Pulmonary embolism and deep vein thrombosis caused by nitrous oxide abuse:A case report
2019-04-22WenSunJiPingLiaoYanHuWeiZhangJingMaGuangFaWang
Wen Sun, Ji-Ping Liao, Yan Hu, Wei Zhang, Jing Ma, Guang-Fa Wang
Wen Sun, Ji-Ping Liao, Yan Hu, Wei Zhang, Jing Ma, Guang-Fa Wang Department of Pulmonary and Critical Care Medicine, Peking University First Hospital, Beijing 100034, China
Abstract
Key words: Nitrous oxide; Pulmonary embolism; Deep vein thrombosis; Homocysteine;Case report
INTRODUCTION
Nitrous oxide (N2O) is a small and simple inorganic chemical molecule that has been used for more than 150 years in clinical practice due to its analgesic, anxiolytic, and anesthetic properties[1].
Pulmonary embolism (PE) refers to obstruction of the pulmonary artery or one of its branches by an embolus such as a thrombus, tumor, air, or fat.Together with deep vein thrombosis (DVT), PE is a form of venous thromboembolism (VTE) that is common and sometimes fatal in acute situations or disabling in the chronic phase[2].
N2O is correlated with hyperhomocysteinemia (HHcy) to some extent, and the latter has been found to be a potential risk factor for VTE.To the best of our knowledge, there are only a few reports of PE and DVT that were possibly triggered by HHcy secondary to N2O use.
CASE PRESENTATION
Chief complaints
Sudden-onset chest pain for 1 d.
History of present illness
A 29-year-old man complained of sudden precordial pain with no remission for 1 d prior to hospital admission but denied hemoptysis, dyspnea, or syncope.He sought evaluation and was admitted for the purpose of intensive examination and treatment.
History of past illness
The patient had been addicted to N2O for 1 year; as a result, peripheral neuropathy had occurred and was treated with vitamin B12 (Vit B12).His medical history included poor blood glucose control and anxiety.However, he had no history of thrombosis,malignant tumor, recent trauma, or prolonged air travel.
Personal and family history
The patient has smoked over 13 pack-years and did not have alcoholism or a positive family history.
Physical examination upon admission
The initial assessment revealed that the patient was overweight and fragile, with a body mass index of 31.12 kg/m2, temperature of 36.3 °C, blood pressure of 110-120/70-80 mmHg, heart rate over 110 beats/min, respiratory rate over 30 breaths/min, and pulse oximetry of 93% when the fraction of inspired oxygen was 49%.Moreover, the physical examination indicated diminished breath sounds on both sides and the accentuation of pulmonic second sounds.
Laboratory examinations
Laboratory data showed a high leukocyte count and an increased hemoglobin level,suggested abnormal liver biochemistry and hyperlipidemia, and revealed HHcy(24.12 µmol/L), high Vit B12(734 µmol/L), and normal folate (14.39 nmol/L).The results of the protein S, protein C, anti-thrombin III antibody, anti-cardiolipin antibody, lupus anticoagulant, anti-β2gp1 autoantibody, and other autoantibodies were within normal limits.The clotting profile was normal, other than the D-dimer value (1.13 mg/L), and thrombophilia screening was negative.As markers in the risk stratification of PE, the levels of brain natriuretic peptide (BNP) and cardiac troponin I(CTnI) were 533 pg/mL and 0.334 ng/mL, respectively.Arterial blood gas levels measured with an oxygen concentration of 49% showed the following:pH, 7.44;PaCO2, 30 mmHg; and PaO2, 74 mmHg.
Imaging examinations
An electrocardiogram (ECG, Figure 1A) showed sinus tachycardia with typical changes on SIQIIITIII (S wave in the aVL lead, Q wave and T wave in the III lead).Ultrasonic cardiogram demonstrated right ventricular enlargement with left ventricular compression.Color Doppler scans were used to explore femoral, popliteal,and calf muscular venous thrombosis in the right extremity.Computed tomographic pulmonary angiography (CTPA) revealed occlusion of the pulmonary artery trunk,lobar artery, and arterial branches on both sides and signs of increased right heart load (Figure 2A and B).
FINAL DIAGNOSIS
Moderate-high risk PE and DVT along with N2O abuse.
TREATMENT
The patient received a diagnosis of acute massive PE and newly discovered proximal and distal DVT, which was treated with a half dose of alteplase (50 mg) intravenously over two hours and anticoagulant therapy, and the HHcy was treated with folate.
OUTCOME AND FOLLOW-UP
After treatment, the laboratory parameters, including D-dimer, CTnI, BNP, and HCY levels, approached normal levels, the abnormal ECG waves disappeared on reexamination (Figure 1B), the areas of embolization were reduced on repeat CTPA(Figure 2C and Figure 2D) and finally disappeared, but the old lower extremity thrombosis still existed.Our patient was no longer taking N2O in regular follow-up.
DISCUSSION
Research reports on the correlation between N2O and VTE are very rare, although the mechanism seems reasonable.Chronic N2O abuse is known to decrease active VitB12levels, inhibit methionine synthase, which plays a key role in the metabolism of methionine and folate, and eventually result in HHcy and thrombosis[3].In a case of aortic arch thrombus caused by N2O, the HCY level was decreased when N2O use was stopped and then increased upon N2O reuse[4].
N2O may be one of the initial factors that caused PE.Previous research[5]supported that elimination of N2O from the inspired gas rapidly reduced pulmonary artery pressure, decreased wasted ventilation, and increased cardiac output in non-lethal venous air embolism.However, the data are insufficient to perform a meta-analysis on the relationship between N2O and PE[6], and ENIGMA-II trials showed that the number of acute HHcy cases after N2O exposure was negligible[7].This obvious inconsistency may be due to different entrance conditions and a lower concentration and shorter duration of N2O in such trials.
HHcy has been found to be an intermediate factor for arterial and venous thrombosis[4,8-10].As a potential risk factor for cardiovascular disease, HHcy may cause endothelium dysfunction, platelet and clotting activation, impaired fibrinolysis, and a shift of the balance of methylation[8,11-12].Meanwhile, the relative risk for VTE in patients with HHcy is 2.5-2.95 that of the normal population[13-14].Approximately 25%of patients with VTE have HHcy.In contrast, approximately one-third of HHcy patients subsequently develop VTE, and these patients have a 3.99-fold increased risk for PE[12].
HHcy may also be a risk factor for recurrent VTE.A multicenter study[15]prospectively followed patients with single idiopathic VTE after oral anticoagulant withdrawal and found that HHcy patients were more likely to have recurrent VTE than those with normal Hcy levels.In addition, other studies[16]have shown that HHcy complicated with PE may lead to aggravated pulmonary circulation and unstable hemodynamics by increasing the release of vascular endothelial inflammatory factors.
In this case, the young man had abused N2O for a long time and had a previous history of peripheral neuropathy.In this patient with no evidence of thrombophilia,PE and DVT were diagnosed successively with the presence of HHcy.Considering his medical history and examination data, we suggested that N2O abuse was possibly a key link to PE, even though other risk factors for venous thrombosis were present,including lack of exercise, smoking, and obesity.
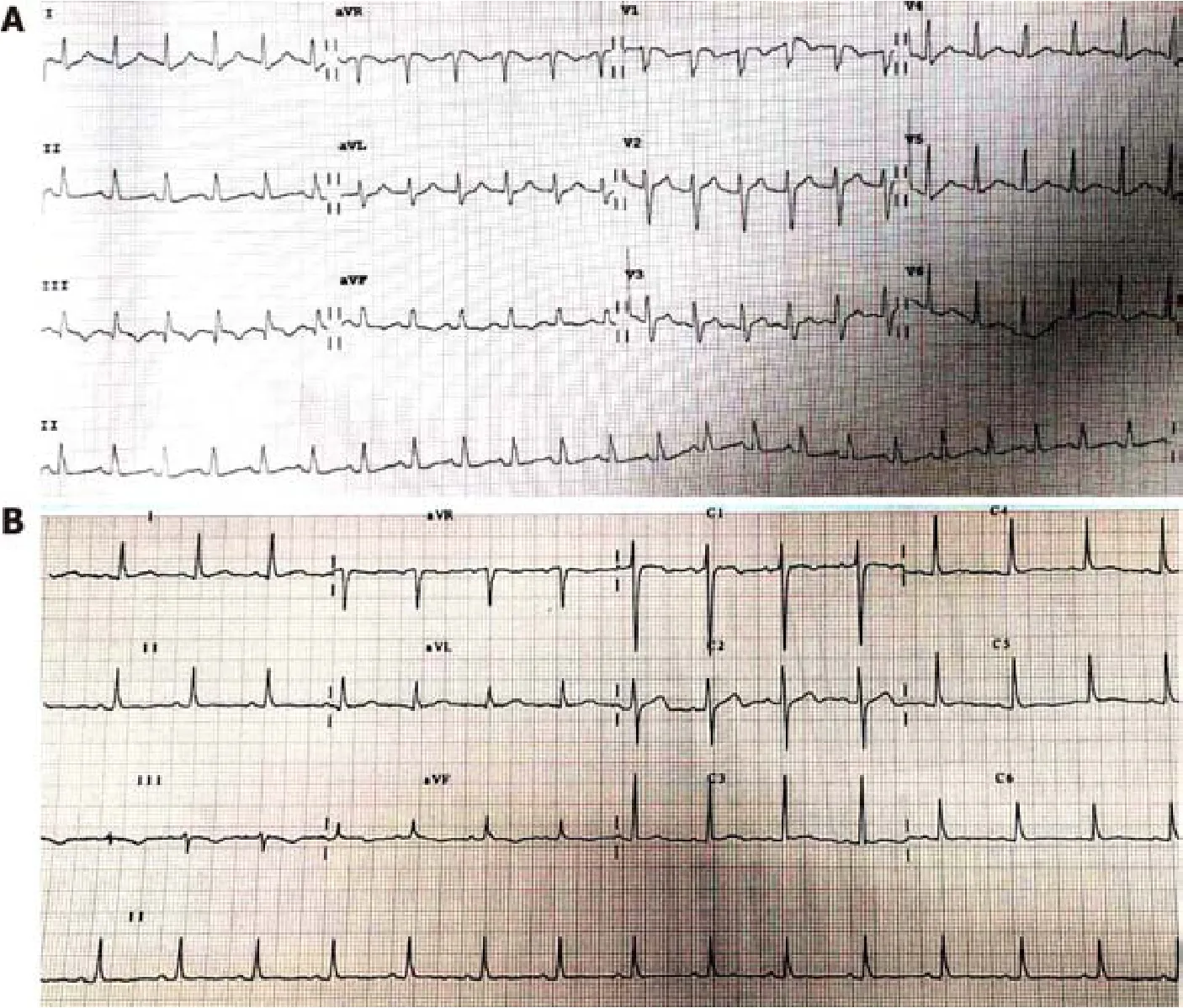
Figure 1 Electrocardiogram before and after thrombolysis.
Although elevated HCY levels can be decreased by 25% by daily use of a folic acid supplement, even at a low dose of 0.5 mg, and by VitB6and B12supplementation,whether this reduces the incidence and recurrence rates of VTE is still controversial[17].In a case[9]of upper extremity deep vein thrombosis and pulmonary embolism in a patient with methylene tetrahydrofolate reductase deficiency-related HHcy, vitamin and folic acid supplementation were suggested until the levels were in normal ranges.Additionally, in another case[10]of pernicious anemia and HHcy in a patient with a G20210A prothrombin gene mutation, no further episodes of thrombosis occurred after treatment with homocysteine-lowering therapy.
However, a secondary analysis of data from the HOPE-2 trial found that homocysteine-lowering therapy (folic acid, VitB6, and B12) did not significantly reduce the incidence rate of venous thromboembolism [hazard rate (HR):1.01; confidence interval (CI):0.66-1.53)[18].
A similar randomized trial enrolling 701 patients showed that vitamin supplementation did not prevent recurrent venous thrombosis (HR:0.84, 95%CI:0.56-1.26)[19].We retrospectively reviewed the case of our patient, who had a high risk of drug relapse and a low likelihood of avoiding other risk factors, and we recommended supplementation with folic acid and vitamins to prevent recurrence of VTE.
CONCLUSION
N2O was likely a direct or indirect factor in the pathogenesis of thrombosis in this case, and Hcy played an intermediate role.Furthermore, N2O can cause fatal embolism in severe cases and during the acute stage.We strongly recommend stopping N2O abuse and supplementation with folic acid and vitamins in patients at a high risk of recurrence, although the treatment protocols remain uncertain.
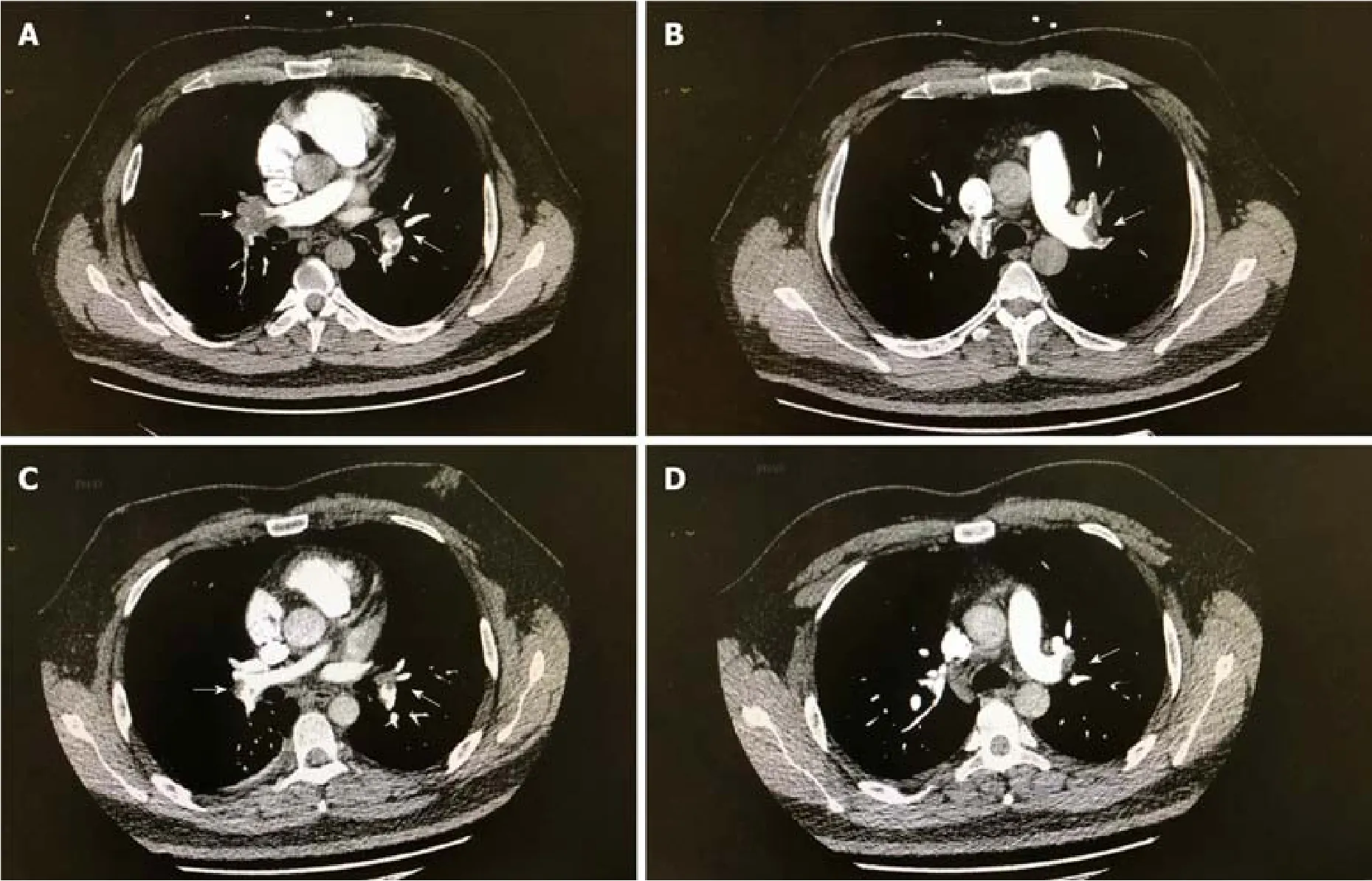
Figure 2 Computed tomographic pulmonary angiography images.
杂志排行
World Journal of Clinical Cases的其它文章
- Overview of organic anion transporters and organic anion transporter polypeptides and their roles in the liver
- Value of early diagnosis of sepsis complicated with acute kidney injury by renal contrast-enhanced ultrasound
- Value of elastography point quantification in improving the diagnostic accuracy of early diabetic kidney disease
- Resection of recurrent third branchial cleft fistulas assisted by flexible pharyngotomy
- Therapeutic efficacy of acupuncture combined with neuromuscular joint facilitation in treatment of hemiplegic shoulder pain
- Comparison of intra-articular injection of parecoxib vs oral administration of celecoxib for the clinical efficacy in the treatment of early knee osteoarthritis
