Microhabitat Segregation of Parapatric Frogs in the Qinling Mountains
2019-03-27ShengnanYANGJianpingJIANGZhenhuaLUOXinYANGXiaoyiWANGWenboLIAOandJunhuaHU
Shengnan YANG, Jianping JIANG, Zhenhua LUO, Xin YANG, Xiaoyi WANG, Wenbo LIAO and Junhua HU*
1 Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China
2 Key Laboratory of Southwest China Wildlife Resources Conservation (Ministry of Education), China West Normal University, Nanchong, Sichuan 637009, China
3 School of Life Sciences, Central China Normal University, Wuhan 430079, China
Abstract Coexistence mechanisms for species with similar ecological traits and overlapping geographic distributions are basic questions in ecology and evolutionary biology. Speci fi c habitat requirements often limit distribution range as well as facilitate partitioning resource utilization in ecological similar species. Understanding niche segregation and differences in microhabitat utilization can contribute to identifying coexistence mechanisms between parapatric species. Feirana quadranus and F. taihangnica are two closely related frog species with parapatric geographic ranges and an elongated contact zone within the Qinling Mountains, which is an important watershed for East Asia. Here,we analysed the difference in microhabitat utilization between the two frog species and explored the key ecological factors that induced their microhabitat differentiation based on quadrats sampled in the contact zone. Our comparison of twenty environmental variables showed that both species used microhabitats with alkalescent warm water and gentle slope conditions. The principal component analysis indicated that climate-related variables, vegetation conditions, and river width were the important factors for microhabitat utilization of these species. These findings contribute to our understanding on the coexistence mechanisms of these two related and parapatric Asian mountain frog species. This study can also be helpful for identifying target habitats to conduct conservation actions and management strategies effectively in the face of environmental changes.
Keywords coexistence, contact zone, microhabitat utilization, niche segregation, Feirana, Qinling Mountains
1. Introduction
How to understand habitat utilization for different species is considered to be a crucial question in ecology and evolutionary biology, and also a critical component and the first step in species conservation and population management (Davidson-Watts et al., 2006; Wiens,1989). Various biotic and abiotic elements, such as food acquisition, predator avoidance, breeding, and especially environmental conditions (e.g. climate variability),contribute to habitat occupancy of species (Barber et al.,2008; Chailleux, 2001; Doligez and Clobert, 2002;Morosinotto et al., 2010). Environmental conditions are not homogeneous across the surface of the Earth, which lead to a situation that diversely combined factors are used by different species and demonstrate complicated and diversified habitat utilization by wild animals(Ficetola et al., 2017; Matthiopoulos et al., 2015). Given the influences on population dynamics, community structures, spatial and temporal variations of distributions,habitat utilization may have important ecological and evolutionary effects on species (Morris, 2003;Rosenzweig, 1981). Small changes on key environmental factors can alter habitat utilization of animals (Cain et al.,2008; Luo et al., 2014). It can be reflected in both macroevolutionary patterns, such as speciation and adaptive radiation, and micro-evolutionary strategies, such as characteristics of population density, persistence duration and community composition (Ficetola et al., 2018;Morris, 2003; Rosenzweig, 1981). Divergent resource utilization can affect the density and persistence of populations, while the difference in niche occupancy may alter the community structure, and lead to a reduction in competitive interactions and promote species coexistence(Ficetola et al., 2018; Rosenzweig, 1981).
Divergence in habitat utilization of congeneric species with overlapping ranges is a classic and important topic for understanding the mechanisms of speciation and species coexistence (Siemers and Schnitzler, 2004; Violle et al., 2011). Although niche conservatism indicates that strong competitive exclusion exists among closely related species and competition intensity increases with phylogenetic relatedness, some species do demonstrate similar distribution characteristics (Michalko and Pekár,2015; Violle et al., 2011). As proposed by Odum (1983),congeneric species with similar diets and foraging behaviours must diverge on their niches (e.g. spatial,trophic, or temporal niches) to facilitate coexistence from competitive exclusion (Davidson-Watts et al.,2006; Michalko and Pekár, 2015). Different habitat utilization is considered as one of the primary driving forces for ecological differentiation, with varied habitat preferences and utilization strategies between parapatric species allowing them to coexist (Pita et al., 2011).Despite habitat utilization and preferences having been investigated across different scales for a various species,many aspects of the complicated coexistence mechanisms remain unclear (Cain et al., 2008; Michalko and Pekár,2015).
Compared with other terrestrial vertebrates, amphibians have limited dispersal capability and show rather poor adaptive responses to environmental changes (Li et al.,2013; Wells, 2007), though many congeneric species possessing parapatric ranges (Fei et al., 2009). Therefore,amphibians are excellent models for exploring ecological separation and habitat utilization differentiation (Cloyed and Eason, 2017; Ficetola et al., 2018). Moreover, habitat change is one of the most important causes of amphibian population declines and local extinctions (Cloyed and Eason, 2017; Jiang et al., 2016; Silvano and Segalla,2010). Understanding the differences and similarities of microhabitat utilization can help to grasp the ecological differentiation of parapatric amphibian populations,enrich our knowledge on their population dynamics and community structures, and provide important insights into conservation plans (Bishop et al., 2012).
Feirana frogs in the family Dicroglossidae are distributed across the Qinling-Daba Mountains (QDM)(Fei et al., 2009; Hu and Jiang, 2018; Wang et al., 2009).Based on phylogeographic analyses, the ancestors of F.quadranus (swelled-vented frog) radiated in the QDM and then expanded to the Longmen-Micang-Daba Mountains,while F. taihangnica (Taihangshan swelled-vented frog) is native to the Qinling Mountains (Wang et al., 2009, 2012,2013). A substantially elongated contact zone between the two species is located in the central Qinling Mountains(Wang et al., 2009; Yang et al., 2011). It is suggested that weak environmental tolerance restricted the dispersal of F. quadranus further north, and interspecific competition was likely preventing the southward expansion of F. taihangnica (Hu and Jiang, 2018). Additionally,these parapatric species have similar natural histories and closely resemble each other ecologically and phenotypically, except for one significantly different trait of breeding males (i.e. F. taihangnica possesses a multitude of tiny granules on the swollen skin of the anal area but F. quadranus does not) (Fei et al., 2009). Thus,the two frog species present a remarkable opportunity to study the microhabitat segregation of congeneric species.In this study, we investigated the differences and similarities in microhabitat utilization between F.quadranus and F. taihangnica. Specifically, our aims were to examine: (1) what different characteristics exist on microhabitat utilization between these two species,(2) how they share homogeneous resources in local assemblages, and (3) what ecological factors influence their microhabitat utilization.
2. Materials and Methods
2.1. Study Area The study area is located in the Qinling Mountains (33°16′-34°05′N, 107°20′-109°04′E; Figure 1),which forms the transition area of subtropical and warm temperate zones in China and is an important watershed for East Asia (Zhang et al., 2017). The north slope of the Qingling Mountains has a warm temperate and semihumid climate because of the cooling dry air from the northwest, while the south slope has a subtropical and humid climate under the effects of the humid air from the southeast (Zhang et al., 2017). There are mainly three vegetation types along the altitudinal gradients (from low to high): subtropical evergreen and deciduous broadleaf forests, temperate deciduous broadleaf and subalpine needle leaf forests, and subalpine scrub meadow (Loucks et al., 2003). Our study was carried out within the region between the Wei River and the Hanshui River in Shaanxi and Gansu provinces, which locates in the contact zone of F. quadranus and F. taihangnica (Figure 1) (Hu and Jiang, 2018; Wang et al., 2009, 2013).
2.2. Data Collection Based on drainage conditions of the study area, the known distributions of Feirana species, and the geographical locations of main rivers in the QDM, seven main tributaries of the Wei River and the Hanshui River were chosen to conduct transect population investigations: the Fengyu, Shitou, Qianyou,Jiaoxi, Xushui, Hongyan and Yanchuan River (Figure 1).They contain different characteristics to be representative of most rivers in the overlapping region. Our field investigations were conducted from August to September in 2010. We searched for the individuals of target frog species along the transects, and 1 m × 1 m quadrats were set as sampling points to measure environment variables,with the individual location as the quadrat center. Then,referring to the previous study (Buckley and Jetz, 2007)and our field experience on target species, 20 environment variables, including air temperature, water temperature,humidity, water depth, pH, slope of riverbank, riparian canopy, distance to water, distance to road, current velocity, river gradient, river width, river width in flood season, sandy component, vegetation coverage,position on slope, and vegetation type of each quadrat were measured and recorded (Table 1). These variables may impose constraints on physiology, distribution, and survival of amphibians (Wells, 2007). We measured 48 quadrats for F. quadranus and 62 quadrats for F.taihangnica, respectively. Since there was only one quadrat that possessed both of the two species, we did not consider niche separation index in this research.

Figure 1 Geographical location of the study area and sampling quadrats for Feirana quadranus and F. taihangnica in the Qinling Mountains.
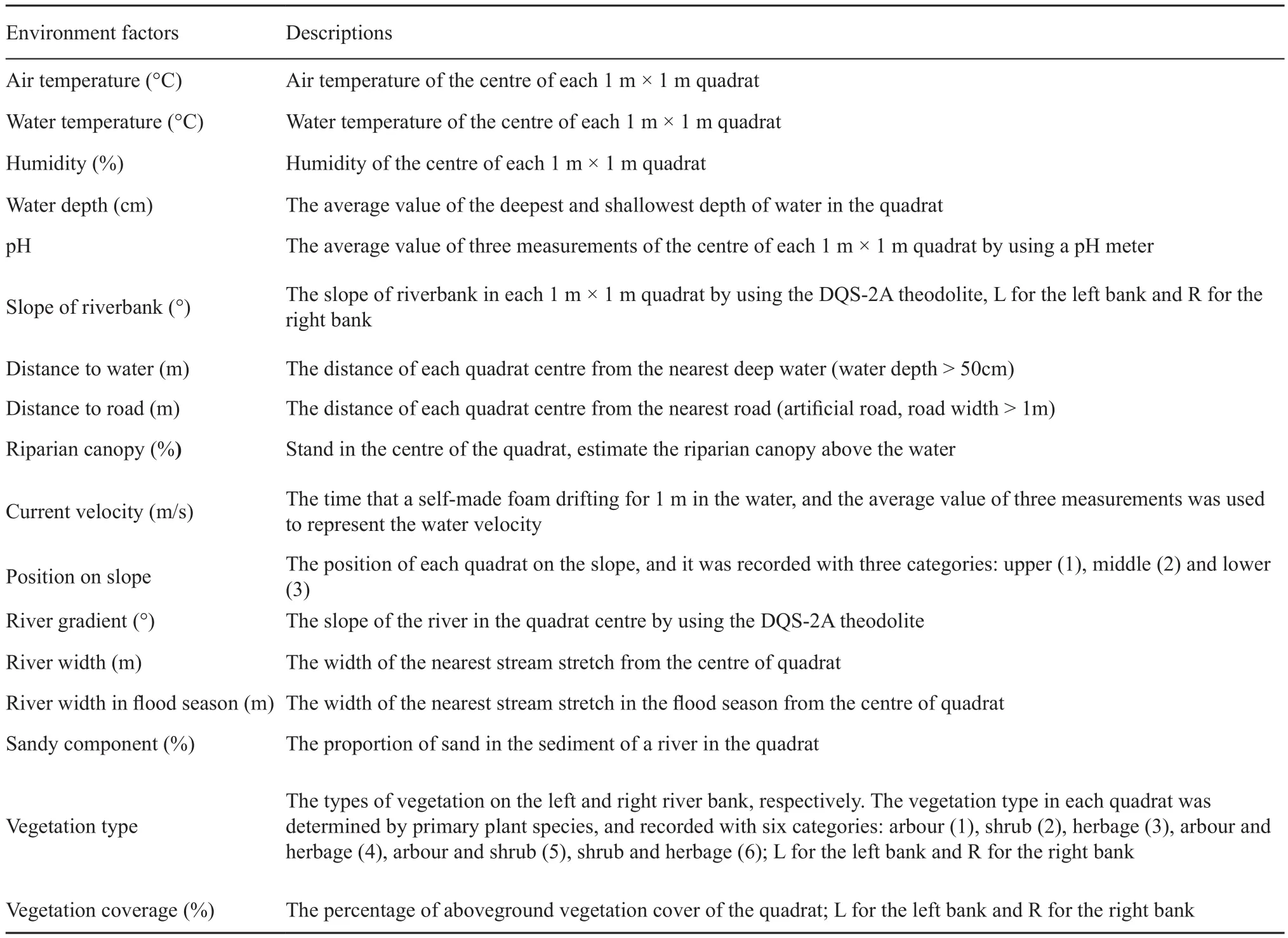
Table 1 Environment factors compiled to depict the microhabitat utilization of Feirana quadranus and F. taihangnica in the Qinling Mountains.
2.3. Statistical Analyses We tested normality of each continuous variable using a Kolmogorov-Smirnov test,and transformed the percentage data by arcsine/squareroots and made ln-transformations for variables that were non-normally distributed. Air temperature, slope of riverbank, distance to water, and river width were lntransformed. If the variable data met the assumptions of normal distribution and equal variance, we performed an independent-samples T test to compare its means between F. quadranus and F. taihangnica; otherwise, we used a Mann-Whitney U test. Additionally, we conducted Chisquare tests to assess whether there were significant differences of the categorical variables (i.e. position on slope and vegetation type) between species.
To reduce the potential collinearities among variables,we conducted Spearman’s correlation tests and excluded humidity data in the later analyses (highly correlated with air temperature, |r| > 0.70; Supplementary Materials, Table S1). Then, we conducted a principal component analysis (PCA) to analyse the effect of each ecological factor on microhabitat utilization of the frogs.Principle components (PCs) that had eigenvalues > 1.0 were extracted for subsequent analyses. We used the independent-samples T tests (for normal data) or the Mann-Whitney U tests (for abnormal data) to detect the interspecific differences of the PCs. All analyses were conducted using SPSS 16.0 (SPSS Inc., Chicago, IL).
3. Results
In general, both of the frog species used fl at habitats (river gradients: F. quadranus/F. taihangnica, respectively,7.09 ± 4.05°/6.18 ± 5.18°; mean ± SD; the same below),with relatively warm (water temperature: 18.72 ± 4.32°C/17.36 ± 3.72 °C) and alkalescent water (pH: 8.36± 0.63/8.13 ± 0.45; Table 2). F. quadranus occupied microhabitats with significant higher humidity, steeper slope of the left riverbank, and faster current velocity than those of F. taihangnica (T-tests, all P < 0.05; Table 2). More sandy component and less vegetation coverageof the right riverbank were found for F. quadranus than F. taihangnica (Mann-Whitney U tests, both P < 0.05;Table 2). No obvious interspecific difference existed in the position on slope or the vegetation type on both riverbanks (Table 2).
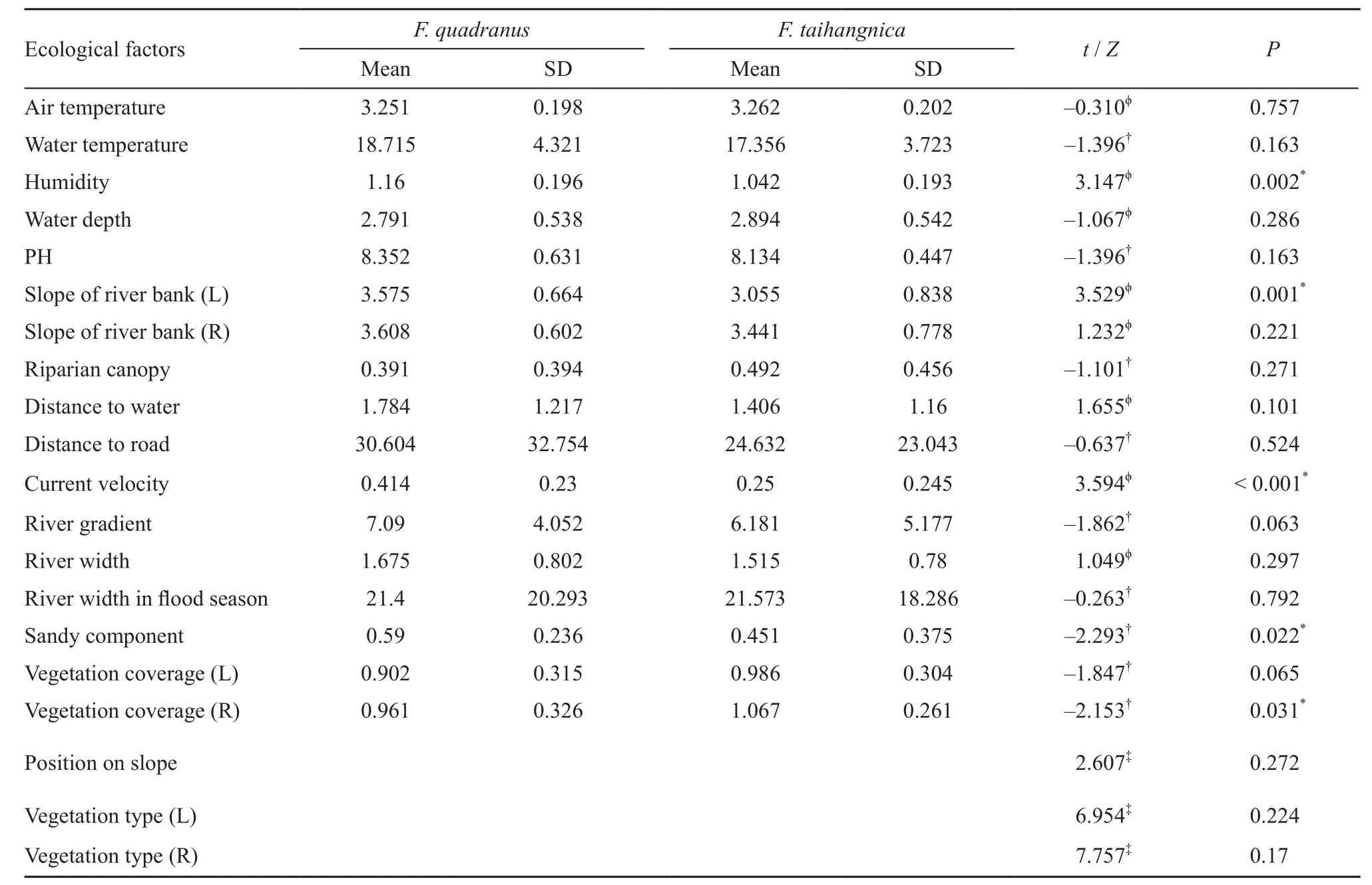
Table 2 Results of the Mann-Whitney U tests and Chi-square tests, showing the differences of the ecological factors affecting microhabitat utilization of Feirana quadranus and F. taihangnica.
Our PCA extracted seven PCs which explained 66%of the total environmental variance, with the 1st and 2nd PCs explaining 19.6% and 11.2%, respectively (Figure 2; Table 3). According to the component matrix, water temperature, air temperature, pH, humidity, riparian canopy, river width, river width in flood season, and vegetation coverage were the key factors that influenced microhabitat utilization (Table S2). The first five PCs contained the information in majority of the variables(55%) and were compared for the interspecific differences(Table S1). Limited differences were displayed in all the first five PCs between F. quadranus and F. taihangnica(Table 3).
4. Discussion
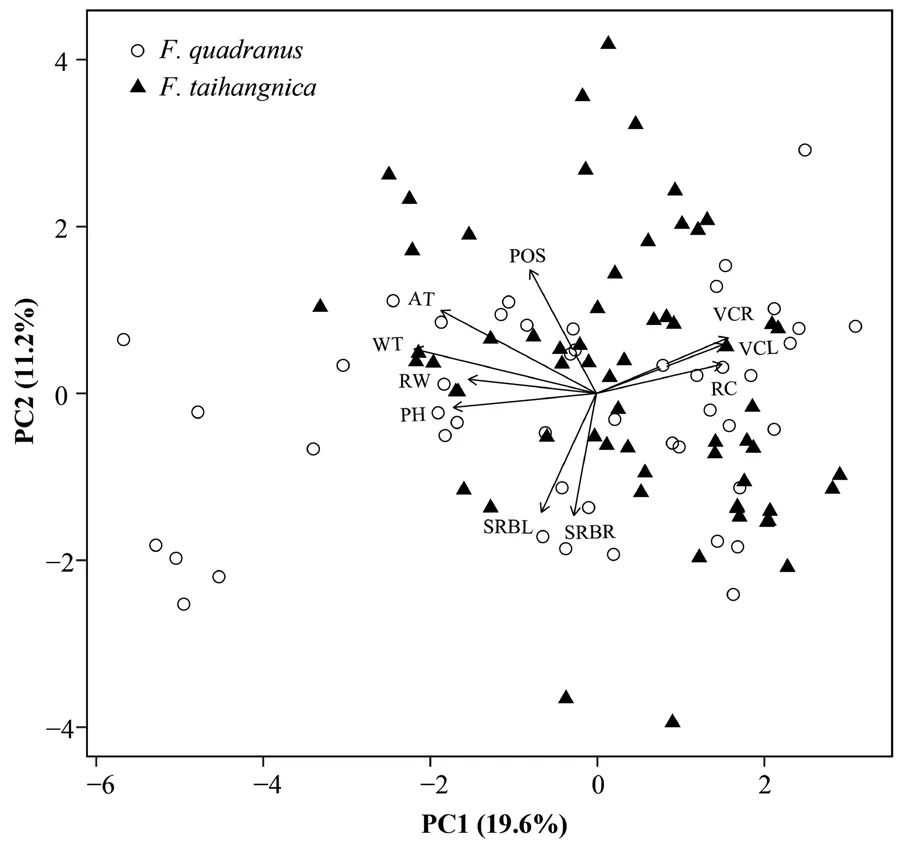
Figure 2 Microhabitat utilization of Feirana quadranus and F. taihangnica based on the first two PC axes. We displayed 10 variables with component loadings ≥ |0.50|, including WT (water temperature), AT (air temperature), pH, VCR (vegetation coverage of the right bank), RW (river width), VCL (vegetation coverage of the left bank), RC (riparian canopy), POS (position on slope),SRBR (slope of the right river bank) and SRBL (slope of the left river bank).
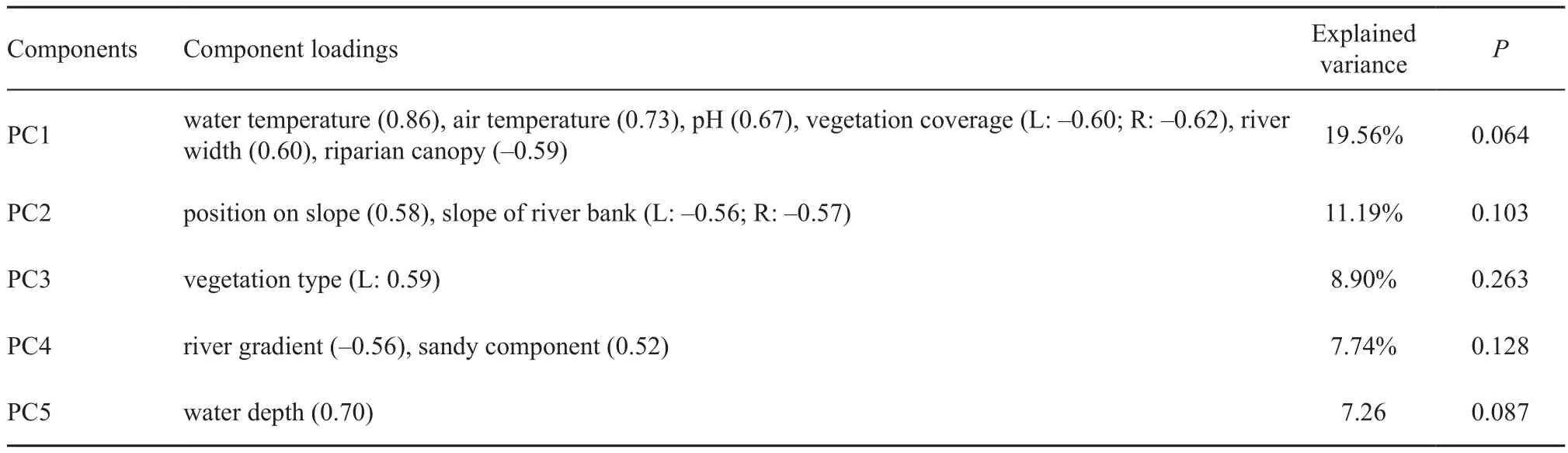
Table 3 Results of the principle component analysis (PCA) for environmental factors and the independent-samples T tests of PCs between Feirana quadranus and F. taihangnica, reporting the main contributors and no significant inter-species difference of the PCs. We only present variables with component loadings ≥ |0.50| and the fi rst fi ve PCs which contained the information in most of the variables.
Parapatric species usually occupy divergent niches,such as segregated microhabitat, to sustain species coexistence over a long term (Morris, 1996; Pita et al.,2011). Our results suggested differences in microhabitat utilization for the two parapatric Asian mountain frog species. In comparison with F. taihangnica, F.quadranus preferred humid microhabitats that were on steeper riverbanks, with less vegetation coverage,faster current velocities, and more sand component on riverbed. Since differences in habitat preference among individuals can lead to disparities on gene frequency within species, habitat complexity plays a major role in speciation process of congeneric species (Graham et al., 2004). Phylogeographic analyses showed that F.quadranus and F. taihangnica were diverged 4.6 million years ago (Wang et al., 2009). Based on the deduced historical expansion, F. taihangnica is considered to be a native species while F. quadranus is probably an external competitor within their overlapping ranges in the central Qinling Mountains (Hu and Jiang, 2018; Wang et al., 2012). Specific habitat requirements often limit distributions of animals as well as facilitate isolation of ecological requirements (Siemers and Schnitzler, 2004).It is suggested that niche differentiation can lead species to complement one another in mixture and, hence, result in better utilization of available resources (Odum, 1983).The difference of microhabitat utilization between the two frog species might demonstrate a result of variable individual preferences on environmental conditions after a long period of evolution.
Since the two congeneric frog species originate from different areas and have some distinct phenotypic features(Fei et al., 2009; Wang, et al., 2009), it is expected that they can occupy different microhabitats in the contact zone to reduce interspecific competition and maintain species coexistence. However, despite slight differences on microhabitat utilization between the two frog species,interspecific divergence on ecological factors generally influencing microhabitat utilization was lacking in this study. Ecological requirements of a species often reflect the balance between the effects of diversified intraspecific competition and constrained interspecific competition,which can not only promote coexistence of different species but also hinder habitat types the animal use(Cadotte and Tucker, 2017; May field and Levine, 2010).Additionally, the niche filtering theory indicates that, in a given environment, the profitale traits are selected by local conditions and some species could be filtered out because they cannot cope with local elements (Michalko and Pekár, 2015). It might then lead to a coexistence of species with similar ecological needs and characteristics.This means that competition can sometimes eliminate taxa with distantly genetic relationships or differently ecological traits (May field and Levine, 2010). The limited segregation of microhabitat utilization in this study supported the expectation that closely related species might have similar ecological niches due to the suites of environments (Hu et al., 2016; Warren et al., 2014).
Leibold and Mcpeek (2006) suggested that weak interspecific competitions would not make parapatric species to narrow their current niches. This deduction is partly supported by the absence of extensive microhabitat segregation between F. quadranus and F. taihangnica,which, might indicate that the available microhabitat resources have not been completely occupied by these frogs in the Qinling Mountains. On the other hand,the phenomenon of relatively large variations in some ecological factors (e.g. river gradients, pH, and water temperature) may lead to a speculation that the frogs are not sensitive to those environmental variables.Microhabitat utilizations investigated by this study are indeed only a part of the realized niches of these frogs. Even though no obvious ecological microhabitat divergence was detected, niche divergence at other levels,such as in their prey selection or reproductive resources,may be at play instead. Remarkably, considering the amount of environmental variables used in this study,the number of sampling quadrats was relatively small.Different types of data for the environmental factors,such as temperature-related variables (continuous data),vegetation conditions (categorical data), and river conditions (percentage data), were included, which could lead to a low interpretation of the PCA and, thus, might cause potential uncertainties of the results. Therefore, we suggest that more extensive surveys should be carried out for understanding microhabitat utilization of these frogs.
Our study indicated that discrepant preferences on environmental conditions are likely to cause microhabitat segregation between congeneric and parapatric species.Several mechanisms of species coexistence can interact,for example, niche partitioning on one dimension may facilitate niche filtering on another (Michalko and Pekár,2015). When availabilities are high for some types of resources, specific preference rather than competition may play roles in microhabitat utilization. However,due to the limited resources, filtering effectiveness on the environmental factors could obviously influence coexistence of ecologically similar species (May field and Levine, 2010).
Amphibians have highly water-permeable skins and are sensitive to variations on ambient environments (Wells,2007). Environmental changes can pose intense impacts on amphibian species. For example, sharp changes in temperature can affect their survival and reproductive processes (Benard, 2015). Studying microhabitat segregation between parapatric amphibian species can be helpful for understanding their ecological niche separations, identifying target habitats, and then providing scientific support to establish efficient management policies and conservation plans for those vulnerable species (Bishop et al., 2012). In this study, microhabitat utilizations for both of the species were highly depended on vegetation and river conditions. This suggested that keeping relatively high vegetation coverage and avoiding encroachment on natural rivers should be effective to protect them. Nevertheless, interspecific comparisons on other ecological traits (e.g. dietary, predator, and reproductive strategy) should be carried out in the future to further understand the coexistence mechanisms of these two parapatric species and identify the protection keystones.
Acknowledgements This study was supported by National Natural Science Foundation of China (31572290,31770568, and 31770427), Youth Innovation Promotion Association CAS (2015304), National Key Research and Development Plan (2016YFC0503303), China Scholarship Council (No. 201706775008), and the project from Qinghai Provincial Communication Department(31118022). We thank M. B. C Andrade for the valuable discussion on this research. All animal handlings were in accordance with the Law of the People’s Republic of China on the Protection of Wildlife and approved by the Animal Care Committee of Chengdu Institute of Biology,Chinese Academy of Sciences.

,)erutarepmet ria(TAgnidulcni ,acingnahiat .F dna sunardauqanarieF fo noitazilitu tatibah ni devlovni srotcaf lacigoloce 02 eht fo stnei cif feoc noitalerroc knar s’namraepS esiwriaP 1S elbaT ot ecnatsid( RD ,)retaw ot ecnatsid( WD ,)yponacnairapir( CR ,)knab thgir eht fo epols( RBRS ,)knab revir tfel eht fo epols( LBRS ,Hp ,)ecnatsid retaw( DW ,ytidimuh ,)erutarepmet retaw(TW ,)knab tfel eht no epyt noitategev(LTV ,)tnenopmoc ydnas( CS ,)nosaes do olf ni htdiw revir( SFWR ,)htdiw revir( WR ,)tneidarg revir( GR ,)epols no noitisop(SOP ,)yticolev tnerruc( RC ,)daor .)knab thgir eht no egarevoc noitategev(RCV dna )knab tfel eht no egarevoc noitategev(LCV ,)knab thgir eht no epyt noitategev( RTV RCVRTVLCVLTVCSSFWRWRGRSOPVCRDWDCRRBRSLBRSHPDWytidimuHTWTA —TA—96.0TW #—14.0-28.0-ytidimuH—31.0-41.071.0DW —70.012.0-26.033.0HP —41.030.0-81.000.001.0-LBRS —53.011.061.091.000.011.0-RBRS —31.0-01.0-62.0-80.0-32.054.0-53.0-CR —20.080.0-41.0-21.0-92.0-62.001.0-02.0-WD —10.022.031.0-91.0-02.0-41.0-90.0-90.0-50.0RD —60.0-12.030.0-22.081.002.001.0-52.040.060.0-VC —41.0-03.001.010.0-10.0-31.0-80.030.071.0-24.083.0SOP —50.0-12.040.020.030.090.032.060.030.0-50.000.040.0GR —70.040.011.070.021.0-64.0-71.010.0-32.070.051.0-53.042.0WR —94.033.0-21.062.0-90.021.0-53.0-30.041.0-41.060.024.0-83.093.0SFWR —11.0-40.0-40.0-70.0-70.0-70.0-70.0-02.0-40.0-51.070.0-12.0-51.010.030.0-CS —60.031.0-30.0-00.041.010.0-20.0-10.0-80.050.030.061.090.020.040.010.0LTV —01.022.0-80.0-70.0-01.0-30.0-11.0-22.070.092.060.0-12.0-70.0-90.051.032.0-52.0-LCV —20.044.030.0-20.0-70.0-70.0-52.010.0-10.0-01.051.0-71.0-20.092.090.0-51.0-32.012.0RTV —81.0-05.000.090.0-20.0-30.0-01.002.0-81.0-12.030.071.031.0-32.0-82.0-90.010.0-13.0-71.0-RCV ,#.noitazilitu tatibah foACP eht ni dedulcxe selbairav :etoN
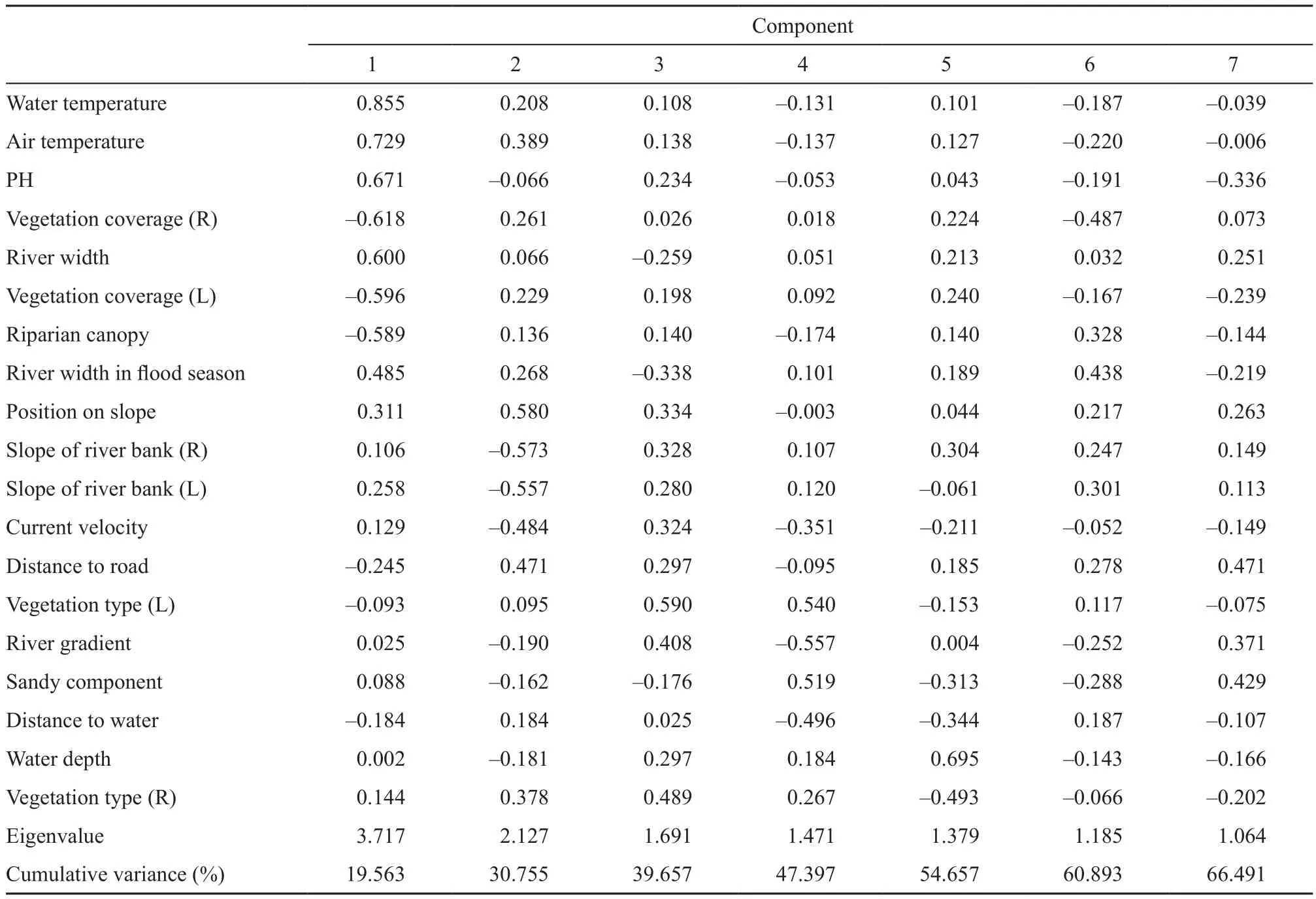
Table S2 Results of the principle component analysis (PCA), explaining variations, eigenvalues, and factor loadings for the seven principal components with eigenvalues > 1.
杂志排行
Asian Herpetological Research的其它文章
- Investigating the Effectiveness of Road-related Mitigation Measures under Semi-controlled Conditions: A Case Study on Asian Amphibians
- Embryonic Growth and Yolk Depletion during Incubation in the Chinese Skink, Plestiodon chinensis
- Macroecological Patterns of Climatic Niche Breadth Variation in Lacertid Lizards
- Female-biased Dispersal of the Emei Moustache Toad(Leptobrachium boringii) under Local Resource Competition
- A New Species of the Genus Trimeresurus from Southwest China(Squamata: Viperidae)
- Resurrection of the Genus Leptomantis, with Description of a New Genus to the Family Rhacophoridae (Amphibia: Anura)
