Embryonic Growth and Yolk Depletion during Incubation in the Chinese Skink, Plestiodon chinensis
2019-03-27LiMAKunGUOShanSUandXiangJI
Li MA, Kun GUO, Shan SU and Xiang JI
Jiangsu Key Laboratory for Biodiversity and Biotechnology, College of Life Sciences, Nanjing Normal University,Nanjing 210023, Jiangsu, China
Abstract We collected 24 gravid female Chinese skinks (Plestiodon chinensis) to study embryonic growth and yolk depletion during incubation. Females laid eggs between late May and mid-June. Eggs were incubated at 24 (± 0.3)°C. One egg from each clutch was dissected at 5-d intervals starting at laying. Embryonic stages at laying varied from Dufaure and Hubert’s (1961) Stage 30-35, with a mean stage of 32.6. Incubation lengths at 24 °C varied from 35.1 to 48.3 d, with a mean of 41.5 d. Based on the derived functions describing instantaneous changes in embryo dry mass and yolk dry mass, we identi fi ed three phases of embryonic growth or yolk depletion in P. chinensis. Phase 1, from Day 0 (at laying) to Day 15 (~36% of the way through incubation), was one of minimal transfer of material from yolk to embryo. Phase 2, from Day 15 to Day 32-33 (~77%-80% of the way through incubation), was characterized by increasingly rapid embryonic growth or yolk depletion. Phase 3, from Day 32-33 to hatching, was characterized by reduced embryonic growth or yolk depletion. The length of the last embryonic stage (Stage 40 = completely differentiated embryos) accounted for about 28% of incubation length, and the dry mass of the smallest embryos of Stage 40 accounted for only ~48% of the hatchling dry mass. Our study adds evidence to the idea that oviposition is not timed to coincide with the onset of rapid embryonic growth in oviparous reptiles, and is fi rst to demonstrate that ~50%embryonic growth occurs in the last quarter of incubation in P. chinensis.
Keywords egg incubation, embryonic growth, embryonic stage, Plestiodon chinensis, scincid lizard, yolk depletion
1. Introduction
As occurring in other animal taxa, embryonic development in reptiles involves differentiation and growth, two distinct processes that are integrated but dissociated to some extent during embryogenesis. Early embryonic development is characterized by differentiation(the origin of tissues and organ systems), whereas late development is characterized by rapid growth (the rapid increase in the size of the embryo) (Andrews, 2004).Oviparous reptiles lay eggs that contain all nutrients necessary for producing a hatchling with some amount of residual yolk (Fischer et al., 1991; Booth and Yu, 2009;Koláčková et al., 2015; Wu et al., 2017; Qu et al., 2019).
During incubation, embryos use yolk as the source of all organic and most inorganic nutrients and eggshell as the additional source of inorganic nutrients such as calcium(Packard et al., 1985; Du et al., 2001; Wu et al., 2017).Most oviparous squamate reptiles (lizards, snakes and amphisbaenians) lay eggs with more advanced embryos than do turtles (gastrulation), tuataras (gastrulation) and crocodilians (neurulation) (Shine, 1983). Embryos of these squamate species have completed a substantial proportion of differentiation prior to oviposition but,because differentiation is well before dramatic embryonic growth (Andrews, 2004), the mass of the embryo accounts for only a very small fraction of the hatchling mass (Shadrix et al., 1994; Ji et al., 1997; Thompson and Stewart, 1997; Cai et al., 2007; Lu et al., 2009).
Studies of lizards such as the Ordos racerunner Eremias brenchleyi (Xu and Wu, 2003), the coral skink Plestiodon (formerly Eumeces) anthracinus (Thompson and Stewart, 1997), the five-lined skink Plestiodon fasciatus (Shadrix et al., 1994) and the northern grass lizard Takydromus septentrionalis (Xu et al., 2004) have showed that dramatic embryonic growth or yolk depletion does not occur until the second quarter of incubation in species where females deposit eggs with embryos at Dufaure and Hubert’s (1961) Stage 26-30. Unfortunately,as mathematical functions describing embryonic growth or yolk depletion during incubation were not provided in these studies, the detailed inverse relationship between two major egg components, embryo and yolk, still remains unknown in lizards. Mathematical functions that have been established for two species of colubrid snakes,the red-necked keelback snake Rhabdophis tigrinus lateralis (Cai et al., 2007) and the checkered keelback snake Xenochrophis piscator (Lu et al., 2009), show three phases of embryonic growth or yolk depletion: Phase 1 is one of the minimal transfer of energy and material from yolk to embryo, Phase 2 is characterized by increasingly rapid embryonic growth or yolk depletion, and Phase 3 is characterized by a gradual reduction in embryonic growth or yolk depletion. Are these three phases detected in snakes generalisable to reptiles? To answer this question,we need to collect independent datasets from different reptilian taxa.
Here, we describe a study dissecting lizard eggs at 5-d intervals starting at oviposition to quantify embryonic growth and yolk depletion during incubation. We used the Chinese skink (Plestiodon chinensis), a medium sized(up to 134 mm snout-vent length, SVL) scincid lizard that is widely distributed in southeastern China (Lin and Ji,2000), as a model animal for two reasons: (1) the skink is among oviparous species with substantial differentiation but negligible embryonic growth prior to oviposition(Ji and Zhang, 2001; Lu et al., 2014; Qu et al., 2014;Shen et al., 2017; Ma et al., 2018); and (2) females often lay at least 10 eggs per clutch, and the larger clutch size provides an opportunity to dissect eggs laid by a single female at different incubation stages. We aim to establish mathematical functions describing embryonic growth, yolk depletion and their instantaneous changes during incubation, to examine whether egg-laying is timed to coincide with the onset of dramatic embryonic growth, and to test the hypothesis that the three phases of embryonic growth or yolk depletion detected in snakes are generalisable to reptiles.
2. Materials and Methods
2.1. Animal collection and care We collected 24 gravid females (> 90 mm SVL) in early May 2015 from Lishui(28°27’N, 119°55’E, ~70 m elevation), Zhejiang, East China. Females were transported to our laboratory in Nanjing, where six were housed in each of four outdoor enclosures (length × width × height: 1.5 ×1.5 ×0.6 m)with a turf-covered soil substrate (~150 mm depth).Females could regulate body temperature by using natural thermal flux. Food, mealworm larvae (Tenebrio molitor), house crickets (Achetus domestica), cockroaches(Blaptica dubia) and water enriched with vitamins and minerals were provided daily. We checked the cages at least thrice daily for freshly laid eggs after the first female laid eggs, thereby collecting and weighing (to the nearest 1 mg) eggs less than 3 h post-laying. The fertility of freshly laid eggs was identified through the eggshell by visual inspection due to the presence of a reddish embryonic disc (Ji and Zhang, 2001). Post-oviposition females were individually measured for SVL and tail length, weighed, and then moved to their own enclosure where they remained until they were released to the field in late July.
2.2. Methods Fertilized eggs were either dissected at laying or incubated individually in covered plastic jars(50 ml) with moist vermiculite at -12 kPa (Ji and Braña,1999). Egg was half-buried in the substrate, with the surface near the embryo being exposed to air inside the jar. Jars were placed in an incubator (Binder, Germany)at 24 ± 0.3 °C. This temperature falls within the range of temperatures optimal for egg incubation in P. chinensis(Ji and Zhang, 2001; Qu et al., 2014; Shen et al., 2017).Jars were weighed at five-day intervals, and distilled water was evenly added into substrates when necessary to compensate for evaporative losses and water absorbed by eggs. We rotated jars daily according to a predetermined schedule to minimize the possible influence of thermal gradients; nevertheless, gradients in temperature inside the incubator were trivial (0.3 °C), as veri fi ed by Tinytalk loggers (Gemini Pty, Australia) placed inside jars.
One egg from each clutch was dissected at five-day intervals starting at laying (Day 0 of incubation). Each dissected egg was separated into embryo and yolk, and its Dufaure and Hubert’s (1961) stage of embryonic development was identified; the two egg components were then dried to constant mass in an oven at 65°C, weighed and preserved frozen for later use. We followed Dufaure and Hubert (1961) to identify embryos at Stage 40 as completely differentiated. Hatchlings were collected, measured for SVL and tail length and weighed immediately after they emerged from the egg.One hatchling from each clutch was dissected, and the remaining hatchlings were released to the site where their mothers were collected. Each dissected hatchling was separated into carcass (including fat bodies) and residual yolk, and the two hatchling components were also dried to constant mass in an oven at 65 °C, weighed and preserved frozen for later use. We extracted lipids from dried samples in a Soxhlet apparatus for 5.5 h using absolute ether as the solvent (Ji and Braña, 1999). The amount of lipids in each sample was determined by subtracting the lipid-free dry mass from the total sample dry mass.
2.3. Statistical analysis We used one-way ANOVA to examine whether eggs dissected at different stages of incubation differed in mean mass. We used non-linear estimation to establish functions and curves describing changes in dry mass and lipid mass, and then established derived functions and curves to show instantaneous variation in tangent slopes of the corresponding curves.Throughout this paper, values are presented as mean ±SE, and significance level is set at α = 0.05.
3. Results
Females laid a single clutch of 10-23 eggs between late May and mid-June. Eggs at laying varied in mass from 0.53-1.00 g, with a mean of 0.73 g. Eggs hatched and dissected at different stages of incubation did not differ from each other in mean mass at laying (F9,230= 0.22, P= 0.99). Eggs steadily gained mass during incubation,and the fi nal mass was about three times heavier than the initial mass (Figure 1). Incubation lengths varied from 35.1-48.3 d, with a mean of 41.5 d. Embryonic stages identified at laying varied from Stage 30-35, with a mean stage of 32.6. Differentiation was nearly completed on Day 30 of incubation (~72% of the way through incubation) when 23 out of 24 embryos were at Stage 40(Figure 2).
Figure 3 shows mean values (± SE) for dry mass and lipid mass of two major egg components (embryo and yolk) at different stages of incubation and their instantaneous changes (tangent slopes) during incubation.There was a clear-cut “ebb and flow” relationship between embryo and yolk; that is, embryonic growth is tightly associated with yolk depletion, and vice versa.Functions describing instantaneous changes in dry mass and lipid mass revealed that embryonic growth or yolk depletion was less striking in the interval from Day 0-15(0-36% of the way through incubation) and that the interval from Day 30-35 (72-84% of the way through incubation) represented the greatest embryonic growth or yolk depletion (Figure 3).
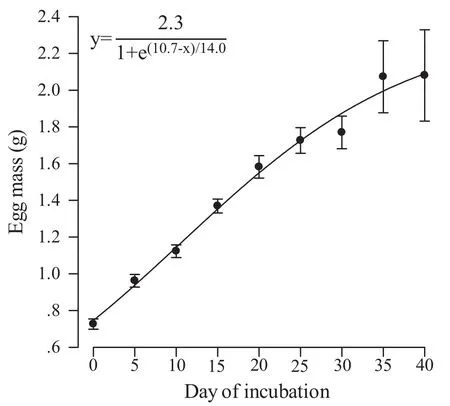
Figure 1 Means (±SE) for egg mass at different days of incubation.The function and curve describing temporal changes in egg mass are provided in the fi gure. N = 24 for each sampling day.

Figure 2 Means (± SE) for Dufaure and Hubert’s (1961) embryonic stage at different days of incubation. The function and curve describing temporal changes in embryonic stage are provided in the fi gure.
4. Discussion
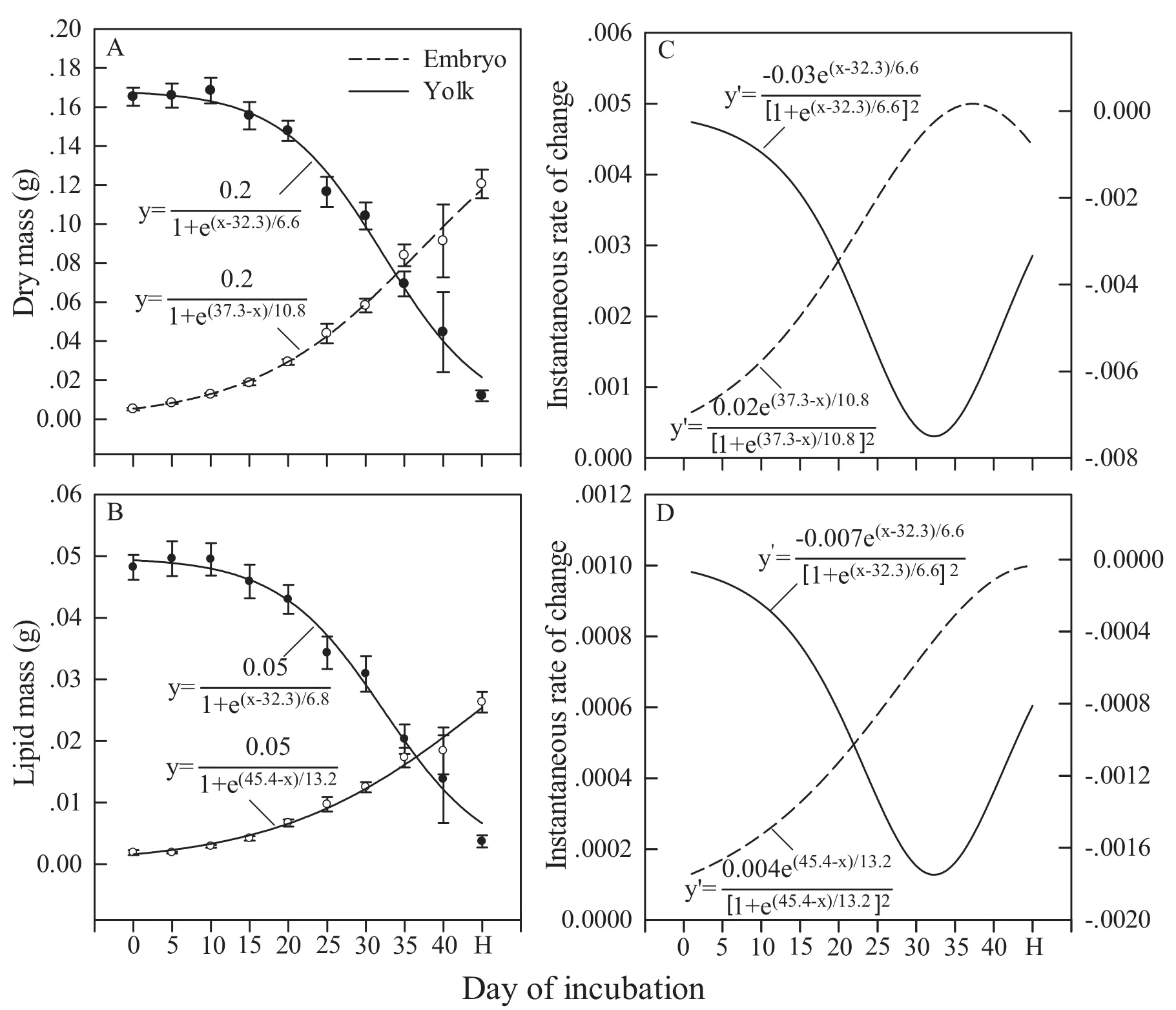
Figure 3 Means (± SE) for dry mass (A) and lipid mass (B) at different days of incubation, and instantaneous rates of changes in dry mass (C)and lipid mass (D). All related functions are provided in the fi gure. H: hatched
It seems likely that oviparous reptiles of different taxa share the same pattern of dynamic changes in embryonic growth and yolk depletion as shown in Figure 3 (lizards:Shadrix et al., 1994; Xu and Wu, 2003; Xu et al., 2004;snakes: Cai et al., 2007; Lu et al., 2009; turtles: Du et al., 2001). Consistent with the results reported for other oviparous squamate reptiles (lizards: Shadrix et al., 1994;Ji and Braña, 1999; Ji et al., 2002; Wang et al., 2013;snakes: Ji et al., 1997, 1999; Cai et al., 2007; Lu et al.,2009), embryos of P. chinensis completed a substantial proportion of differentiation at laying but had negligible dry mass in comparison to the rest of the egg (Figure 3). Here, we did not observe rapid embryonic growth or yolk depletion in the interval from Day 0 (Stage 30-35)to Day 15 (Stage 36-38) of incubation. Accordingly,we conclude that, as in other lizards such as the bearded dragon Amphibolurus barbata (Packard et al., 1985), E.brenchleyi (Xu and Wu, 2003), P. anthracinus (Thompson and Stewart, 1997), P. fasciatus (Shadrix et al., 1994)and T. septentrionalis (Xu et al., 2004), the timing of oviposition does not precede immediately an interval of rapid embryonic growth in P. chinensis. Our study therefore adds evidence to the idea that a dramatic change in the pattern of embryonic growth or yolk depletion is not the proximate cause for egg-laying in oviparous reptiles (Shadrix et al., 1994; Thompson and Stewart,1997; Du et al., 2001; Cai et al., 2007; Lu et al., 2009).
Based on the derived mathematical functions and curves (Figure 3), we can also identify three phases of embryonic growth or yolk depletion in P. chinensis. The first phase, from Day 0 to Day 15 (~36% of the way through incubation), is an interval within which the rate of embryonic growth or yolk depletion does not rise steeply and embryonic growth is therefore slow. The second phase, from Day 15 to Day 32-33 (~77-80%of the way through incubation), is characterized by increasingly rapid embryonic growth or yolk depletion,with the greatest rate of embryonic growth or yolk depletion occurring at the end of the phase. The third phase, from Day 32-33 to hatching, is characterized by a gradual reduction in the rate of embryonic growth or yolk depletion. The first phase ends at an earlier incubation stage in P. chinensis than in P. fasciatus (~50%; Shadrix et al., 1994), presumably because females oviposit at a later mean embryonic stage in the former (Stage 30-35,with a mean stage of 32.6) than in the later (Stage 30;Shadrix et al., 1994) species. That the fi rst phase ends at an earlier incubation stage in species with more advanced embryos at laying has also been documented in snakes.For example, the fi rst phase ends at an earlier incubation stage in red-necked keelback snake Rhabdophis tigrinus lateralis (~36% of the way through incubation; Cai et al.,2007) than in the checkered keelback snake Xenochrophis piscator (~41% of the way through incubation; Lu et al., 2007), simply because females oviposit at a later embryonic stage in R. t. lateralis (Stage 26-27; Cai et al., 2007) than in X. piscator (Stage 25; Lu et al., 2009).The second phase identified in this study ends at an incubation stage (~80% of the way through incubation)surprisingly consistent with that reported for two snakes,R. t. lateralis (~81 of the way through incubation; Cai et al., 2007) and X. piscator (also ~81 of the way through incubation; Lu et al., 2009). This consistency, together with the similarity of the three phases among reptiles of different taxa (Shadrix et al., 1994; Du et al., 2001; Xu et al., 2004; Cai et al., 2007; Lu et al., 2009), suggests that the pattern of embryonic growth or yolk depletion during embryogenesis is common among reptiles.
Our data showed that differentiation did not complete until ~72% of the way through incubation (Day 30), and that the length of the last embryonic stage (Stage 40)accounted for ~28% (11.5/41.5) of incubation length(Figure 3). The mean dry mass of the smallest fully differentiated embryos (Stage 40) accounted for only~48% (58/120) of the hatchling mean dry mass (Figure 3). Taken together, these finding suggest that ~50%embryonic growth occurs in the last quarter of incubation in P. chinensis. Of the earlier 50% embryonic growth,~10%, ~15% and ~25% occur in the first, second and third quarters of incubation, respectively.
Acknowledgements This work was carried out in compliance with laws on animal welfare and research in China. We thank Guo-Hua Ding, Zhi-Hua Lin and Shu-Zhan Zhao for assistance during the research. For funding,we thank the National Natural Science Foundation of China (31470471).
杂志排行
Asian Herpetological Research的其它文章
- Investigating the Effectiveness of Road-related Mitigation Measures under Semi-controlled Conditions: A Case Study on Asian Amphibians
- Microhabitat Segregation of Parapatric Frogs in the Qinling Mountains
- Macroecological Patterns of Climatic Niche Breadth Variation in Lacertid Lizards
- Female-biased Dispersal of the Emei Moustache Toad(Leptobrachium boringii) under Local Resource Competition
- A New Species of the Genus Trimeresurus from Southwest China(Squamata: Viperidae)
- Resurrection of the Genus Leptomantis, with Description of a New Genus to the Family Rhacophoridae (Amphibia: Anura)
