Human podocyte injury in the early course of hypertensive renal injury
2019-04-22DaSunJiaoJiaoWangWeiWangJuanWangLiNingWangLiYaoYingHuiSunZiLongLi
Da Sun, Jiao-Jiao Wang, Wei Wang, Juan Wang, Li-Ning Wang, Li Yao, Ying-Hui Sun, Zi-Long Li
Da Sun, Jiao-Jiao Wang, Wei Wang, Juan Wang, Li-Ning Wang, Li Yao, Zi-Long Li, Department of Nephrology, The First Hospital of China Medical University, Shenyang 110001, Liaoning Province, China
Ying-Hui Sun, Department of Experimental Medicine, General Hospital of Northern Theater Command, Shenyang 110016, Liaoning Province, China
Abstract
Key words: Podocyte; Hypertension; Hypertensive renal injury; Microalbuminuria;Nephrin; CD2-associated protein
INTRODUCTION
It is generally accepted that renal morphology and function are dependent on the maintenance of normal blood pressure.Long-standing uncontrolled hypertension can lead to hypertensive renal injury, which is a major cause of chronic kidney disease and accounts for significant mortality[1,2].
Glomerular podocytes are highly differentiated cells that cover the outermost layer of the glomerular basement membrane.Podocytes and their foot processes form tight interdigitating networks that contribute to the hydraulic permeability of the glomeruli and play a crucial role as a filter for macromolecules[3].
Due to the biological and morphological characteristics of podocytes, podocyte injury is recognized as a central feature in the development of many types of renal disease[4,5].In fact, podocyte injury and loss has been proved to be causally related to glomerulosclerosis in a variety of kidney diseases such as diabetic nephropathy and minimal change disease[6-9].
In the last 25 years, progressive observations in animal models have shown that podocyte injury is probably another key event in the pathogenesis of hypertensive nephrosclerosis[10-14], subsequent to the lesions of glomerular ischemia resulting from narrowing and hyalinization of interlobular and afferent arterioles.A possible explanation is that elevated systemic blood pressure can give rise to Bowman’s capsule hypertension, which may cause podocyte injury in a mechanical-stretchdependent manner.However, it remains unclear whether podocyte-associated proteins are involved in the regulation and control of podocyte injury and loss.It is also unknown whether podocyturia can replace proteinuria for early prediction of hypertensive renal injury.
To address these problems, we detected urinary podocytes and obtained human data on podocyte injury and loss under hypertensive conditions.We also observed alterations of podocyte-specific proteins, nephrin and CD2-associated protein(CD2AP) in glomeruli in an attempt to understand the molecular mechanism of podocyte injury during chronic hypertension.
MATERIALS AND METHODS
Patients, urine samples, and renal specimens
This study was approved by the Clinical Institutional Review Board for Human Research (Ethics Committee) of China Medical University and conducted in the Department of Nephrology and Urological Surgery.Written informed consent was obtained from all participants prior to enrollment in the study.
The hypertension group comprised 18 patients with chronic hypertension and microalbuminuria (9 male and 9 female; mean age, 63.2 years; range, 44–81 years).No patient had any signs of infection.Smokers and patients with a history of primary glomerular disease, cardiac insufficiency, diabetes, tumors, or rheumatoid diseases were excluded.Control group 1 comprised 10 healthy volunteers (5 male and 5 female; mean age, 60.1 years; range, 57–79 years) recruited from friends and family of laboratory staff.Control group 2 comprised 16 patients who underwent partial nephrectomy due to renal trauma (with no previous hypertension or proteinuria) in the Department of Urological Surgery during the study period.
Urine samples voided in the morning were collected in sterile containers and processed within 1 h.Freshly obtained urine specimens (100 mL) were transferred to tubes and centrifuged at 1500 r/min for 10 min.Urinary supernatant was carefully aspirated and the sediment pellet was mixed and cytospun onto slides.Subsequently,the slides were air-dried at room temperature, baked at 60°C for 2 h, and fixed with acetone at 4°C for 10 min.
Renal biopsy was performed in the hypertension group.The normal parts of nephrectomized kidney in control group 2 were used for comparison of pathological changes in kidney tissue.The renal specimens were subjected to routine fixation procedures.The pieces of tissue were immersed in 4% paraformaldehyde at room temperature for 24 h, followed by gradual dehydration in a series of alcohols and then transferred into xylene.Finally, the samples were embedded in paraffin wax.The remaining kidney tissue from both groups was maintained at -80°C for biochemical examination.
Albuminuria, glomerular filtration rate and renal injury
Albuminuria was evaluated by measuring the urinary albumin-to-creatinine ratio(ACR) from a single morning urine specimen.Microalbuminuria was defined as ACR 30–300 mg/g.Glomerular filtration rate (GFR) was estimated (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration equation.Renal injury was defined as either microalbuminuria or eGFR < 60 mL/min/1.73 m2.
Antibodies
The primary antibodies used were:Rabbit polyclonal anti-nephrin (Boster, Wuhan,China); mouse monoclonal anti-CD2AP (Santa Cruz Biotechnology, Santa Cruz, CA,United States).The secondary antibodies used were:biotinylated sheep anti-rabbit IgG and biotinylated rabbit anti-mouse IgG (Sigma, St.Louis, MO, United States),tetramethylrhodamine (TRITC)-labeled anti-rabbit IgG and fluorescein isothiocyanate(FITC)-labeled anti-mouse IgG (Abcam, Cambridge, MA, United States).
Immunostaining of deparaffinized sections
The paraffin-embedded tissues were cut into 3-μm thick sections and were deparaffinized with xylene and a graded series of alcohols.Some of the sections were stained with hematoxylin and eosin (HE) to observe morphology.Other sections were incubated with 3% hydrogen peroxide to block the nonspecific reactivity of endogenous peroxidase.After washing with phosphate-buffered saline (PBS), the samples were retrieved with sodium citrate buffer at high pressure and were incubated with blocking buffer (PBS containing 5% bovine serum albumin; Boster) at 37°C for 1 h.The sections were subsequently incubated with the primary antibodies(anti-nephrin or anti-CD2AP) at a dilution of 1:100 overnight at 4°C.
On the second day, the specimens were recovered at room temperature for 30 min and divided into two groups:(1) Some of the sections were used for immunohistochemistry staining.After washing in PBS, the corresponding secondary antibody was added at 37°C for 30 min.The sections were then incubated with horseradish peroxidase-conjugated avidin-biotin complex at 37°C for 20 min and visualized with metal-enhanced 3’3-diaminobenzidine (DAB) for 5 min.To obtain a clearer structure, the sections were counterstained with hematoxylin for 50 s and then dehydrated in a graded series of hydrochloric acid, alcohols and xylene.Finally, the sections were sealed with neutral balsam, air-dried and viewed with a light microscope (Eclipse 80i, Nikon, Japan).The integral optical density (IOD) of each positive stain was analyzed using Image Pro Plus 6.0, and the result was determined as the sum of the glomeruli.IOD was defined as the sum of the ODs of all the positive pixels in the image, which represented the quality of the targeted protein; and (2)Other sections were used for immunofluorescent staining.The sections were incubated with TRITC-labeled donkey anti-rabbit IgG and FITC-labeled goat antimouse IgG at a dilution of 1:200 at 37°C for 1 h in a moist chamber, followed by washing in PBS.Finally, the specimens were sealed with 50% glycerin and viewed with a confocal laser scanning microscope (FV 10-ASW2.1 Viewer).
Immunoelectron microscopy
To detect ultrastructural changes in the glomeruli and immunolocalization of specific podocyte proteins under various hemodynamic conditions, the extracted renal tissues were fixed in 1% osmium tetroxide for 20 min at 4°C after DAB staining, dehydrated through an acetone gradient, and finally inversion-embedded with Epon 812 and polymerized at 60°C for 48 h.Ultrathin sections (70 nm) were cut with an ultramicrotome and stained with 4% uranyl acetate and 1% lead citrate, and then ultrastructural images were obtained with a transmission electron microscope(HT7650; Hitachi, Tokyo, Japan).
Immunostaining for urinary podocytes
The acetone-fixed slides were stained in a similar manner to the renal tissue described above, and viewed under a light or confocal laser scanning microscope.Nucleated,nephrin-positive or CD2AP-positive cells were considered to be podocytes.Two to four slides were examined per patient sample.
Statistical analysis
The quantitative data are presented as the mean ± SD.Statistical analysis was performed using SPSS version 24.0 statistical software.For mean comparisons between the two groups, the Mann–Whitney test was used.For qualitative data comparison, theχ2test was used.P< 0.05 was considered statistically significant.
RESULTS
Clinical data
Clinical characteristics including age, gender, body mass index (BMI), blood pressure,urinary ACR, serum creatinine, and eGFR were obtained by chart review (Table 1).There were no significant differences in age, BMI, serum creatinine and eGFR between the hypertension group and control group 1.The levels of systolic and diastolic blood pressure (including blood pressure under medical treatment) were significantly higher in the hypertension group than in control group 1 (P= 0.000).ACR in the hypertension group fulfilled the criteria for microalbuminuria, and was significantly higher than in control group 1 (P= 0.000).Of note, the absolute values of urinary albumin in 11 of 18 patients enrolled in our study were < 10.6 mg/L (4.6–33 mg/L),and undetectable in routine urine tests.
Podocytes in urinary sediments
To determine whether podocytes are lost from urine, we examined urine sediments for podocyte-specific proteins (nephrin and CD2AP) under different hemodynamic conditions.Nucleated cells expressing nephrin or CD2AP were assumed to be podocytes (Figure 1).In the hypertension group, 55.6% of urine samples had nephrinpositive cells present on cytospins, and 33.3% of urine samples had CD2AP-positive cells.Approximately 50% of samples positive for nephrin also stained for CD2AP.Double immunofluorescence staining showed partial colocalization of nephrin and CD2AP (Figure 2).However, nephrin and CD2AP were barely detected in control group 1 (Table 2).
Histological analysis of glomeruli
To evaluate glomerular lesions in patients with chronic hypertension, we performed histological examination after HE staining.General morphology of the glomeruli from nephrectomized kidneys was normal (Figure 3).The capillary loops were smooth and plump under normotensive conditions (Figure 3A).However, the glomeruli revealed severe atrophy under chronic hypertension.The capillary loops became severely wizened or obliterated, while Bowman’s capsule and the luminal space of tubules became more dilated than in normal glomeruli (Figure 3B).
Expression and distribution of nephrin and CD2AP
Nephrin localized to the slit diaphragm (SD) was strongly detected and stained continually and evenly along the extra-glomerular capillary loops in control group 2(Figure 4A), but was stained weakly and intermittently in the hypertensive group(Figure 4B).CD2AP is a multifunctional adaptor protein localized in the cytoplasm and membrane ruffles in podocytes.In the glomeruli of control group 2, the appearance of CD2AP was interrupted in a linear-like pattern and distributed diffusely (Figure 4C), but they became more scattered and decreased concomitantly with attenuation of nephrin in the hypertension group (Figure 4D).Figures 4E and F show the IODs of each protein in various groups.
Double immunofluorescent staining showed CD2AP (green) expression in theglomeruli.CD2AP was partially co-localized with nephrin (red) resulting in a yellow overlap (Figure 5).The changes in nephrin and CD2AP were confirmed by confocal microscopy (Figure 5).Under hypertensive conditions, the changes in nephrin and CD2AP were similar to those observed by immunohistochemistry.
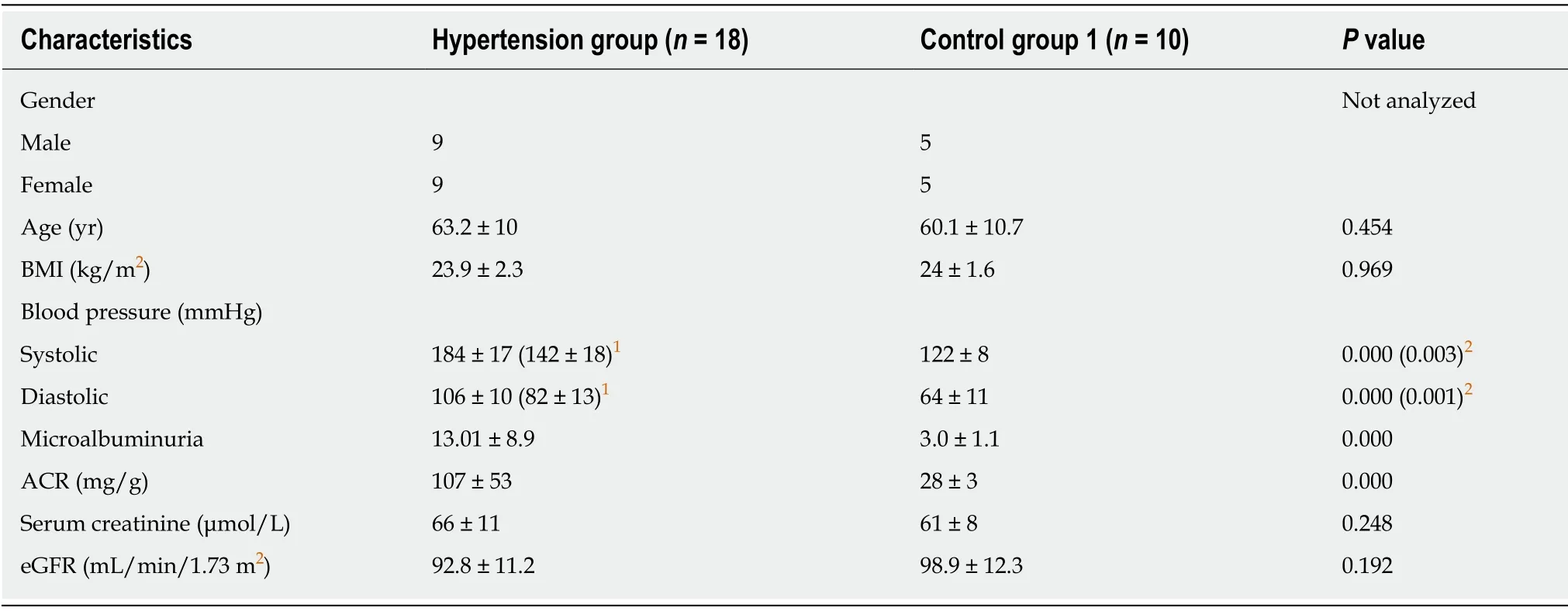
Table 1 Clinical characteristics
Transmission electron microscopy
To visualize the distribution of nephrin and CD2AP under different hemodynamic conditions, we detected them using transmission electron microscopy.In control group 2, the foot processes had an intact arrangement and were displayed as interdigitating (Figures 6A and B).In contrast, the foot processes showed different degrees of fusion and partial foot process effacement in the hypertensive group(Figures 6C and D).Nephrin was distributed on the cytoplasmic face of foot processes in many filtration slits (Figure 6A), and CD2AP had a similar distribution (Figure 6B).In the hypertension group, staining of nephrin and CD2AP was scattered and uneven,and accumulated in some fusion foot processes, but the overall level decreased(Figures 6C and D).
DISCUSSION
Podocyte loss due to severe podocyte injury (including change in expression of podocyte-associated proteins and ultrastructure) has been observed in the early course of nephropathy in rat models of hypertension[10,13,15].However, human data on podocyte injury and loss in hypertensive renal injury are limited.
In the present study, the detection of urinary podocytes was consistent with podocyte loss from the glomeruli and indicated that podocyte injury might occur in the early course of hypertensive renal injury.This result was similar to those in previous studies of human and experimental hypertensive nephrosis[13,16,17].Several studies have also detected podocytes in the urine of healthy subjects, but the number of podocytes shed by healthy controls is significantly lower than in patients with active glomerular diseases[17,18].The possible explanation for these findings may be natural cell senescence and apoptosis[17,19], which will need to be confirmed in a greater number of healthy participants.
Another study has suggested that urinary podocyte loss occurs prior to the onset of proteinuria in the 5/6 nephrectomy model[20], characterized by systemic hypertension and continuing glomerular hyperfiltration.More importantly, podocyturia does not subside and mirrors proteinuria throughout the disease course.Although the level of ACR fulfilled the diagnostic criteria for microalbuminuria, we found that the absolute values of urinary albumin in most patients were in the normal range (< 10.6 mg/L),and could not be detected in routine urine tests.In contrast, podocytes were detected in the urine sediments in the same patients.These findings revealed that podocyturia is a sensitive marker, reflecting active ongoing glomerular damage, and is better than proteinuria.Detection of urinary podocytes may be used for predicting hypertensive renal injury earlier.
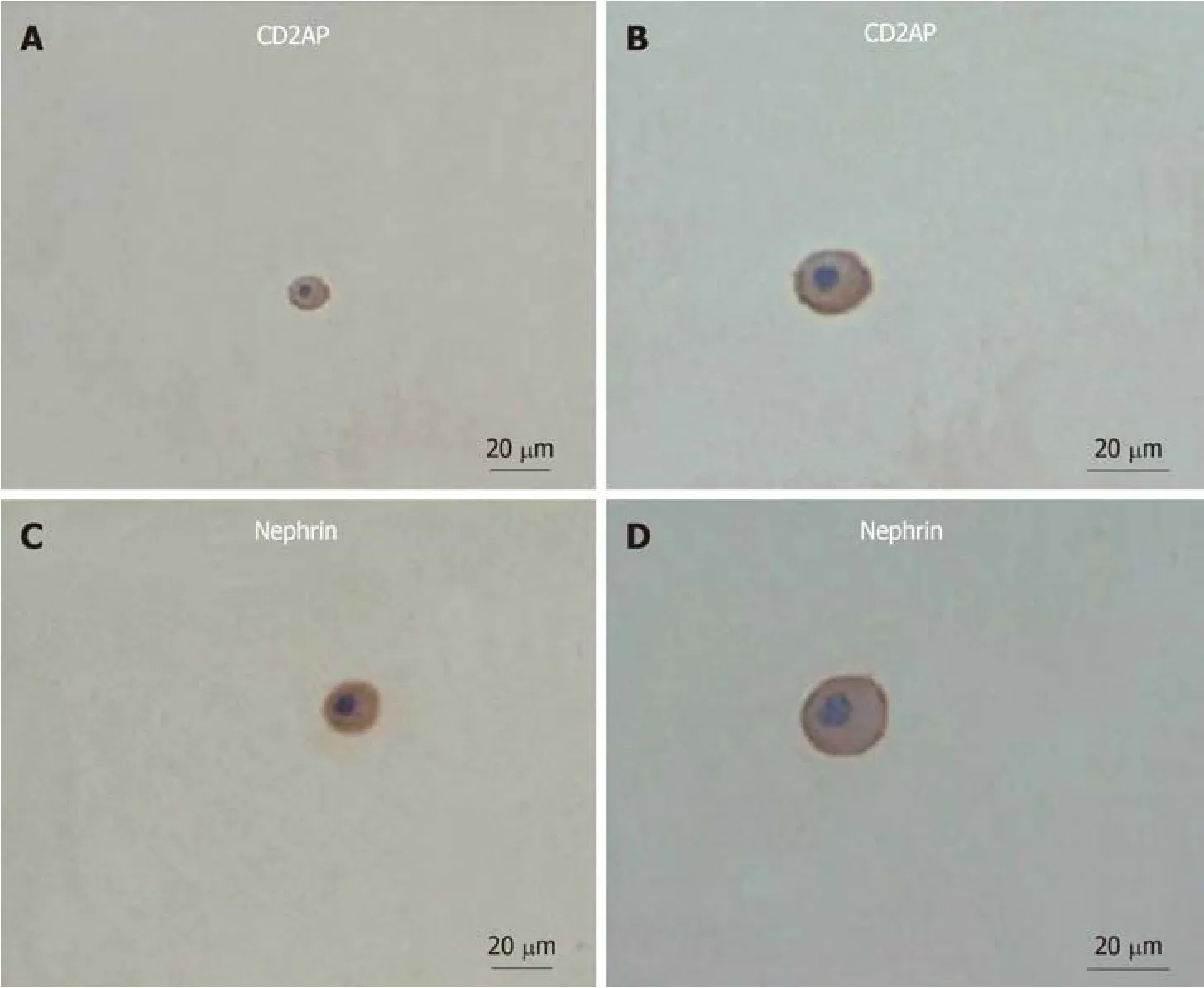
Figure 1 Immunocytochemical staining of urinary podocytes in the hypertension group.
Elevated systemic blood pressure can give rise to Bowman’s capsule hypertension and glomerular hyperfiltration, which has been suggested to cause podocyte injuries in a mechanical-stretch-dependent manner[16].However, the detailed changes in podocytes morphology in humans have not been clearly illustrated before.In the present study, we observed alterations of podocyte-specific proteins nephrin and CD2AP in glomeruli in an attempt to determine the injury sites of podocytes during chronic hypertension.The results demonstrated that expression of nephrin and CD2AP decreased under chronic hypertension conditions.In addition, the transposition and redistribution of nephrin and CD2AP occurred concomitantly with staining intensity attenuation.
Nephrin is a transmembrane protein expressed by glomerular podocytes and is predominantly localized at the SD[21].Immunoelectron microscopy has revealed that neprhin bridges the filtration slit to form a 35-nm-long globular cross-strand that contributes to a porous SD scaffold[22], which plays a crucial role in controlling glomerular permeability of plasma proteins[23,24].
CD2AP is primarily expressed in podocytes at the cytoplasmic face of the SD, and serves as a linker protein between cell-membrane receptors and actin-modifying proteins[25,26].ViaCD2AP, nephrin is anchored to the SD on the actin cytoskeleton to adjust the arrangement of the cytoskeleton[27,28].
Neprhin and CD2AP are critical to the integrity of the glomerular filtration barrier,and our findings indicate that downregulation and distribution of nephrin and CD2AP are causally related to podocyte injury.Consistent results were obtained in experimental hypertensive nephrosis, such as salt-loaded Dahl rats and malignant stroke-prone spontaneously hypertensive rats, which showed decreased expression of nephrin with podocyte injury[29].Furthermore, reduced nephrin expression was also found in renal biopsy specimens from women with pre-eclampsia, a pregnancyspecific disorder characterized by hypertension and proteinuria[30].However, direct evidence of the expression of CD2AP in glomeruli under chronic hypertension is limited.In previous studies, reduced expression of CD2AP has been demonstrated in humans with minimal change nephrotic syndrome and IgA nephropathy[31].Surprisingly, the staining intensity of CD2AP was undiminished in passive Heymann nephritis, a rat model of human membranous nephropathy, although the staining appeared to be more condensed and uneven[32].
One concern raised is that nephrin and CD2AP may also play a crucial role inexacerbating podocyte injury.Recent studies have suggested that podocytes exposed to high concentrations of protein actively endocytose extracellular albumin in a timedependent manner.When the endocytosed albumin is accumulated and out of the podocytes’ handling capacity, it causes endoplasmic reticulum (ER) stress and triggers apoptosis of podocytes.Of interest, CD2AP can inhibit the apoptosis of podocytes induced by ER stress[33].Besides, nephrin and CD2AP also bind to a subunit of phosphoinositide 3-kinase (PI3K), and together they stimulate PI3K-dependent AKT signaling and prevent Bad-mediated apoptosis of podocytes in culture[34,35].Thus,we speculate that reduced and altered expression and distribution of nephrin and CD2AP are not only the results of podocyte injury, but also promoting factors in the progression of hypertensive podocyte damage.
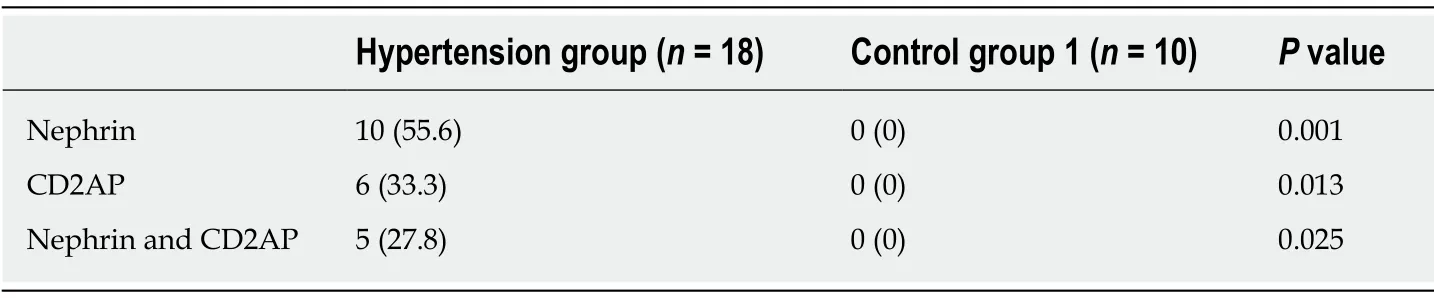
Table 2 Expression of nephrin and CD2-associated protein in urinary sediments, n (%)
All these findings improve our understanding of the mechanism of podocyte injury during chronic hypertension.However, there were several limitations in our study.First, this was a pure observational study and does not suggest a cause-and-effect relationship between urinary podocyte loss and progression of hypertensive renal injury.Second, the possibility that some of the podocyte loss may have resulted from apoptosis could not be excluded.Finally, our study population was complicated with microalbuminuria, so the earlier changes in podocytes before the onset of proteinuria were not observed.
In summary, urinary podocytes were detected in patients with chronic hypertension and microalbuminuria, with immediate evidence of loss of podocytes.This supports podocyturia as an important contributor to podocyte injury and a potentially valuable diagnostic tool; in particular, given its potential to predict hypertensive renal injury earlier than proteinuria can.In addition, we visualized changes in podocyte-specific proteins, neprhin and CD2AP, which located the sites of podocyte injury during hypertensive renal injury.Our next step is to investigate the associated molecules in the neprhin and CD2AP signaling pathway, which should provide new insights into the regulatory mechanisms during hypertensive renal injury.
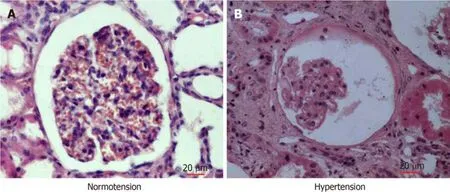
Figure 3 Light micrographs of human renal cortical tissues stained with hematoxylin and eosin.
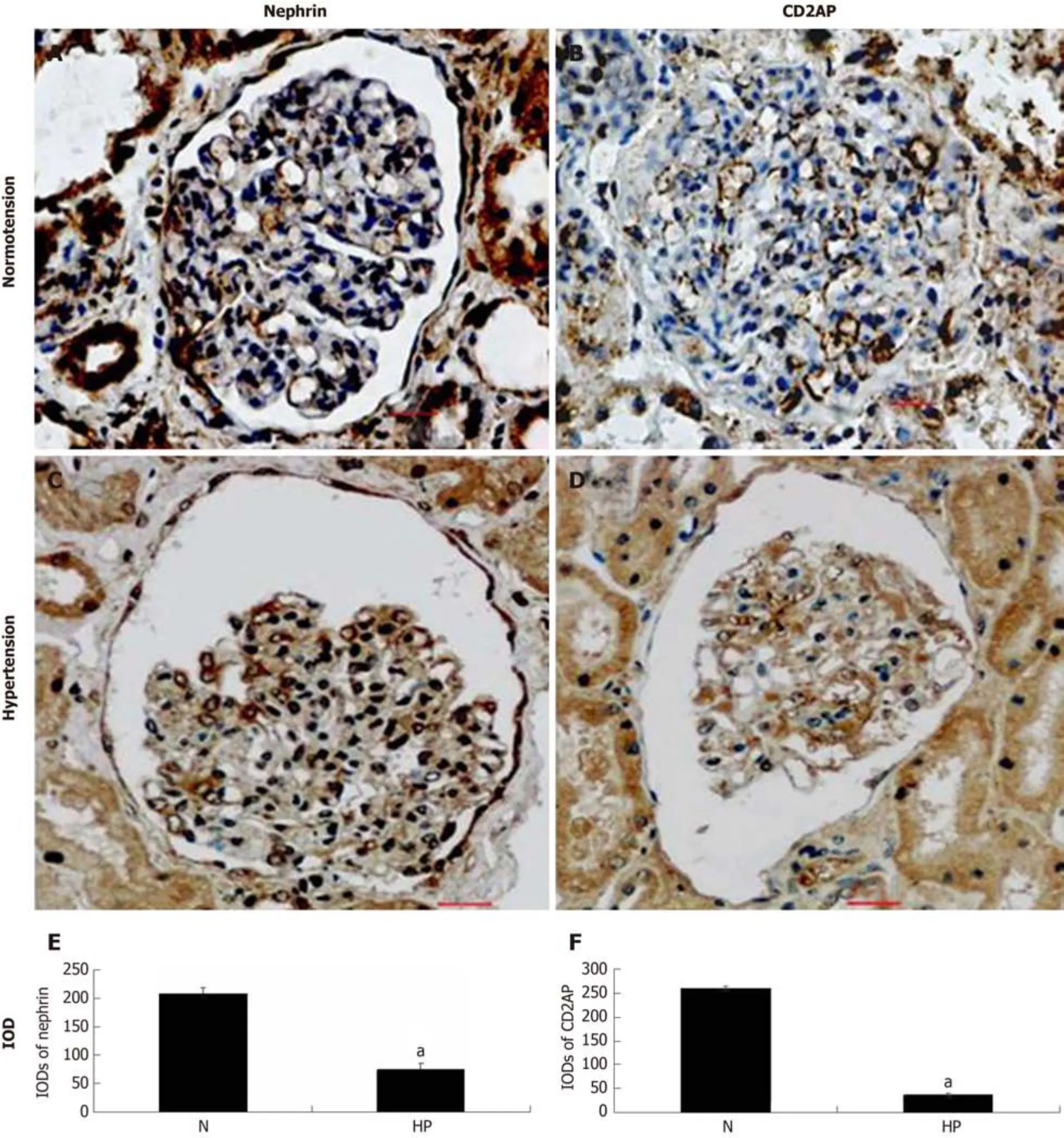
Figure 4 Expression of nephrin and CD2-associated protein in human renal tissue by immunohistochemical staining.
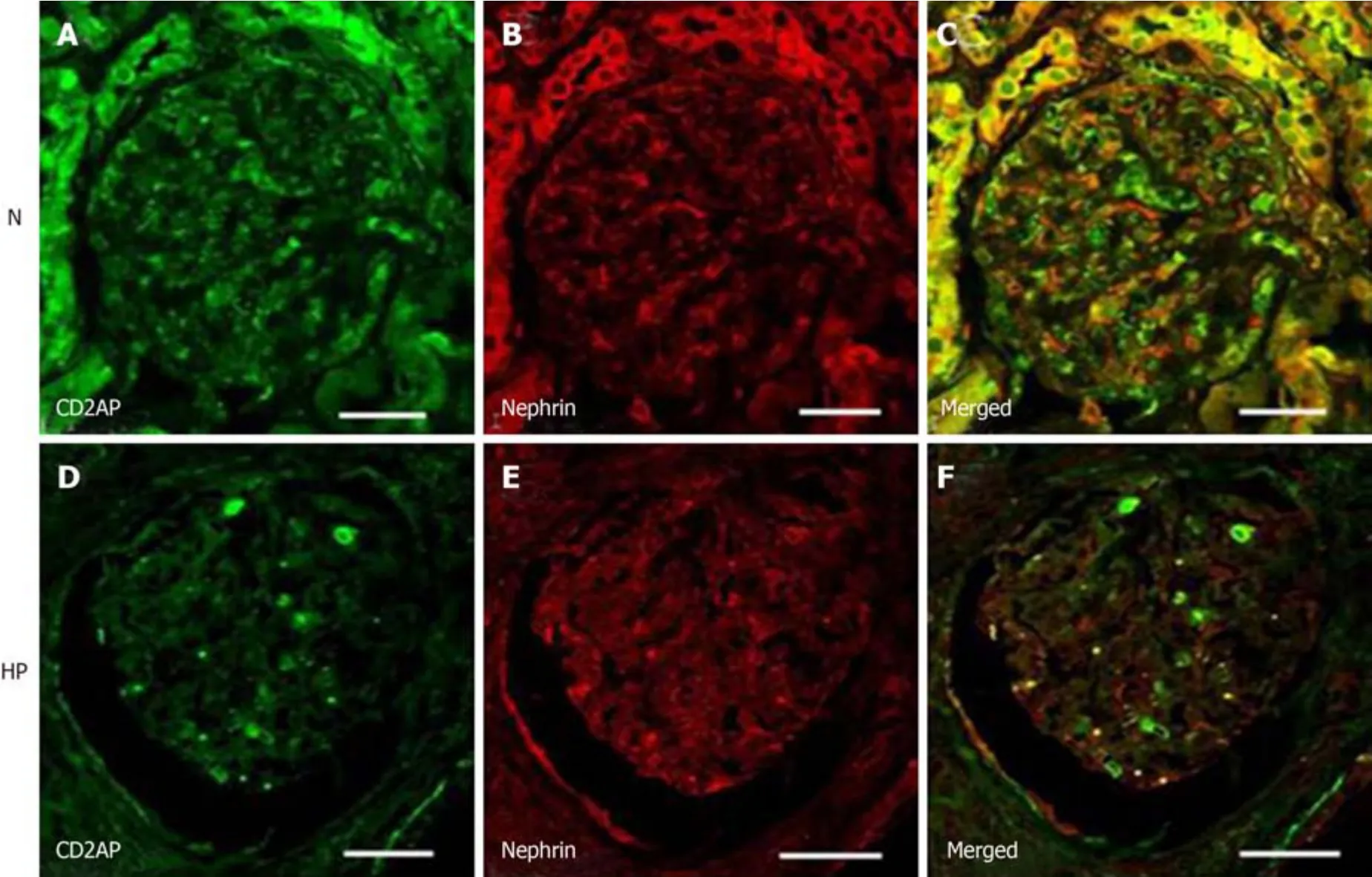
Figure 5 Confocal laser scanning micrographs show double immunofluorescence of nephrin–CD2-associated protein under normotensive and hypertensive conditions.

Figure 6 Immunoelectron micrographs of nephrin and CD2-associated protein in glomeruli under normotensive and hypertensive conditions.
ARTICLE HIGHLIGHTS
Research background
Emerging evidence has shown that podocyte injury contributes to the development of renal damage associated with hypertensive kidney injury; however, the mechanism remains unclear and human data are limited.
Research motivation
This study aimed to investigate human podocyte injury induced by hypertension in the early course without massive proteinuria or decreased renal function.Based on the information obtained in this study, it is expected to provide novel diagnostic methods and therapeutic strategies for hypertensive renal injury in the future.
Research objectives
In this study, we summarized the method for detecting urinary podocytes, excluded the interference of physiological shedding, and verified the evaluation significance of urinary podocytes for hypertensive renal injury in the clinic.In addition, we preliminarily investigated the molecular mechanism of podocyte injury in the early course of hypertensive renal injury.
Research methods
Human urine specimens and kidney tissue were used in this study.Urinary podocytes were observed under light microscopy and confocal microscopy.The intrarenal expression of podocyte-specific proteins, nephrin and CD2-associated protein (CD2AP) were detected by immunohistochemical techniques under different hemodynamic conditions and quantified by integral optical density values.The ultrastructure of human glomeruli was examined using transmission electron microscopy.
Research results
Nucleated cells expressing nephrin or CD2AP were defined to be podocytes, and were detected in urine sediment in the hypertension group.By contrast, few podocytes were observed in control group 1.Moreover, podocyte foot process fusion and significantly decreased nephrin and/or CD2AP expression in glomeruli were observed in the hypertension group compared with control group 2, indicating podocyte injury and detachment from glomerular basement membrane, which was consistent with urinary detection of podocytes.
Research conclusions
Decreased expression and the abnormal redistribution of neprhin and CD2AP are causally related to the disruption of podocyte morphology, and together with urinary podocytes lost from the glomerulus, confirm that podocyte injury is a key event in the progression of hypertensive kidney injury.Furthermore, podocyturia appears early in the course of hypertensive renal injury, and may be a sensitive marker for early prediction of hypertensive renal injury.
Research perspectives
Calcium channels, associated signaling pathways and downstream cytokines are closely related to the regulatory mechanisms of podocytes, which is the research focus of hypertensive renal injury in future studies.
ACKNOWLEDGEMENTS
We thank the Department of Immunodermatology, the First Hospital of China Medical University for providing us with confocal laser microscopy.Thanks also to the Cytobiology College, China Medical University for providing us with transmission electron microscopy.
杂志排行
World Journal of Clinical Cases的其它文章
- Colorectal cancer:The epigenetic role of microbiome
- Relationship between acute hypercarbia and hyperkalaemia during surgery
- Surgical treatment ofpatients with severe non-flail chest rib fractures
- Super-selective arterial embolization in the control of acute lower gastrointestinal hemorrhage
- End-stage liver disease score and future liver remnant volume predict post-hepatectomy liver failure in hepatocellular carcinoma
- Treatment of hemorrhoids:A survey of surgical practice in Australia and New Zealand
