Utility of linked color imaging for endoscopic diagnosis of early gastric cancer
2019-03-22ToshihisaFujiyoshiRyojiMiyaharaKoheiFunasakaKazuhiroFurukawaTsunakiSawadaKeikoMaedaTakeshiYamamuraTakuyaIshikawaEizaburoOhnoMasanaoNakamuraHirokiKawashimaMasatoNakaguroMasahiroNakatochiYoshikiHirooka
Toshihisa Fujiyoshi, Ryoji Miyahara, Kohei Funasaka, Kazuhiro Furukawa, Tsunaki Sawada, Keiko Maeda,Takeshi Yamamura, Takuya Ishikawa, Eizaburo Ohno, Masanao Nakamura, Hiroki Kawashima,Masato Nakaguro, Masahiro Nakatochi, Yoshiki Hirooka
Abstract BACKGROUND Linked color imaging (LCI) is a method of endoscopic imaging that emphasizes slight differences in red mucosal color.AIM To evaluate LCI in diagnostic endoscopy of early gastric cancer and to compare LCI and pathological findings.METHODS Endoscopic images were obtained for 39 patients (43 lesions) with early gastric cancer. Three endoscopists evaluated lesion recognition with white light imaging(WLI) and LCI. Color values in Commission Internationale de l'Eclairage (CIE)1976 L*a*b* color space were used to calculate the color difference (ΔE) between cancer lesions and non-cancer areas. After endoscopic submucosal dissection,blood vessel density in the surface layer of the gastric epithelium was evaluated pathologically. The identical region of interest was selected for analyses of endoscopic images (WLI and LCI) and pathological analyses.RESULTS LCI was superior for lesion recognition (P < 0.0001), and ΔE between cancer and non-cancer areas was significantly greater with LCI than WLI (29.4 vs 18.6, P <0.0001). Blood vessel density was significantly higher in cancer lesions (5.96% vs 4.15%, P = 0.0004). An a* cut-off of ≥ 24 in CIE 1976 L*a*b* color space identified a cancer lesion using LCI with sensitivity of 76.7%, specificity of 93.0%, and accuracy of 84.9%.CONCLUSION LCI is more effective for recognition of early gastric cancer compared to WLI as a result of improved visualization of changes in redness. Surface blood vessel density was significantly higher in cancer lesions, and this result is consistent with LCI image analysis.
Key words: Linked color imaging; Early gastric cancer; Endoscopic submucosal dissection; Vessel density; Color difference
INTRODUCTION
The mortality of gastric cancer has been decreasing worldwide, but there are still more than 800000 deaths due to this disease. Gastric cancer is the third leading cause of death, followed by lung and colorectal cancer, in overall mortality worldwide[1]. In Japan, the morbidity of gastric cancer ranks first in males and third after breast and colorectal cancer in females[2]. Since advanced gastric cancer has a poorer prognosis,early detection and treatment is required to overcome the disease.
Endoscopy is an essential modality for detection of early gastric cancer. Recent advances and innovations in endoscopic devices have provided scopes with a high pixel count, a smaller diameter, and various optical technology. In particular, imageenhanced endoscopy (IEE) that uses imaging and analysis of wavelengths of visible light with illumination has a key role in detection of early gastrointestinal cancer.Among IEE methods, narrow-band imaging (NBI) and blue light imaging (BLI) are commonly used. NBI is effective for observation of capillaries on mucosal surfaces through emission of narrow band light[3-5]and can be used for qualitative diagnosis(differentiation of cancer from non-cancer tissues, and the extent of cancer)[6-8]in the oropharynx and hypopharynx[9], esophagus[10,11]and stomach. BLI allows observation of surface capillaries[12], in magnified observation, there is little difference in the diagnostic performances of BLI and NBI[13].
A new IEE method, referred to as linked color imaging (LCI), with an endoscopic light source was recently introduced by Fujifilm Corp. (Tokyo, Japan). LCI observation is interchangeable with white light imaging (WLI)/BLI with just one push of a button. LCI images are created by emission of white light and narrow-band shortwave light, and processing images to make a well-separated red area. The most important wavelengths for diagnosis of normal mucosa and atrophic mucosa are concentrated around the red color. LCI emphasizes slight differences in the “red”color of the mucosa by image processing, including enhancement of differences in chroma and hue of the red mucosal color. As a result, red mucosa is visualized as redder and white mucosa as whiter. LCI has a color shade that is closer to WLI than NBI and BLI and processes images to separate the mucosal color more effectively;therefore, LCI is a suitable imaging method to identify early gastric cancer.
The utility of LCI for diagnosis of Helicobacter pylori gastritis has been well described[14], but there are few studies of LCI for diagnosis of gastrointestinal cancer and, to our knowledge, parallel analyses of LCI, WLI, and pathology samples have not been reported. Therefore, the objectives of this study are to evaluate the utility of LCI for endoscopic diagnosis of early gastric cancer and to examine if pathological findings can explain the changes in red shade in LCI.
MATERIALS AND METHODS
Materials
The subjects were 39 patients (43 lesions) with early gastric cancer who underwent endoscopic submucosal dissection (ESD) of the stomach. WLI and LCI at the same time point were used for comparison of endoscopic images. All procedures were performed in the Department of Gastroenterology and Hepatology, Nagoya University Graduate School of Medicine from September 2016 to January 2018. This study was conducted after approval by the ethics committee of Nagoya University,and was performed in accord with the Helsinki Declaration. All subjects gave informed consent to participation after receiving a written explanation of the study.
Methods
Equipment and setting: An ELUXEO™ system (video processor VP-7000, light source BL-7000) and a Video Endoscope EG-760Z (Fujifilm) were used in the study. In WLI,the structure emphasis was set at H+1+3 (SE = Hi, DH = +1, DL = +3). In LCI, the structure emphasis was set at A6 and the color emphasis was set at C1. Endoscopic images were stored as uncompressed TIFF files. Images were 1280 × 1024 pixels and RGB 24-bit color.
Image assessment by endoscopists: WLI and LCI images were taken before endoscopic therapy and not magnified in the study. Lesions were imaged with WLI and LCI at the same timepoint, and the images were placed in a file in random order.These images were then evaluated by three endoscopists who routinely perform endoscopic therapy and are board-certified fellows of the Japan Gastroenterological Endoscopy Society. The evaluators were blinded to clinical data and pathological results. Each evaluator viewed one WLI and one LCI image simultaneously and assessed which image was superior for lesion recognition using a 5-point Likert scale:+2, very much; +1, somewhat; 0, undecided; -1, not really; -2, not at all. The same display was used for assessment. The evaluators assessed images alone without discussion with other evaluators.
Color assessment of endoscopic images: Two regions of interest (ROI, diameter 24 pixels) were picked from each lesion. One was from the cancer lesion and the other from surrounding non-cancer mucosa. Non-cancer mucosa was normal mucosa without ulceration or other specific changes. After ESD, the identical ROI was marked in the ESD resected sample and evaluated pathologically. ROI color in the endoscopic image was assessed using Commission Internationale de l'Eclairage (CIE) 1976 L*a*b*color space. This is a three-dimensional space for presenting a color with axes of L*(from black to white; white is highest), a* (from green to red; red is highest), and b*(from blue to yellow; yellow is highest). The difference between two colors (color difference) was approximated by the distance in the CIE 1976 L*a*b*color space[15].L*a*b* values of the ROI in cancer and non-cancer tissues were measured using Adobe Photoshop CS4 (Adobe Systems Inc., San Jose, CA, United States) to estimate the color difference (Figure 1).
Histopathological assessment: Resected gastric tissue was fixed in 10% neutral buffered formalin and embedded in paraffin. Routine hematoxylin and eosin staining(Figure 2A) and immunohistochemical staining were performed using 3-μm sections.CD31 immunohistochemistry (Figure 2B) was used to highlight the blood vessel wall,using a commercial primary antibody (clone JC/70A, 1:1200; Dako, Glostrup,Denmark). The blood vessel area and its proportion of the total area were analyzed based on photomicrographs for each ROI at ×100 magnification. An area of 350 μm depth from the surface of the mucosa was trimmed on the photomicrograph (Figure 2C) and the blood vessel area was defined as the sum of the CD31-positive area and the area encircled by CD31-positive endothelial cells (Figure 2D and E). Recognition and calculation of the positive area was performed automatically using image analysis software (WinRoof v.3.9.0, Mitani Co., Tokyo, Japan).
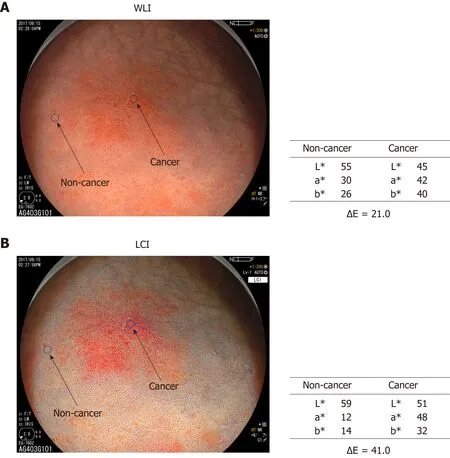
Figure 1 Color values and color differences between cancer and non- cancer areas. A region of interest(diameter: 24 pixels) was established in each lesion. Color values (L*, a*, b*) were measured using Adobe Photoshop CS4 to estimate color differences (ΔE) between cancer and non-cancer areas. The results are shown in Table 2.WLI: White light imaging; LCI: Linked color imaging.
Statistical analysis: Statistical analysis were performed using SPSS v.22.0 for Windows (IBM Japan, Tokyo, Japan) and SAS 9.4 (SAS Institute, Inc.). Variables are expressed as medians (range). Differences in paired continuous variables were compared by Wilcoxon signed-rank test, and differences in independent continuous variables were compared by Wilcoxon rank sum test. ROC curves were created with values in CIE 1976 L*a*b* color space to differentiate between cancer and non-cancer lesions. Diagnostic performance was estimated based on the area under the curve(AUC), cut-off values, sensitivity and specificity. The significance level was set at a two-tailed P value < 0.05.
RESULTS
The background and clinicopathological characteristics of the patients and lesions are shown in Table 1. Most lesions were differentiated tissue type (well or mod) and intramucosal carcinoma. All cases were completely resected with a free margin.Evaluation by three endoscopists indicated that LCI was superior to WLI for recognition of lesions of early gastric cancer (P < 0.0001). This finding being particularly strong for flat/depressed lesions compared to elevated lesions (Figure 3).The color difference (ΔE) between cancer and non-cancer areas was significantly higher in LCI than in WLI [29.4 (9.4-63.9) vs 18.6 (5.1-38.4), P < 0.0001] (Table 2) and thus LCI could differentiate a cancer lesion from a non-cancer area better than WLI. In LCI, using a cut-off from ROC curves of ≥ 24 for detection of a cancer lesion, the diagnostic performance had sensitivity of 76.7%, specificity of 93.0%, positive predictive value (PPV) of 91.7%, negative predictive value (NPV) of 80.0%, and accuracy of 84.9%. In pathological assessment, the blood vessel density in the surface layer was significantly higher in cancer lesions than in non-cancer areas [5.96% (2.17-17.08) vs 4.15% (1.71-8.22), P = 0.0004] (Figure 4). Surface blood vessel density exceeding 9% was only found in cancer lesions. The relationship between the value in CIE 1976 L*a*b* color space and blood vessel density in WLI and LCI is shown in Figure 5.
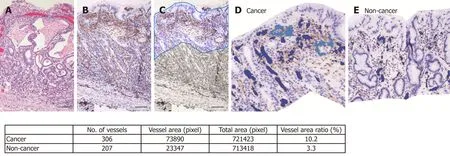
Figure 2 An example of blood vessel density calculation by imaging analysis software. A: Hematoxylin and eosin staining of a cancer lesion (case 43, original magnification × 100, scale bar 100 μm). B: CD31 immunohistochemistry highlights blood vessels. C: A depth of 350 μm was trimmed manually. Automatically recognized blood vessels are colored in the cancer lesion (D) and non-cancer area (E). The calculated blood vessel area and ratio in this case are shown in the table.
DISCUSSION
The results of this study show the utility of LCI for screening endoscopy of early gastric cancer and the concordant change in blood vessel density with redness in the LCI image. To our knowledge, this is the first study to compare objective color evaluation of early gastric cancer using the CIE 1976 L*a*b* color space in LCI with a pathological analysis of blood vessel density. This study showed that the large color difference in LCI may be due to the surface blood vessel density. The key results of the study were as follows: LCI recognized early gastric cancer lesions more effectively than WLI; LCI gave better contrast than WLI for the color difference between cancer lesions and the surrounding non-cancer area; an a* cutoff ≥ 24 for the value in CIE 1976 L*a*b* color space had good sensitivity and specificity for diagnosis of early gastric cancer; and surface blood vessel density in cancer lesions was significantly higher than that in non-cancer areas.
Based on an assessment by three endoscopists, LCI recognized early gastric cancer lesions more easily than WLI. A previous study also indicated that LCI gave images that were easier to review than WLI for both experts and non-experts in > 70% of patients with early gastric cancer[16]. Endoscopists attempt to find a lesion using the ruggedness of the mucosa and changes in color shade when observing inside the stomach, but this requires appropriate technology to show slight differences in color shade. Most lesions with slight differences in color shade can be detected by WLI using innovations such as adjusted air volume and progression in lesions. However, it is difficult to recognize a flat lesion without mucosal ruggedness or a slightly recessed lesion. To recognize such lesions, a change in color shade between a cancer lesion and a non-cancer area is important.
Subjective descriptions of color are commonly used in conventional endoscopic findings, and consequently, it is difficult to make an objective evaluation. In this study, CIE 1976 L*a*b* color space was used to assess the color objectively. The results showed that LCI gave better contrast than WLI for the color difference between cancer and non-cancer areas. These results are consistent with the assessment that LCI is a better imaging method than WLI for recognition of early gastric cancer, especially for flat and recessed lesions, which are likely to be assessed based on color difference,rather than on mucosal ruggedness.
The relationship of cancer and the microvascular architecture has been widely examined[17-20], with reports of an irregular pattern and heterogeneity of the vasculature, and microvascular dilatation. In our study, we used non-magnified endoscopy to analyze the gross vascular effect, rather than the single vessel architecture. Since irregular running and vessel dilation can increase the blood vesseldensity, our results are closely related to previous studies using magnified endoscopy. The endoscopic color of early gastric cancer is widely distributed from strong reddening to fading. Some previous studies have mentioned the role of the number of capillaries in defining the color of the mucosa[21-23]. An increase in number,as might be assumed, can increase the blood vessel density. Furukawa et al[24]also mentioned the significance of the blood vessel density. Given the depth that endoscopic light can reach, we examined blood vessel density from the surface to a depth of 350 μm[25]. For as objective an evaluation as possible, the blood vessel density was assessed using pathological imaging analysis software (WinRoof v.3.9.0). This analysis showed that the blood vessel density was significantly higher in cancer lesions than in non-cancer areas, and exceeded 9% in all cancer lesions in the study.These results are consistent with findings showing that the blood vessel density in cancer lesions is higher than that in non-cancer areas in differentiated gastric cancer[24].
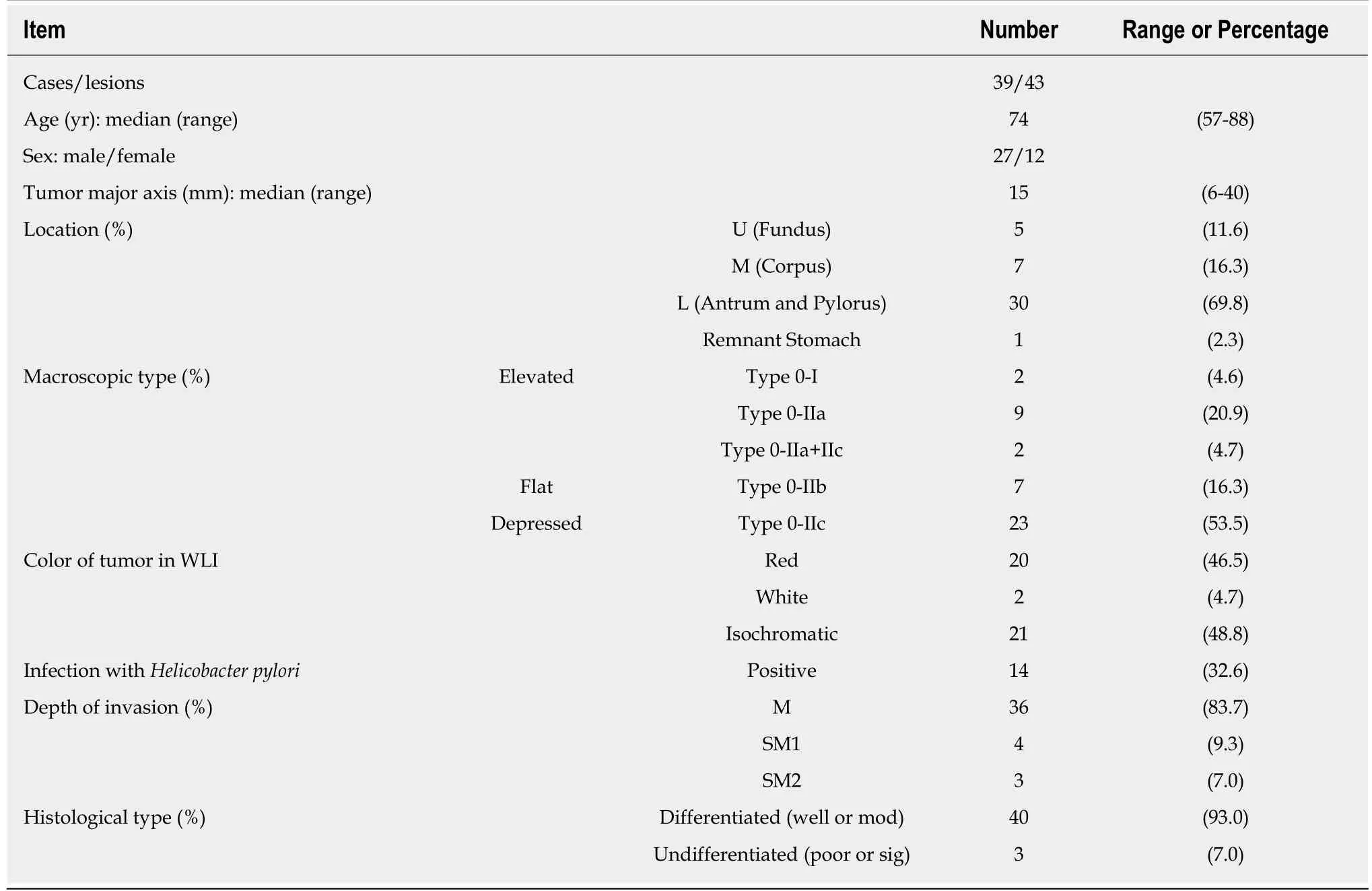
Table 1 Characteristics of the patients and tumors
Changes in redness between cancer and non-cancer areas in pathological images were assessed objectively by estimating a value in CIE 1976 L*a*b* color space. In LCI,ROC curves drawn using this value estimated an AUC of 0.86 at an a* cut-off value of 24. Values of ≥ 24 and < 24 indicated cancer and non-cancer areas, respectively, with a diagnostic sensitivity of 76.7%, specificity of 93.0%, PPV of 91.7%, NPV of 80.0%, and accuracy of 84.9%. We believe that our finding can lead to improved diagnosis of cancer lesions, based on objective evaluation using CIE 1976 L*a*b* color space. If these color values are evaluable in real time, artificial intelligence may permit automatic recognition and diagnosis of lesions.
This study has several limitations. First, it was a single-center and small-scale study. Second, the subjects were patients with early gastric cancer who underwent ESD, and consequently, most had differentiated intramucosal carcinoma. To include patients with undifferentiated cancer and early gastric cancer invading the submucosa, patients who underwent surgical resection would need to be added.Third, in this study, we focused only on cancer, and we did not evaluate inflammation or adenoma. Fourth, because recognition of WLI or LCI is obvious for expert endoscopist, bias is inevitable in the evaluation. To overcome this bias, we used objective color evaluation based on CIE 1976 L*a*b* color space.
In conclusion, LCI was useful for recognition of early gastric cancer lesions because this method provides good contrast in color differences between lesions andsurrounding tissue. Blood vessel density from the surface to a depth of 350 μm was higher in cancer lesions than in non-cancer areas, and LCI clearly shows this feature as a change in redness.
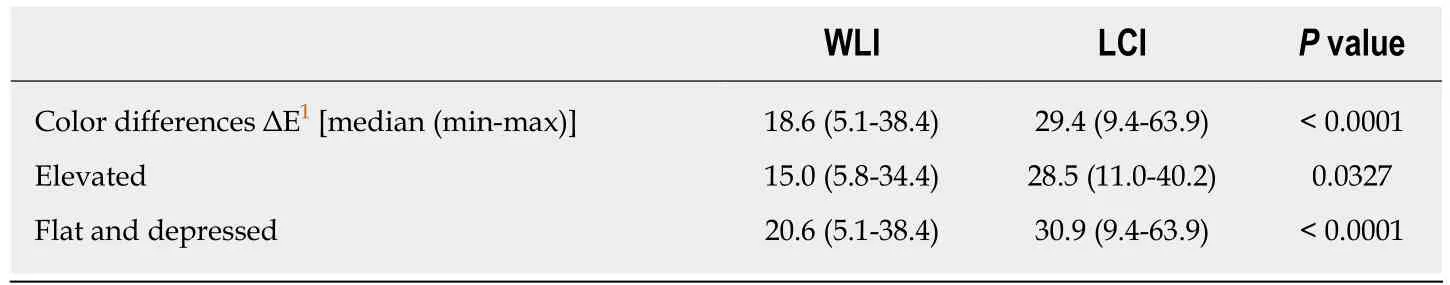
Table 2 Color differences between cancer and non-cancer areas in white light imaging and linked color imaging
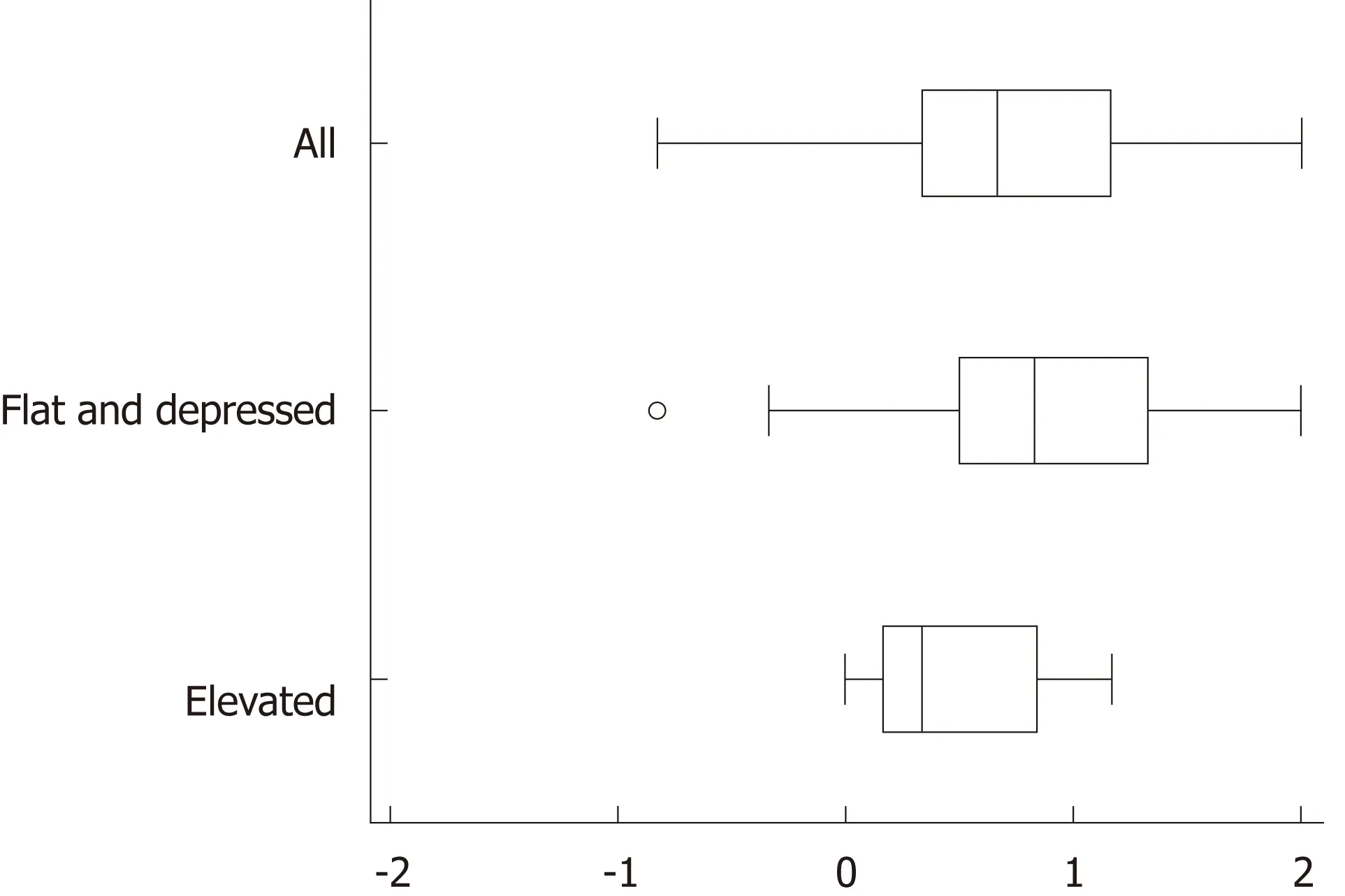
Figure 3 Recognition of early gastric cancer lesions using a Likert scale. Linked color imaging was superior to white light imaging for recognition of early gastric cancer lesions based on evaluations by three endoscopists (P < 0.0001). This effect was stronger for flat/depressed lesions than for protruding lesions.
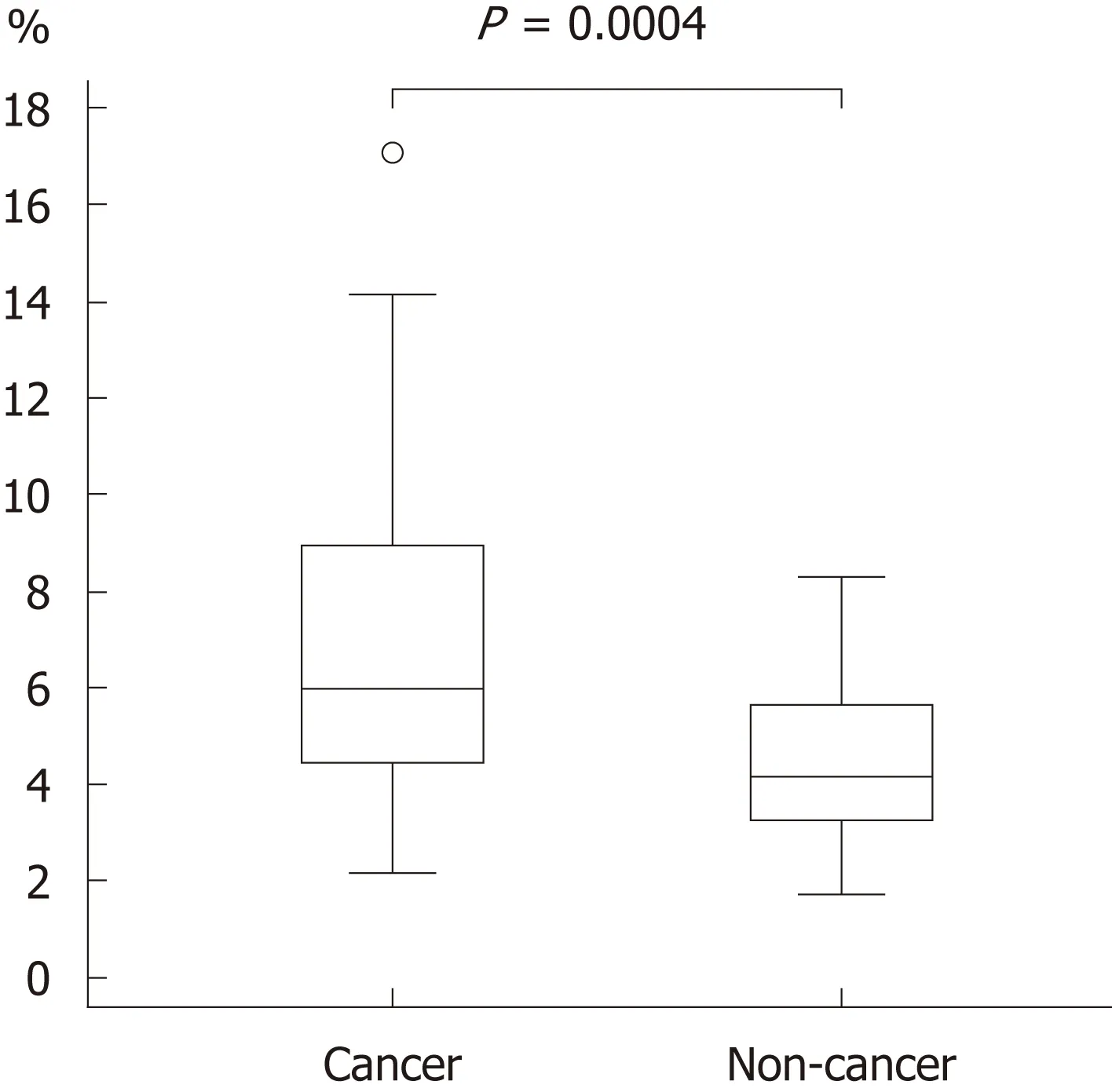
Figure 4 Blood vessel densities from the surface layer to a depth of 350 μm in cancer and non-cancer areas. The blood vessel density was significantly higher in cancer than in non-cancer areas [median (range): 5.96% (2.17-17.08) vs 4.15% (1.71-8.22), P = 0.0004].
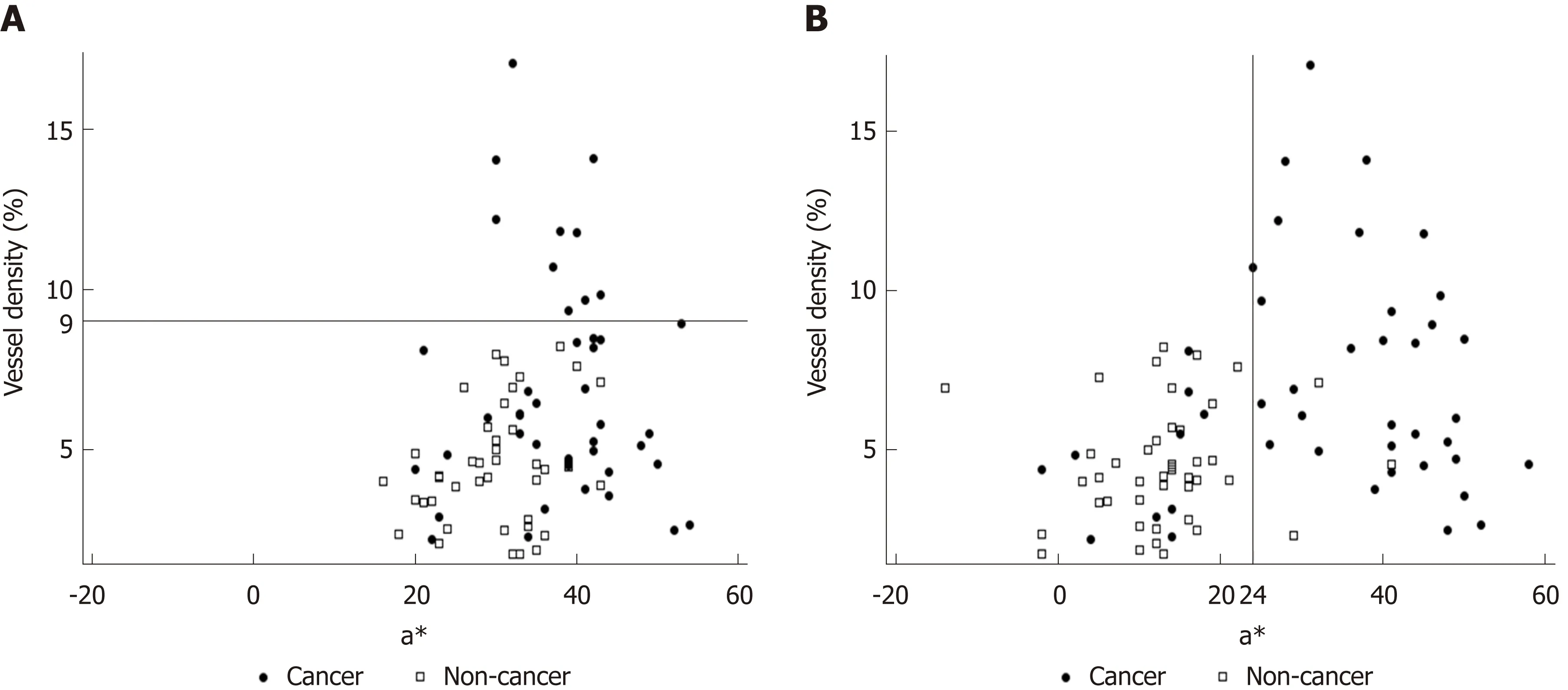
Figure 5 Relationship of a* in Commission lnternationale de l'Eclairage 1976 L*a*b* color space (X axis) with blood vessel density from the surface layer to 350 μm (Y axis) using (A) white light imaging (WLl) and (B) linked color imaging (LCl). Cancer and non-cancer areas were better differentiated with LCI compared to WLI. The blood vessel density was ≥ 9% in all cancer lesions. Using a cut-off value of ≥ 24 for cancer lesions, the diagnostic performance with LCI had sensitivity of 76.7%, specificity of 93.0%, positive predictive value of 91.7%, negative predictive value of 80.0%, and accuracy of 84.9%. WLI: White light imaging; LCI:Linked color imaging.
ARTICLE HIGHLIGHTS
Research background
Linked color imaging (LCI) emphasizes slight differences in the “red” color of the mucosa by image processing, including enhancement of differences in chroma and hue of the red mucosal color.
Research motivation
The utility of LCI for diagnosis of Helicobacter pylori gastritis has been well described, but there are few studies of LCI for diagnosis of gastrointestinal cancer and, to our knowledge, parallel analyses of LCI, white light imaging (WLI), and pathology samples have not been reported.
Research objectives
The objectives of this study are to evaluate the utility of LCI for endoscopic diagnosis of early gastric cancer and to examine if pathological findings can explain the changes in red shade in LCI.
Research methods
Three endoscopists evaluated lesion recognition with WLI and LCI. Color values in Commission Internationale de l'Eclairage (CIE) 1976 L*a*b* color space were used to calculate the color difference (ΔE) between cancer lesions and non-cancer areas. After endoscopic submucosal dissection, blood vessel density in the surface layer of the gastric epithelium was evaluated pathologically. The identical region of interest was selected for analyses of endoscopic images(WLI and LCI) and pathological analyses.
Research results
LCI gave better contrast than WLI for the color difference between cancer lesions and surrounding non-cancer tissue; an a* cutoff ≥ 24 for the value in CIE 1976 L*a*b* color space had good sensitivity and specificity for diagnosis of early gastric cancer; and surface blood vessel density in cancer lesions was significantly higher than that in non-cancer areas.
Research conclusions
LCI was useful for recognition of early gastric cancer lesions because this method provides good contrast in color differences between lesions and surrounding tissue. Blood vessel density from the surface to a depth of 350 μm was higher in cancer lesions than in non-cancer areas, and LCI clearly shows this feature as a change in redness.
Research perspectives
If these color values are evaluable in real time, artificial intelligence may permit automatic recognition and diagnosis of lesions.
杂志排行
World Journal of Gastroenterology的其它文章
- Nutritional and vitamin status in patients with neuroendocrine neoplasms
- Personalized medicine in functional gastrointestinal disorders:Understanding pathogenesis to increase diagnostic and treatment efficacy
- Quest for the best endoscopic imaging modality for computerassisted colonic polyp staging
- Short hairpin RNA-mediated knockdown of nuclear factor erythroid 2-like 3 exhibits tumor-suppressing effects in hepatocellular carcinoma cells
- MicroRNA-596 acts as a tumor suppressor in gastric cancer and is upregulated by promotor demethylation
- Performance of risk stratification systems for gastrointestinal stromal tumors: A multicenter study
