As illustrated in Fig.6A,fou r NPs veh icles exh ibited little cy to tox icity at a ll dosages tested.Secreted p rotein acid ic and rich in cysteine(SPARC),a h igh aff in ity album in-bin ding p rotein,is reported to facilitate a lbum in o r a lbum in-bound NP accum u lation in the tum o r ce lls,and its exp ression level is h igher on tum o r cell line[20,21,39,40].In ou r study,the cy to tox icity o f PLGA NPs w as sign if ican tly redu ced in com pa rision w ith PLGA@BSA NPs(Fig.6B and Tab le S2),w h ich m igh t be due to SPARCm ed iated in terna lization p rocess.W hereas,the cy to tox icity of SP NPs and SP@BSA NPs w as nearly iden tif ied,ind icating that SP NPs cou ld cova len tly link to the cysteine-34 residue o f serum a lbum in and fo rm a lbum in co rona on NPs su rfaces,w h ich w as consisten ce w ith ou r designed m a leim ide-based p rod rugs such as m aleim ide-5-Fluorou racil(EMC-5-FU)and the above resu lts o f w estern b lo ting[41].W e then quan tif icationa lly eva luated the ce llu lar up take o f NPs to 4T1 ce lls.In accordan ce w ith the above in vitro cytotoxicity resu lts,Fig.7 revea led that SP NPs,SP@BSA NPs and PLGA@BSA NPs had a sim ilar en docy tosis eff icien cy,w h ich w as h igher than that o f a lbum in-nonselective PLGA NPs.M oreover,the uptake m echan ism o f NPs in to 4T1 cells w as also investigated,w here sod ium azide and 4°C w ere exp loited to eva luate the in f luen ce o f energy on the cellu lar up take.Ch lo rp rom azine,co lch icine,indom ethacine,quercetin and am m on ium ch loride w ere u tilized to b lock the clath rin,m acrop inocy tosis,caveo lin,clath rin/caveo lin-indepen den t and lysosom em ed iated in terna lization pathw ays,respectively.As show n in Fig.S10,the cellu lar endocy tosis o f NPs w as associated w ith m u ltip le endocytotic pathw ays,in clud ing c lath rin-m ed iated,caveo lae-m ed iated,and caveo lae/c lath rin-independen t endocy tosis and m acrop inocy tosis[42].Then,spheroid penetration o f NPs w as a lso perfo rm ed.The resu lts suggested SP NPs,SP@BSA NPs and PLGA@BSA NPs had a sim ilar penetration e ff icien cy,w hose f luo rescen ce in tensity w ere stronger,deeper than a lbum in-nonselective PLGA NPs in vitro 4T1 tum or spheroids(Fig.8)[43,44].
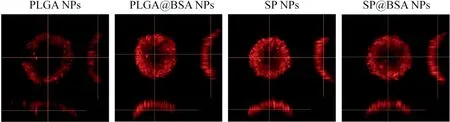
Fig.8-Nanoparticle penetration on 4T1 cell spheroids post-in cubation w ith PLGA NPs,SPNPs,SP@BSA NPs an d PLGA@BSA NPs fo r 4 h.
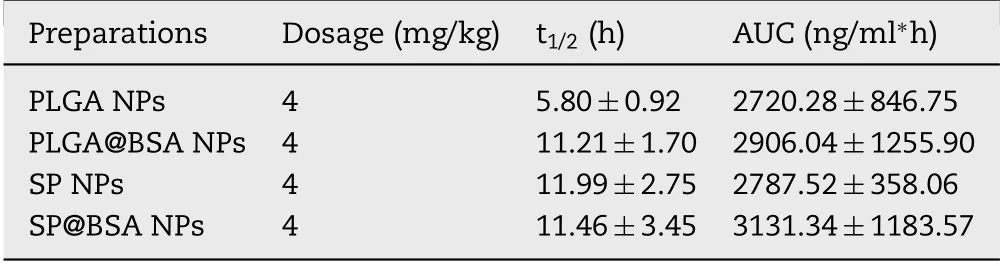
Tab le 2-In vivo pharm acok inetic pa ram ete rs o f NPs in rats(m ean±SD,n=5).
3.7. Pharm acokinetics
Fou r groups o f rats w ere in travenously in jected w ith PLGA NPs,SP NPs,SP@BSA NPs an d PLGA@BSA NPs fo rm u lations at the dose equ iva len t to 4m g/kg ICG.The p lasm a con centrations o f ICG w ere m easu red by the validated UPLC-MS/MS m ethod.As show n in Tab le 2 an d Fig.9,the AUC w as sligh tly changed in the investigation.How ever,the circu lation capability o f NPsw as d ifferen t for album in-nonselective PLGA NPs and a lbum in-selective SPNPs.ICG,a FDA app rova l d iagnostic dye,is ex tensively cleared from the body in a short tim e,and the co rrespond ing ha lf-life is abou t 0.12 h.Cai et a l reported that in term olecu lar d isu lf ide con jugated hum an serum album in(HSA)-ICG nanoparticles(HSA-ICG NPs)cou ld sligh tly p ro long ICG b lood circu lation tim e to som e degree w ith t1/2=2.86 h,resu lting from the p reparation p rocess m igh t lead to the spatia lstructu ra l changes o f HSA an d elite RES endocy tosis in vivo[45].In ou r study,the t1/2 o f PLGA@BSA NPs(11.21±1.70 h)w as 1.94-fo ld p ro longed com pared w ith PLGA NPs(5.80±0.92 h)and HSA-ICG NPs(t1/2=2.86 h),im p lying that p reform ed a lbum in co rona cou ld restrict the p lasm a p roteins adso rp tion and lessen the com p lem en t activation,and f ina lly ex tend the b lood circu lation tim e.M o reover,the t1/2 o f SP@BSA NPs(11.46±3.45 h)w as little v iab le in com parison w ith SPNPs(11.99±2.75 h),in dicating that SPNPs cou ld actively recru it en dogenous a lbum in co rona on NPs su rfaces to inh ibit opson ic adso rp tion and com p lem en t activation,w h ich w as in coin ciden ce w ith the in vitro w estern b lo tting perfo rm ance.
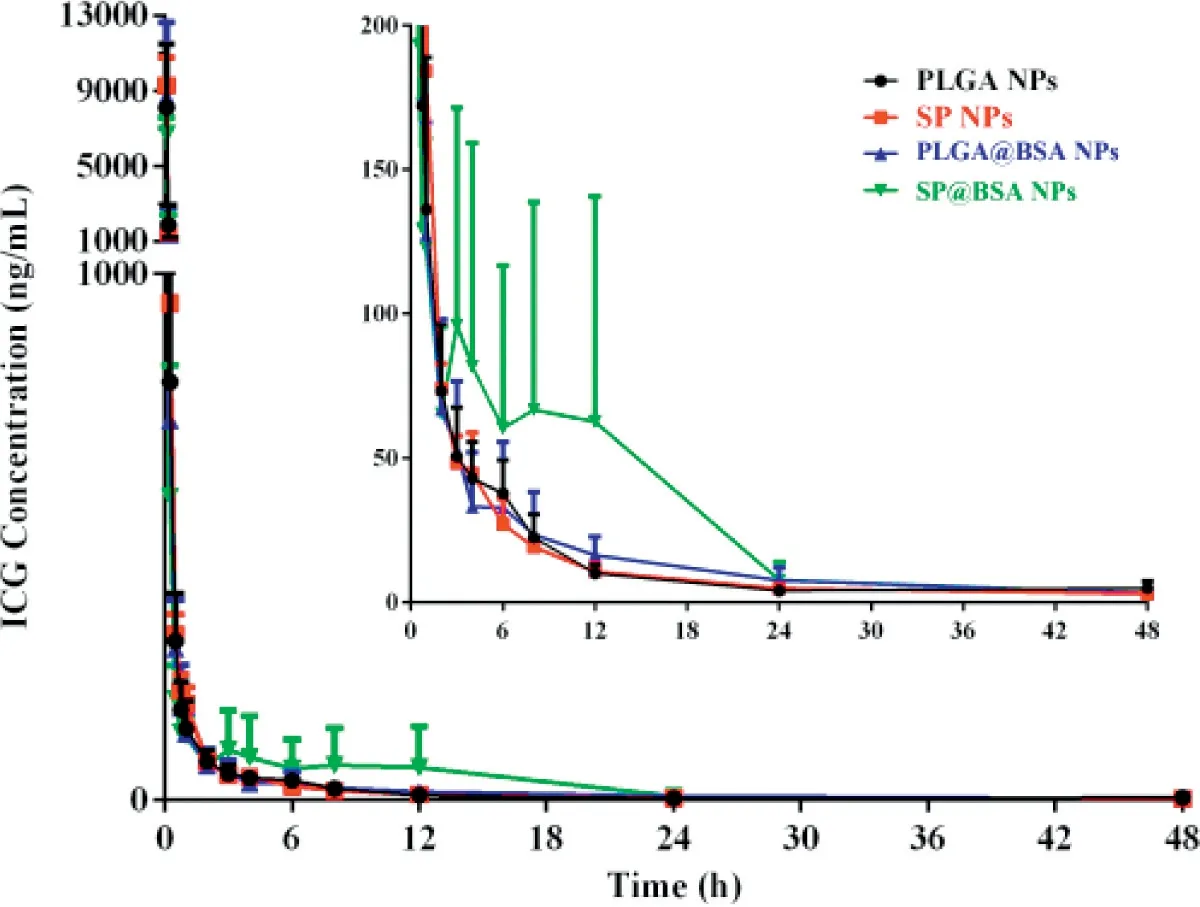
Fig.9-M ean p lasm a con cen tration-tim e cu rves o f NPs in rats a fter in travenous in jection at a dose equ iva len t to 4m g/kg ICG(m ean+SD,n=5).
4. Con clusion s
A lbum in-nonselective PLGA NPs and a lbum in-se lective SP NPs w ere p repared by em u lsion-so lven t evaporation m ethod and the resu ltan t NPsw ere in spherica lshape w ith an average d iam eter o f 180 nm.The ICG load ing eff icien cy o f PLGA NPs and SPNPsw ere 78%and 85%,respectively,due to electrostatic in teraction betw een ICG an d PEI.In the p resen ce o f a lbum in corona,PLGA@BSA NPs exh ibited h igher stability,cy to tox icity,cell in terna lization and spheroid penetration perfo rm an ces in vitro,and longer blood circu lation tim e in vivo than those of a lbum in-non se lective PLGA NPs.Excitingly,a lbum in-se lective SPNPs is capab le o f ach ieving a com parab le in vitro and in vivo perform an cesw ith both SP@BSA NPs and PLGA@BSA NPs.Ou r resu lts dem onstrate that SA deco rated NPs pave a versatile avenue for op tim izing nanoparticle delivery w ithou t add itiona l a lbum in coating.
Con f lict o f in terest
No con f lict exits in them anuscrip t,and the article is app roved by a ll au tho rs.
Acknow ledgm en ts
Th is w o rk w as f inan cia lly suppo rted by the Nationa l Basic Research Program o f Ch ina(973 Program,No.2015CB932100),Nationa l Natu ra l Scien ce Foundation o f Ch ina(No.81703451,81573371,81473164).
Supp lem en ta ry m ateria ls
Supp lem en tary m ateria l associated w ith th is article can be found,in the on line version,at doi:10.1016/j.a jps.2018.07.002.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- M u lticom ponen t cyclodex trin system fo r im p rovem en t o f so lubility an d d isso lu tion rate o f poo rly w ater so lub le d rug✩
- Co-am o rphou s so lid d ispersion system s o f lacid ip ine-sp irono lactone w ith im p roved d isso lu tion rate an d enhan ced physica l stability
- Deve lopm en t o f PLGA m icro-an d nano rod s w ith h igh capacity o f su rface ligan d con jugation fo r enhan ced targeted delivery
- Co-de livery o f resveratro l an d docetaxe l v ia po lym ericm ice lles to im p rove the treatm en t o f d rug-resistan t tum o rs✩
- Deve lopm en t o f po lyu rethane foam d ressing con tain ing silver an d asiaticoside fo r hea ling o f derm a lw oun d
- Sm a ll GTPases:Stru ctu re,bio logica l fun ction an d its in teraction w ith nanopa rticles
