Identification of genomic features associated with immunotherapy response in gastrointestinal cancers
2019-04-24YinHeZhiXianLiuZeHangJiangXiaoShengWang
Yin He,Zhi-Xian Liu,Ze-Hang Jiang,Xiao-Sheng Wang
Abstract
Key words: Gastrointestinal cancer;Tumor immunity;Tumor immunotherapy;DNA mismatch repair;Tumor mutation burden;Tumor aneuploidy
INTRODUCTION
Gastrointestinal (GI) cancers,including malignancies of the esophagus,stomach,biliary system,pancreas,small intestine,large intestine,rectum,and anus are the most prevalent malignant carcinomas globally and account for a large number of cancer deaths[1].Traditional treatment strategies for GI cancers include surgery,chemotherapy,radiotherapy,and targeted therapy[2].However,for the refractory or metastatic GI malignancies these traditional treatment strategies often have a limited therapeutic effect[3].Recently,immunotherapy has demonstrated rapid success in treating various refractory malignancies,such as melanoma[4],non-small cell lung cancer (NSCLC)[5],head and neck cancer[6],renal cell carcinoma[7],leukemia[8],and lymphoma[9].In particular,the immune checkpoint blockade (ICB) that targets molecules involved in mediating antitumor immunosuppression has been used clinically for treating diverse cancers[10].Several immune checkpoint inhibitors have been approved by the Food and Drug Administration in clinically treating cancer,including ipilimumab (anti-CTLA4),nivolumab and pembrolizumab (anti-PD1),and atezolizumab and avelumab (anti-PD-L1).However,there is currently no immunotherapeutic drug specifically used for treating a GI cancer,although pembrolizumab is being used for treating DNA mismatch repair-deficient GI cancers.
Abundant evidence shows that the response to ICB is associated with certain genomic features[4,11,12].These include DNA mismatch repair deficiency[13],tumor mutation burden (TMB)[14]or neoantigen load[15],and tumor aneuploidy[16].Several studies have revealed that the colorectal cancers (CRCs) with mismatch repair deficiency (dMMR) were more sensitive to anti-PD-1 therapy than those with mismatch repair proficiency (pMMR)[17,18].Similar results have been demonstrated in other cancer types including ovarian[19],endometrial[20],and gastric cancers[21-23].In addition,previous studies found that higher TMB was associated with a more favorable response to ICB in diverse cancer types,indicating the potential role of TMB in predicting ICB efficacy[14].A recent study revealed an inverse correlation between tumor aneuploidy and immunotherapy response[16].These prior studies suggest that it is significant to identify the genomic features associated with immunotherapy response for treating diverse refractory malignancies,including a large number of GI cancers.
In this review,we examined the associations between three genomic features(dMMR or microsatellite instability (MSI) status,TMB,and tumor aneuploidy) and tumor immunity in GI cancers.
ASSOCIATION BETWEEN DMMR/MSI AND TUMOR IMMUNITY IN GI CANCERS
DNA mismatch repair is a highly conserved system for repairing the errors of deletion,insertion,and mismatch occurring in DNA replication and recombination[24].It plays a key role in maintaining genomic stability[25].dMMR is associated with genome-wide instability and can lead to tumorigenesis and cancer development[26].dMMR may cause a high increase in the frequency of insertion and deletion mutations in simple repeat (microsatellite) sequences,a phenomenon known as MSI[27,28].Plentiful evidence shows that MSI can trigger hyperimmunity in a tumor that may promote response to ICB therapy.First,tumors with MSI are hypermutated and thus generate many neoantigens to incite the tumor immune response[13].In fact,MSI is associated with the increased infiltration of tumor-infiltrating lymphocytes (TILs) in the tumor[29,30].It has been shown that MSI CRCs exhibited high infiltration of activated CD8+cytotoxic T lymphocytes and activated Th1 cells[31].Second,MSI tumors often exhibit the elevated expression of immune checkpoint molecules such as PD-L1 that may increase the sensitivity to immunotherapy[32].
Several GI cancers harbor a comparatively high proportion of MSI-high (MSI-H)tumors,including gastric cancer (GC) (22%),hepatocellular carcinoma (16%),CRC(13%),and esophageal adenocarcinoma (ESCA) (7%)[33].A number of studies have revealed that MSI-H GI tumors are more responsive to ICB[13,17,21].In the clinical trial study (KEΥNOTE-012)[21],two out of four MSI-H GC patients responded to pembrolizumab.In a phase 2 clinical study of CRC treatment with pembrolizumab[13],the objective response rate (ORR) in dMMR CRCs was 40%vs0% in pMMR CRCs[13].Pancreatic cancer generally has a poor response to immunotherapy[34].However,a study showed that six pancreatic cancer patients with dMMR or MSI exhibited an objective response rate of 83% to pembrolizumab[17].The high TMB,neoantigen load,and TIL infiltration in MSI-H GI cancers could explain why this subtype has a favorable response to immunotherapy.Furthermore,because the expression of PD-L1 in this subtype is common[35],and anti-PD-1/PD-L1 therapy may achieve a higher response rate in PD-L1-positive tumors than in PD-L1-negative tumors[36],MSI-H GI cancer patients are likely to respond to immunotherapy.Therefore,dMMR/MSI is an important predictive biomarker in GI cancer immunotherapy.
To further investigate the relationship between MSI status and tumor immunity in GI cancers,we downloaded RNA-Seq gene expression (Level 3) and clinical data from The Cancer Genome Atlas (TCGA) project (https://portal.gdc.cancer.gov/).We first quantified the enrichment levels of six immune signatures (B cells,CD8+T cells,cytolytic activity,human leukocyte antigen (HLA),interferon response,and natural killer (NK) cells) (Table 1)[37]in each GI sample using the single-sample gene-set enrichment analysis score[38].We compared the enrichment levels between MSI-H GI cancers and MSI low (MSI-L) or microsatellite stability (MSS) GI cancers.We observed a significant upregulation of the six immune signatures in the MSI-H colon adenocarcinoma (COAD)vsthe MSI-L/MSS COAD (Mann-WhitneyUtest,P< 0.05)(Figure 1A).Similar results were observed for stomach adenocarcinoma (STAD)(Figure 1B).Next,we used the ESTIMATE algorithm[39]to evaluate the immune score for each GI sample,which represents the degree of immune cell infiltration in the tumor.We found that the MSI-H COAD had significantly higher immune scores than the MSI-L/MSS COAD (Mann-WhitneyUtest,P< 0.05) (Figure 1A).Collectively,these results confirmed that MSI-H GI tumors tend to have stronger tumor immunity compared to MSI-L/MSS GI tumors,suggesting that the MSI-H GI cancer subtype could be more responsive to immunotherapy.
ASSOCIATION BETWEEN TMB AND TUMOR IMMUNITY IN GI CANCERS
TMB represents the overall load of somatic mutations in the tumor.Tumor somatic mutations may produce neoantigens to drive antitumor immune responses[40].Therefore,the increase in TMB would result in elevated tumor immunogenicity as well as antitumor immunity[41,42].Previous studies have shown that TMB varies with cancer type[43,44].Some cancer types generally have high TMB,such as skin cutaneous melanoma,lung cancer,esophageal carcinoma,and bladder urothelial carcinoma,and some have low TMB,such as leukemia.Most GI cancers have a medium level of TMB(defined as total somatic mutation counts) (Figure 2A).However,some GI cancer subtypes,such as the MSI-H subtype,often have high TMB (Figure 2A).
The high TMB cancer types,such as melanoma,NSCLC,and MSI-H cancers,are more sensitive to the ICB therapy because they can produce more neoantigens[17,45-47].As a result,high TMB is associated with long-term clinical benefit to anti-CTLA-4[46,48]and anti-PD-1/PD-L1[49-51]therapy.In GI cancers,the high TMB in ESCA was associated with clinical benefit of the ICB therapy[52],the EBV+(Epstein–Barr virus+)and MSI+molecular subsets of GC with high TMB showed increased immune cell infiltration and PD-1/PD-L1 pathway activation[53],and the high TMB CRC patients were commonly responsive to PD-1/PD-L1 blockade[14].Interestingly,a high TMB in pancreatic cancer was negatively associated with T cell activity and a worse overall survival[54].
We evaluated the correlation between TMB and tumor immunity in GI cancers based on the TCGA data.We found that the six immune signatures showed significant positive correlations with TMB in COAD (Spearman's correlation test,P<0.05) (Figure 2B).In STAD,the cytolytic activity and NK cell signatures were significantly positively associated with TMB (Figure 2B).These data confirmed that TMB is likely to be positively associated with tumor immunity in GC cancers.

Table 1 Six immune signatures and their associated gene sets
ASSOCIATION BETWEEN TUMOR ANEUPLOIDY AND TUMOR IMMUNITY IN GI CANCERS
Tumor cells often display a high degree of chromosomal instability (CIN)[55].CIN refers to distortions in the number of chromosome (aneuploidy) or the chromosomal structure (translocation,inversion,and duplication)[56].Aneuploidy,also known as somatic copy number alterations (SCNAs),is a common characteristic present in 88%of solid tumors[57]and plays a key role in tumor development[58-61].Numerous studies have revealed a significant correlation between tumor aneuploidy and tumor immunity[16,57].Davoliet al[16]found that tumors with a high level of aneuploidy inversely correlated with cytotoxic immune infiltration and that the tumor patients with high aneuploidy had a poor survival prognosis.Tayloret al[57]demonstrated that aneuploidy was negatively associated with tumor immune activity.Moreover,tumor aneuploidy is likely to increase intratumor heterogeneity[62,63],which may inhibit tumor immunity[64,65].
In GI cancers,the genomic feature of ESCA resembles the CIN subtype of GC[66].The CIN subtype,characterized with a high degree of SCNAs[53,67,68],accounts for nearly half of all GC cases[53].Our previous study showed that immune signatures were significantly downregulated in the CIN subtype versus the genomically stable subtype in GC and COAD[69].
To further explore the relationship between tumor aneuploidy and tumor immunity in GI cancers,we used the absolute algorithm[70]to assess the ploidy score for each GI sample in TCGA,and evaluated the correlations between the six immune signatures and the ploidy scores in GI cancers.We found that diverse immune signatures were significantly inversely correlated with the ploidy scores in GI cancers,including all six immune signatures in STAD,five in liver hepatocellular carcinoma,and four in COAD (Spearman's correlation test,P< 0.05) (Figure 3).We also found that the immune scores were inversely correlated with the aneuploidy in these GI cancer types (Figure 3).Collectively,these results confirmed the negative correlation between tumor aneuploidy and tumor immunity in GI cancers and suggested an important role for aneuploidy in predicting the immunotherapy response in GI cancers.

Figure 1 Association between mismatch repair deficiency/microsatellite instability and tumor immunity in gastrointestinal cancers.
CONCLUSION
Due to the limited therapeutic effect of traditional treatment strategies on refractory or metastatic GI cancers,immunotherapy could be an alternative approach for these cancers.Immunotherapy is likely to be effective for the “hot” tumors whose microenvironment has dense T cell infiltration[71].The tumors with MSI,high TMB,or low aneuploidy are often “hot” tumors that respond to immunotherapy.Nevertheless,immunotherapy often has poor efficiency for the “cold” tumors that lack immune infiltration[71].To improve the immunotherapy response in “cold”tumors,a combination of different treatment strategies may convert “cold” tumors into “hot” tumors.The combined treatment strategies could be the combination of different immunotherapeutic methods[72-75]or the combination of immunotherapy with other therapeutic approaches[76-79].
Besides the genomic features,mutations in some specific genes may suggest an immunotherapy response.Our previous study showed thatTP53mutations were associated with depressed tumor immunity in STAD and COAD[69],suggesting that theTP53mutation status may predict the response of STAD and COAD patients to immunotherapy.In addition,KRASmutations are associated with suppressed immune activity in CRC[80].

Figure 2 Association between tumor mutation burden and tumor immunity in gastrointestinal cancers.
Previous studies revealed that ICB was effective in a subset of GI cancer patients,but the response rate in an unselected GI tumor cohort was modest[81].It suggests that the predictive genetic and genomic features are important for stratifying GI cancer patients responsive to immunotherapy.A large volume of cancer genomics data has been produced through the advancement of next-generation sequencing technology,enabling us to investigate the cancer genomic features associated with tumor immunity and immunotherapy response.The dMMR/MSI status,TMB,and tumor aneuploidy are genomic features associated with tumor immunity and immunotherapy response.Generally speaking,the high TMB tumors are more likely to respond to immunotherapy[14].However,the association between TMB and immunotherapy response is not absolutely positive.Some responders have a low TMB and some non-responders have a high TMB[12].One possible explanation is that the intratumor heterogeneity confounds the mutation landscape,affecting tumor immunity[82].In fact,a previous study has shown that it is clonal neoantigens(generated by mutations identified in distinct regions of a tumor) that associate with immunotherapy response rather than subclonal neoantigens (generated by mutations identified in only a subset of regions of a tumor)[83].Tumor aneuploidy is another genomic feature that is associated with the antitumor immune response,and could be a stronger predictor of tumor immune infiltration than TMB[16].
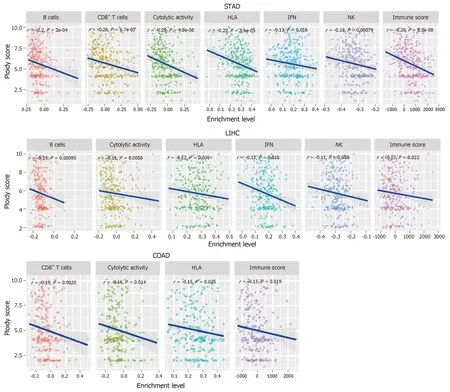
Figure 3 Tumor aneuploidy is negatively associated with tumor immunity in gastrointestinal cancers.
In conclusion,dMMR/MSI,TMB,and tumor aneuploidy are the well-recognized genomic features that are associated with the immunotherapy response of GI and other cancers.To improve the potential of immunotherapy for GI cancers,more genomic features need to be identified,and the relevant investigations should remain a high priority.
杂志排行
World Journal of Gastrointestinal Oncology的其它文章
- Up-regulation of tumor necrosis factor-α pathway survival genes and of the receptor TNFR2 in gastric cancer
- Predictive factors of histological response of colorectal liver metastases after neoadjuvant chemotherapy
- Iodine-125 implantation with transjugular intrahepatic portosystemic shunt for main portal vein tumor thrombus
- Validated model for prediction of recurrent hepatocellular carcinoma after liver transplantation in Asian population
- Significance of HER2 protein expression and HER2 gene amplification in colorectal adenocarcinomas
