Nutritional and vitamin status in patients with neuroendocrine neoplasms
2019-03-22DominiqueSVMClementMargotETTesselaarMoniquevanLeerdamRajaventhanSrirajaskanthanJohnRamage
Dominique SVM Clement, Margot ET Tesselaar, Monique E van Leerdam, Rajaventhan Srirajaskanthan,John K Ramage
Abstract Symptoms of gastroenteropancreatic located neuroendocrine neoplasms (GEPNENs) are often related to food intake and manifest as abdominal pain or diarrhoea which can influence patients nutritional status. Malnutrition is common in cancer patients and influences quality of life, treatment options and survival but is also present in up to 40% of patients with GEP-NENs. As part of malnutrition there are often deficiencies in fat-soluble vitamins, mainly vitamin D. Little knowledge exists on trace elements. Several factors influence the development of malnutrition such as size and localisation of the primary tumour as well as metastases, side effects from treatment but also hormone production of the tumour itself. One of the main influencing factors leading to malnutrition is diarrhoea which leads to dehydration and electrolyte disturbances. Treatment of diarrhoea should be guided by its cause. Screening for malnutrition should be part of routine care in every GEP-NEN patient. Multidisciplinary treatment including dietician support is necessary for all malnourished patients with GEPNENs.
Key words: Neuroendocrine neoplasm; Nutrition; Malnutrition; Vitamin deficiency;Diarrhoea; Steatorrhoea
INTRODUCTION
Neuroendocrine neoplasms (NENs) are rare neoplasms arising from cells of the diffuse neuroendocrine system. NENs commonly arise from the gastroenteropancreatic (GEP) or bronchial tract[1]. The incidence of NENs is growing worldwide[2-4].Based on recent analysis of the United States SEER database this has arisen since 1973 from 1.03 to 6.98 per 100000 in 2012[2]. In the United Kingdom similar growth in incidence has been demonstrated from 3.9 per 100000 in 2001 to 8.8 per 100000 in 2015[5]. NENs are classified based on the World Health Organisation (WHO) 2010 classification, based on morphological criteria and proliferative activity (Ki-67 index or mitotic count). The grades are G1 Ki-67: < 2% and mitotic count < 2/10 mm2, G2 Ki-67: 3%-20% or mitotic count 2-20/mm2neuroendocrine tumours (NETs) and G3 Ki-67:> 20% neuroendocrine carcinoma (NECs)[6]. In 2016 the WHO has updated this classification for pancreatic neoplasms and differentiates G3 NETs from small- or large cell NECs[7]. NENs are difficult to diagnose and have metastasized in around 50% of cases at diagnosis[8]. GEP-NENs may present a heterogenous clinical behaviour but many well differentiated tumours (G1-G3) are indolent or slow growing with a 5 year survival which can be up to 50%-70%[2,9]. Well differentiated NETs can be functional, secreting hormones [the most common is carcinoid syndrome (CS)], or non-functioning[10].
Surgical removal of the primary tumour is the preferred treatment where it is possible but it can also be considered in metastatic disease and this may have survival benefits for some sites[11,12]. In the metastasized setting long-acting somatostatin analogues are often the first line of treatment in cases with positive somatostatin receptor imaging[10]. Due to the position of the tumour within the GEP tract, patients with GEP-NENs can experience gastrointestinal (GI) symptoms like bloating,diarrhoea, abdominal pain and weight loss. Treatments for GEP-NENs can also have side effects such as diarrhoea or steatorrhea. These factors can influence the weight,nutritional and vitamin status of patients with GEP-NENs. Malnutrition influences quality of life but also reduces tolerance to anti-cancer therapy and reduces survival in patients with cancer[13,14]. Currently the nutritional and vitamin status is a neglected area in patients with GEP-NENs[15].
This review will discuss the current knowledge regarding nutritional, vitamin and trace element status in patients with GEP-NENs and factors contributing to malnutrition. One of the main influencing factors is diarrhoea and we will discuss ways to analyse the causes of diarrhoea as well as treatment modalities. We will comment on any means of improving nutritional status.
NUTRITIONAL STATUS DEFINITION MALNUTRITION
Nutritional status can be measured based on anthropometric data [weight, height,body mass index (BMI)], biochemical markers like serum proteins (albumin or transferrin) or body composition measures[16]. There are several definitions for malnutrition from the literature and health care organisations. The WHO, National Health Service and European Society of clinical nutrition and metabolism (ESPEN)nutrition in cancer guideline uses definitions based on intake and metabolic effects[13,17,18]. The American Society for Parenteral and Enteral Nutrition and ESPEN guidelines on malnutrition include definitions based on BMI, unintentional weight loss and loss of body composition parameters such as fat-free mass or muscle mass[19,20].
In patients with cancer as part of their disease and malnutrition a syndrome called cancer cachexia can develop. This is defined as weight loss > 5% in past 6 mo without starvation or weight loss < 2% and BMI < 20 kg/m2or weight loss > 2% and sarcopenia (defined as appendicular skeletal muscle index males < 7.26 kg/m2and females < 5.45 kg/m2)[14]. Malnutrition can exist even in the absence of weight loss.These broad definitions can be difficult to measure objectively.
MALNUTRITION IN PATIENTS WITH GEP-NENs
Several recent studies show 30%-50% of patients visiting an oncology clinic for the first time are malnourished[21-23], but not every clinician is aware of this phenomenon.Caccialanza et al[24]performed a survey among all Italian oncologists and only 28% of oncologists reported performing nutritional assessments (based on weight loss, BMI,screening tools or intake) as part of their routine care. About 40% of the oncologists within this survey denied the use of available specialist nutrition teams. A recent abstract from Lim et al[25]reports that less than 50% of patients in NEN clinic had their weight measured, and BMI was available in only 14% of these patients. Forty-three percent of all NEN patients within an outpatient clinic in Denmark were reported to have weight loss at some point during their disease. This is significantly more common in patients with GEP-NENs compared to patients with bronchial NENs or unknown primary[26].
There are several studies reporting malnutrition in NEN at first or follow up visits which are summarized in Table 1. The range of reported malnutrition is 4.9%-38%[26,27].One study reports no malnourished patients[15]whereas the TELECAST study, a prospective study on diarrhoea, reports 58% of patients with a NET and CS to have metabolic and nutritional disorders[28]. The studies in Table 1 report on different patient populations. Borre et al[26]included only 70% GEP-NEN patients, Maasberg et al[29]included 77% GEP-NEN patients while Qureshi et al[30]and Robbins et al[27]included only GEP-NEN patients. The patient populations underwent different forms of therapy. The patients in the Qureshi et al[30]and Robbins et al[27]groups had previous surgery in 60% of cases versus 48% in the Borre et al[26]group. Somatostatin analogues were administered in 30% of patients within the Robbins et al[27]group, 41.6% within the Qureshi et al[30]group and 50% in the Maasberg et al[29]and Borre et al[26]groups.Thirty percent of patients within the Maasberg et al[29]group were treated with systemic chemotherapy (this percentage was unknown for the other groups).
The type of tumours differs within the above-mentioned groups. The Borre et al[26],Robbins et al[27]and Qureshi et al[30]groups report 60%-70% G1 tumours versus 32% G1 tumours in the Maasberg et al[29]group. Robbins et al[27]mentions 37.5% of patients to have functional symptoms (diarrhoea and or hot flushes) whereas Maasberg et al[29]mentions 24.1% of patients to have these. The studies reporting on malnutrition used different screening methods. The malnutrition universal screening tool, nutritional risk screening, subjective global assessment are clinical tools based on BMI, weight loss, dietary intake and severity of illness. The international classification of diseases 9 scores were used in 1 study[31]. Currently a universal screening method for malnutrition in patients with GEP-NENs is lacking. Due to different screening methods, patient selection and different stage of NEN, the published data are too heterogenous to compare.
EFFECTS OF MALNUTRITION ON OUTCOMES
Some studies have reported the correlation of malnutrition to outcomes in terms of response to treatment, length of hospital stay or survival. Maasberg et al[29]reports on the nutritional status of NEN inpatients admitted for staging examinations or therapeutic interventions. Malnourished patients had a longer length of hospital stay compared to well-nourished patients (8 vs 4 d). The survival of malnourished patients was shorter although the malnourished patient group was comprised of a high percentage of NECs.
Glazer et al[31]identified 22096 patients with an abdominal NEN within the national inpatient sample database. In this group malnutrition was associated with a higher inpatient complication rate of 15% compared to 10% in well-nourished patients.Obesity was associated with lower inpatient mortality rates while malnutrition was associated with higher inpatient mortality rates. Ekeblad et al[32]reported being underweight at diagnosis (BMI < 20kg/m2) of a pancreatic NEN was related to a poorer prognosis. Marrache et al[33]studied 67 patients with liver metastasis from aNEN undergoing transarterial chemoembolization and found that the BMI was a factor predicting tumour response and associated with delayed progression.
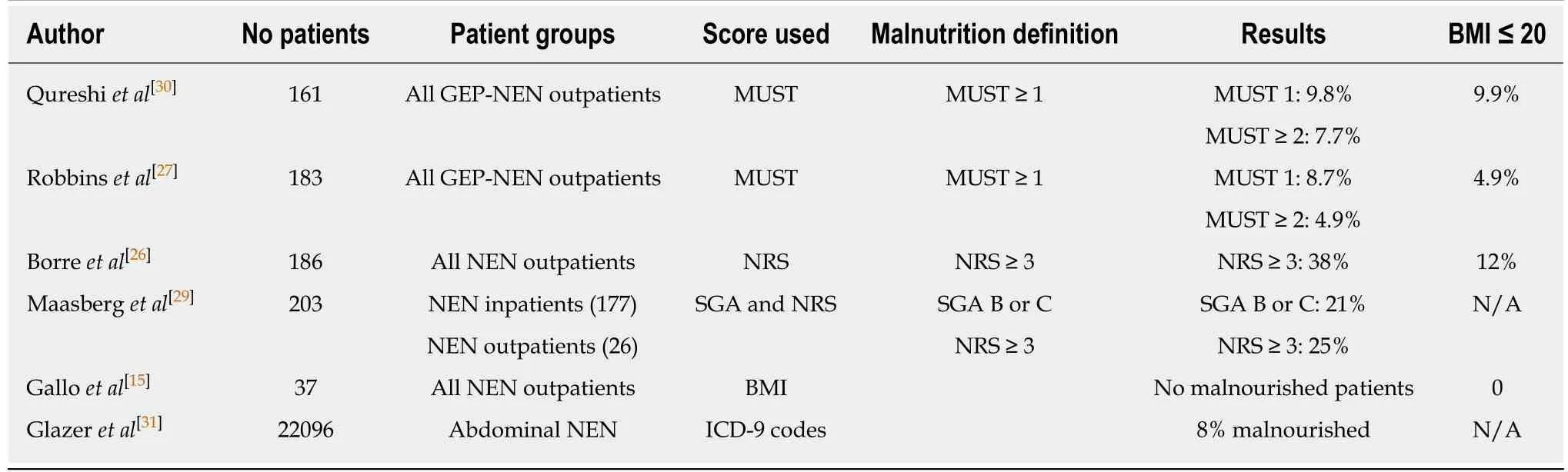
Table 1 Summary of available studies regarding malnutrition in patients with gastroenteropancreatic neuroendocrine neoplasms
FAT SOLUBLE VITAMIN STATUS IN PATIENTS WITH GEPNENs
Vitamin status can be considered as part of nutritional status. Two studies report on the status of fat-soluble vitamins in patients with NEN. Fiebrich et al[34]analysed the fat soluble vitamin status of 35 patients with metastatic small intestinal NEN on treatment with somatostatin analogues for at least 18 mo. Eighty percent of patients showed abnormally low levels of at least 1 fat soluble vitamin and 32% of patients showed multiple deficiencies. Vitamin deficiencies measured in plasma were reported in 9% of patients for vitamin A, 31% for vitamin D, 14% vitamin E and 69% vitamin K1. In 12% of patients vitamin K1 deficiency resulted in prolonged prothrombin time.Increased stool frequency was not associated with lower vitamin levels. De Hosson et al[35]described 15 patients with a NET that had been on a somatostatin analogue for more than 6 mo. Nine out of fifteen patients had vitamin deficiencies (vitamin A, D, E,K, B12 and B3) and after 10 wk of nutritional intervention and supplementation 7 patients still had deficient vitamin levels.
VITAMIN D STATUS IN PATIENTS WITH GEP-NENs
Vitamin D status has received increasing attention in cancer patients and it is suggested it may play a possible role in the development of different types of tumours[36]. Vitamin D deficiency (defined as 25 OH vitamin D levels ≤ 20 ng/mL) is described in between 46% and 81% of patients with NENs[27,36-38]. The study populations reporting on the prevalence of vitamin D deficiency could not be compared directly since Motylewska et al[37]included GEP-NENs as well as lung NENs and other NENs, Lind et al[38]included only small intestine NENs while Massironi et al[36]and Robbins et al[27]included all GEP-NENs.
Two studies included the vitamin D status of healthy volunteers. Motylewska et al[37]reported a vitamin D deficiency of 89% in healthy volunteers. Massironi et al[36]found significantly higher vitamin D level (median 23.9 ng/mL) in healthy volunteers compared to GEP-NEN patients (median 12.9 ng/mL). One study describes the overall and progression free survival (OS and PFS) in 138 GEP-NEN patients and found a negative correlation between low vitamin D levels and OS and PFS. Vitamin D supplementation improved the OS in patients with vitamin D deficiency compared to patients with vitamin D deficiency without supplementation.
Vitamin D supplementation with over-the-counter vitamin D preparations improves the vitamin D levels in most GEP-NEN patients. Robbins et al[27]showed a decrease in numbers of patients with vitamin D deficiency from 66.8% at baseline to 44.4% after 1 and 2 years of starting supplementation, although supplementation of vitamin D had not normalised the level in all patients. Lind et al[38]showed only 28%of small intestine NEN patients on oral vitamin D supplementation to be vitamin D deficient as compared to 46% of patients without supplementation. Vitamin D supplementation does improve the bone mineral density in patients with small intestine NEN. Lind et al[38]describes 2 cohorts of 25 patients with a small intestinal NEN who all had prior surgery. Within the first cohort baseline vitamin D levels and dual-energy x-ray absorptiometry (DEXA) scan were performed. The second cohort was advised to take oral substitution of vitamins and minerals. After 6-15 mo vitamin D levels were measured and a DEXA scan was performed. The DEXA scan results showed low bone density in 76%, osteoporosis in 32% and osteopenia in 44% for the first cohort and respective values of 26%, 24% and 36% in the second cohort.
VITAMIN B3/NIACIN STATUS IN PATIENTS WITH GEPNENs
Tryptophan is a precursor of serotonin. In healthy individuals only 1% of tryptophan will be used to make serotonin. In patients with NETs and CS up to 60% of tryptophan is used for serotonin production leading to tryptophan deficiency.Pellagra as a result of tryptophan deficiency with symptoms of dermatitis, diarrhoea and dementia can develop in about 5% of patients with CS[39-41].
Shah et al[42]analysed the niacin levels in blood of 36 patients with CS, 32 patients with a carcinoid tumour but without CS and 24 non-carcinoid patients (patients with pancreatic NEN, other cancers, GI diseases and healthy volunteers). With a cut-off level of 130, 28% of patients (10/36) with CS were niacin deficient and 12.5% (4/32)without CS compared to none in the non-carcinoid patients. Serotonin and tryptophan levels were measured: in patients with increased serotonin levels a decreased niacin and tryptophan level was observed.
Bouma et al[43]identified 42 patients with serotonin producing NEN [based on 5-hydroxyindolacetic acid (5-HIAA) > 3.8 mmol/mol creatinine and/or platelet serotonin > 5.4 nmol/109platelets] and low tryptophan levels (< 40 umol/L) and/or pellagra-like symptoms. Urine N1-NM (N1-Methylnicotinamide) levels were measured prior and after starting niacin supplementation. Forty five percent of patients showed low N1-NM levels and after starting niacin supplementation all urine levels normalised. 5-HIAA levels showed a negative correlation with niacin status,but the 5-HIAA level did not correlate with the plasma tryptophan level. In view of the difficulties measuring niacin, nicotinamide should be prescribed if pellagra is suspected.
TRACE ELEMENTS IN PATIENTS WITH GEP-NENs
Malnutrition can also result in low levels of trace elements, like cobalt, copper,fluorine, iodine, selenium and zinc. In patients with cancer low levels of selenium and zinc were described and could affect wound healing, cause depressive symptoms and compromise the immune response[13,44]. Little is known about other trace elements. The role of trace elements in patients with NENs is unknown. One study exists evaluating the selenium level of patients with NENs having peptide receptor radionuclide therapy (PRRT) treatment. Four wk prior to PRRT 5 out of 21 included patients showed normal selenium levels. Four wk after PRRT 18 patients showed significant decrease in selenium levels[45]. There may be significant issues with blood selenium levels which may not represent whole body stores of selenium.
FACTORS INFLUENCING MALNUTRITION
In patients with NENs several factors can contribute to the development of malnutrition as summarized in Figure 1. In cancer patients the protein, carbohydrate and lipid metabolism are altered resulting in increased metabolic resting rate, insulin resistance, lipolysis and proteolysis. This can lead to weight loss as a sign of malnutrition[13]. If this process continues, cancer cachexia as a result of decreased energy intake and increased total body energy expenditure (TEE), can develop[14].Tumour metabolism and inflammation increase the TEE cytokines and factors in animal models involved are for example IL-6, IL-1, TNF-α, IFN-γ, Leukaemia inhibitor γ factor, GDF15, TWEAK, TRAF, oncostatin M, TNFSF12 and PGE2. Patient data regarding these cytokines and factors are lacking[46]. In patients with pancreatic adenocarcinoma elevated C-reactive protein levels are a marker for cachexia and predict poor prognosis[47]. Little knowledge regarding the development of cachexia and prognosis exists in patients with GEP-NENs. Due to the slow growing nature of GEP-NENs the risk of developing cachexia may be reduced[29]. In patients with NET and CS, diarrhoea as a result of excessive hormone secretion also contributes to malnutrition[48-51]. Several foods containing high levels of amines such as mature cheese or chocolate may provoke symptoms in these patients. They tend to avoid these foods which can contribute to decreased nutritional intake[49,52]. Small intestine NENs can cause strictures or retroperitoneal fibrosis in the GI tract resulting in symptoms such as bowel obstruction, influencing patients’ food intake and nutritional status[53,54].
Uncontrolled diarrhoea leads to dehydration, electrolyte abnormalities, vitamin deficiencies and thus influence malnutrition[55]. Surgical treatment of a GEP-NEN can result in diarrhoea for several reasons. Loss of absorptive surface after small bowel or Whipple’s resection can lead to diarrhoea and malabsorption. In cases of small bowel resection when < 200 cm small bowel remains, short bowel syndrome with inability to maintain fluid- and nutritional status can develop[15,53,56]. Another cause is removal of a part of the terminal ileum which can result in vitamin B12 deficiency and bile acid malabsorption leading to diarrhoea[48,53,55,57]. A third cause is following small intestine or Whipple’s resection, bacterial overgrowth can develop resulting in diarrhoea with malabsorption and maldigestion as well as malabsorption of fat soluble vitamins[48,53,55,58]. Resection of small or large bowel as well as Whipple’s resection can also influence the bowel transit time with diarrhoea and possible malnutrition as a result[15,49].
Treatment with somatostatin analogues can result in malnutrition for several reasons. Diarrhoea is one of the most commonly reported side effects due to decreased duodenal absorption of carbohydrates and triglycerides[59,60]. In addition decreased pancreatic enzyme release in response to a meal is seen as an effect from somatostatin analogues resulting in pancreatic exocrine insufficiency with steatorrhea and malnutrition[15,55,57]. Chemotherapy can lead to malnutrition due to its side effects such as loss of taste, oral problems, diarrhoea, nausea and vomiting[13,15,49].
ANALYSING CAUSES OF DIARRHOEA
Diarrhoea is an important factor contributing to malnutrition in patients with GEPNENs as summarized in Figure 1. Figure 2 summarizes diagnostic approaches to diarrhoea. CS is prevalent is 20% of patients with NETs[61]. CS is a clinical diagnosis with symptoms of diarrhoea (80% of patients), hot flushes (50%-85% of patients) and wheezing (10%-20% of patients) often in the presence of liver metastasis[48,62,63].Twenty-four h urine collection for 5-HIAA, a breakdown product of serotonin, has a sensitivity and specificity around 90% for small intestine NENs[64]. Certain serotoninrich foods (bananas, avocados, plums, eggplant, tomatoes, plantain, pineapples, kiwis and nuts) and medications (analgesic acetaminophen, cough syrups and warfarin) can increase urinary 5-HIAA levels and should be avoided 24 h before and during specimen collection.
In case of previous surgery on either small intestine or pancreas, diarrhoea could be caused by bile acid malabsorption, short bowel syndrome, bacterial overgrowth or pancreatic exocrine insufficiency. A Selenium homotaurocholic acid conjugated with taurine (SeHCAT) scan is the gold standard for diagnosing bile acid malabsorption with a sensitivity 100% and specificity 89%[65]. A capsule containing radiolabelled SeHCAT is ingested and after 1-3 h and after 7 d a gamma scan is performed to measure the SeHCAT retention in the body. Levels of 10%-15% retention are mild, <10% as moderate and < 5% severe bile acid malabsorption[66,67]. A study in 57 patients with GEP-NENs showed 80% of patients with bile acid malabsorption[68].
Following small intestine resection, a short bowel syndrome can develop and this is usually the case when < 200 cm small bowel remains[69,70]. Adaptation in the postoperative period over 1-2 years is possible[70]. No data exists on the prevalence of short bowel syndrome in patients with GEP-NENs. Small bowel bacterial overgrowth develops in the case of blind small bowel loops or strictures. A gold standard for the diagnosis of small bowel bacterial overgrowth is lacking. Quantitative culture of jejunal aspirate is frequently described but is an invasive procedure with upper endoscopy and lacks a sterile environment to obtain a culture[71]. Hydrogen breath tests are a widely used alternative for diagnosing small bowel bacterial overgrowth with a sensitivity of 63% and specificity 83% compared to a jejunal culture[72,73]. In the earlier mentioned study with 57 patients with GEP-NENs 62% of patients were diagnosed with bacterial overgrowth[68].
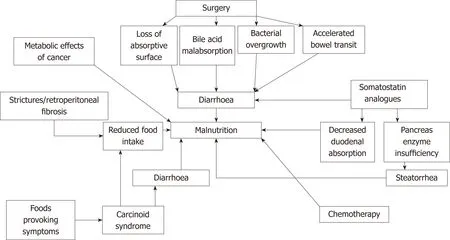
Figure 1 Factors influencing malnutrition in patients with gastroenteropancreatic neuroendocrine neoplasms. Summary of factors influencing the development of malnutrition in patients with gastroenteropancreatic neuroendocrine neoplasms.
Partially or total resection of the pancreas leads to decreased synthetic capacity of pancreatic enzymes resulting in pancreatic exocrine insufficiency[74,75]. Faecal elastase can be measured as a marker for pancreatic exocrine insufficiency with a cut-off < 200 μg/g stool. In patients with chronic pancreatitis the sensitivity of faecal elastase diagnosing pancreatic exocrine insufficiency is 63%-100% with a specificity of 83%-93%[76-78]. In patients with GEP-NENs there is controversy about the role of faecal elastase. In a study of 57 patients with GEP-NENs only 17% of patients had a low faecal elastase despite symptoms of steatorrhoea[68]. Chaudhry et al[79]described 32 patients with GEP-NENs (27 small intestine, 5 pancreas and 7 other) on treatment with a somatostatin analogue and 82% of patients had symptoms of steatorrhoea but the faecal elastase was low in only 6 patients. The sensitivity of faecal elastase in this study was only 15.4%. Another study on 50 patients with a metastatic NEN (n = 30 small intestine, n = 11 pancreas, n = 6 lung or n = 3 other) on somatostatin analogue treatment the faecal elastase was a good marker for pancreatic exocrine insufficiency although faecal samples were not available from all patients[80]. Faecal elastase could be a useful marker to diagnose pancreatic exocrine insufficiency although data in small groups of patients with NENs are conflicting.
Steatorrhoea could be one of the side effects of treatment with somatostatin analogues due to the inhibition of pancreatic exocrine secretion[81,82]. A recent study on 50 patients starting with a somatostatin analogue showed that 24% developed pancreatic exocrine insufficiency after a median of 2.9 mo, although the study lacks data about previous surgery and altered anatomy of the GI tract[80].
TREATMENT OPTIONS
Malnutrition should be treated according to its causes as summarised in Figure 2. In patients with diarrhoea due to CS, treatment should be to try to reduce hormone levels[48]. A first step should be to optimise the dose of long acting somatostatin analogues, and this could be achieved by increasing the dose, shortening the interval or adding a short acting dose[83-85]. Another step could be palliative debulking surgery or liver directed therapy with chemoembolization of the hepatic artery, radiofrequency ablation/microwave ablation or selective intra-hepatic radio-embolization to lower the tumour burden and reduce hormone production[86,87]. Clinical(symptomatic) responses after liver directed therapy are reported in up to 75%[88-91].Recently telotristat ethyl, an oral inhibitor of tryptophan decarboxylase, has become available for symptomatic patients for whom monotreatment with somatostatin analogues not enough is to control diarrhoea. The TELESTAR trial was a phase III double blind placebo-controlled trial in 135 patients with CS (defined as > 4 bowel movements a day) were randomized to receive 250 mg telotristat ethyl tablet, 500 mg telotristat ethyl tablet or placebo three times daily for 12 wk. There was a statistically significant reduction in bowel movements in 42%-44% of patients (250 mg or 500 mg)compared to 20% reduction in placebo patients after 12 wk. A significant reduction in urine-5 HIAA level and improvement in quality of life scores was seen[92]. A companion trial (TELECAST) looking at 126 patients with CS and less than 4 bowel motions a day and with either loose stools or daily > 2 flushing episodes or abdominal pain or nausea in > 20% of days or 5-HIAA urine levels above normal limits were randomised to telotristat ethyl 250 mg, 500 mg or placebo for 12 wk. This trial showed good safety data and classified 40% of patients on telotristat ethyl as durable responders[28].
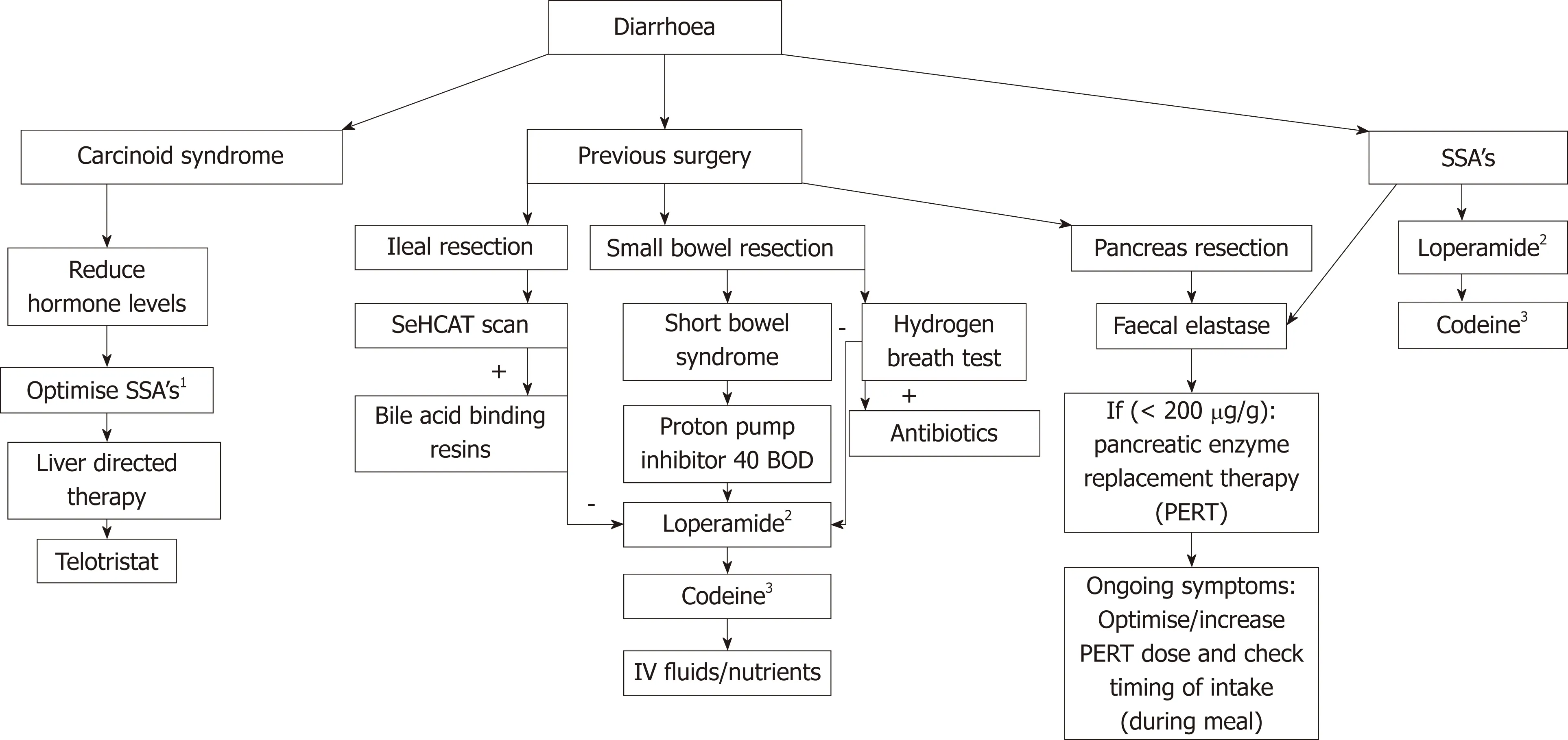
Figure 2 Approach to patients with diarrhoea. Summary of causes of diarrhoea in patients with gastroenteropancreatic neuroendocrine neoplasms, how to analyse and treatment advise. 1Optimise SSA’s: Increase dose, shorten interval or add short acting dose; 2Advise loperamide: Increasing dose 2-4-8 mg 4 times a day, up to 12-24 mg 4 times a day in short bowel syndrome; 3Advise codeine: 15-60 mg 4 times a day; Advise PERT: 1 × 25000 units of lipase per small meal 2 × 25000 units lipase per large meal, titrate up may need > 80000 units per large meal. SSA’s: Somatostatin analogue’s; SeHCAT: Selenium homotaurocholic acid conjugated with taurine; BOD: Twice a day; PERT: Pancreatic enzyme replacement therapy.
Another study published by Weickert et al[93], investigated the details of 120 patients participating in the TELESTAR trial and telotristat ethyl’s effect on nutritional status after 12 wk of treatment. Weight gain and improvement in nutritional markers as albumin, cholesterol levels and triglycerides were seen in patients on telotristat ethyl but not in patients on placebo. In two small studies with 11 and 14 patients with small intestine NENs, liver metastases and CS there were reports of the positive effect of ondansetron reducing diarrhoea[94,95]. If a SeHCAT shows bile acid malabsorption,treatment with bile acid binding resins is advised. In patients with bile acid malabsorption without NEN a response to colesevelam is reported in 50%-96% of cases[66,67,96].
Short bowel syndrome should ideally managed by a dedicated multidisciplinary team due its prolonged course and intensive fluid and nutritional management[69].Medical treatment includes proton pump inhibitors to reduce gastric acid production in the maximum dose of 40 mg twice daily. Loperamide is the cornerstone of treatment to reduce small bowel mobility. A starting dose of 2 mg 4 times a day(breakfast, lunch, diner, bedtime) is advised. This can be increased to 8 mg 4 times a day but in case of short bowel up to 12-24 mg 4 times a day. Codeine phosphate is less effective than loperamide but can be combined with loperamide to reduce small bowel mobility. Doses starting with 15 mg 4 times a day gradually increasing up to 60 mg 4 times a day are advised. Octreotide 50-200 μg subcutaneously twice daily can be tried for 3-5 d to reduce diarrhoea but many patients with NENs are already receiving treatment with long acting somatostatin analogues. In addition intravenous fluids and total parenteral nutrition (TPN) can be necessary[70,97].
Small bacterial overgrowth should be treated with antibiotics but there are no guidelines as to which antibiotic is preferable. Broad spectrum antibiotics which affect enteric aerobes and anaerobes are used and rifaximin may be preferable since it is not absorbed (although not licensed for this indication)[71,98].
Pancreatic exocrine insufficiency as result of pancreatic surgery or a side effect of somatostatin analogue treatment should be treated with pancreatic enzyme replacement. The dose of pancreatic enzymes needs to be individually adjusted based on dietary fat intake[99]. This would be ideally managed by a specialized dietician. A suggestion for a starting dose could be 25000 units lipase (or equivalent) with a snack and 2 × 25000 units lipase (or equivalent) with a large meal. This could gradually be increased to 1-2 × 400000 units lipase with a small meal and 120000 units lipase with a large meal. If symptoms persist compliance and timing of ingestion of pancreatic enzymes should be checked. Acid reduction using proton pump inhibitors may also help to increase efficacy of supplements[99,100].
A more common side effect from somatostatin analogues can be diarrhoea[101,102].These side effects often occur for 2-3 d after the injection but can occur daily and decrease 6-12 mo after starting[103]. A first step in treatment can be loperamide 2-4 mg 4 times a day and can be increased to 8 mg 4 times a day[104,105]. Codeine can be added 15 mg 4 times a day and gradually increased to 60 mg 4 times a day[104,105]. In cases of steatorrhoea pancreatic enzyme replacement therapy as described above can be started.
Malnutrition should also be treated with nutritional support to increase body weight. Several authors advise to include a dietician specialised in NENs to be part of the multidisciplinary team[49,53]. No systematic guidelines for nutritional support exist in patients with NENs. The American carcinoid cancer foundation published a nutritional guideline in 2000 and updated version in 2009[106]. This guideline, intended for patients, advises all patients to increase protein intake to restrict carbohydrate servings (fruit, vegetables and whole grains) to 5-10 portions/day, moderate to low fat intake (25%-30% of daily calories) and eat a variety of foods. In patients with CS,foods provoking symptoms containing high levels of amines are advised to be avoided. Examples of foods with high levels of amines are: Mature cheeses (cheddar,camembert, stilton), alcohol beverages, smoked/salted or pickled fish or meat, liver,caffeine containing drinks, chocolate, nuts, bananas, avocado, raspberries[106].
The ESPEN guideline on nutrition[13]in cancer recommends a total energy intake ranging between 25-30 kcal/kg/day with a protein intake of 1 g/kg/day but ideally 1.5 g/kg/day. If oral nutrition remains inadequate despite interventions, enteral nutrition is the first choice. Only in the case of severe intestinal insufficiency(radiation enteritis, short bowel syndrome, peritoneal carcinomatosis or chylothorax)should parenteral nutrition (TPN) be considered. The role of parenteral nutrition in patients with cancer is controversial. Economics, traditions and ethical issues will differ between countries. In some countries feeding of palliative cases is essential.These countries tend to report survival benefits for TPN and in selected cases TPN improves quality of life[107-112]. There are risks of life- threatening catheter infections and septicaemia in the use of TPN[70]. There is one case report on the use of TPN in a patient with a NEN[113]. The decision to start enteral nutrition should be made by the multidisciplinary team if malnutrition is not improving with maximal oral and medical support for 3-6 mo. The decision on starting TPN should only be made in highly selected cases.
RECOMMENDATIONS
Screen patients with GEP-NENs for malnutrition every clinic visit (weight, BMI and nutrition screening tool). Screen patients with GEP-NENs on treatment with somatostatin analogues for fat soluble vitamin deficiencies once a year and start supplementation. Screen patients with GEP-NENs and previous small bowel surgery,without treatment somatostatin analogues once a year for fat soluble vitamin deficiencies and start supplementation. Data is lacking on screening of trace elements.In patients with diarrhoea try to analyse the cause and base the treatment on its cause.A patient with GEP-NENs and malnutrition (BMI < 18.5 kg/m2or > 5% weight loss in 3 mo) could benefit from dietician input.
CONCLUSION
There are multiple definitions of malnutrition and not all suitable for use in clinical practice. Although the definition of malnutrition is not clearly defined, up to 40% of patients with GEP-NENs are malnourished. Malnutrition is associated with longer hospital stay, higher complication rate and lower response on treatment in a few small studies. As part of the nutritional status fat soluble vitamin deficiencies are often present in patients with GEP-NENs. Supplementation does not normalise vitamin levels in every patient. The influence of vitamin deficiencies on survival is less clear. Several factors may influence the nutritional status and diarrhoea is the main one. Diarrhoea can have multiple causes in patients with GEP-NENs and requires systematic investigation and treatment. Multidisciplinary care with a dietician is necessary for every malnourished patient with GEP-NENs.
杂志排行
World Journal of Gastroenterology的其它文章
- Personalized medicine in functional gastrointestinal disorders:Understanding pathogenesis to increase diagnostic and treatment efficacy
- Quest for the best endoscopic imaging modality for computerassisted colonic polyp staging
- Short hairpin RNA-mediated knockdown of nuclear factor erythroid 2-like 3 exhibits tumor-suppressing effects in hepatocellular carcinoma cells
- MicroRNA-596 acts as a tumor suppressor in gastric cancer and is upregulated by promotor demethylation
- Performance of risk stratification systems for gastrointestinal stromal tumors: A multicenter study
- Utility of linked color imaging for endoscopic diagnosis of early gastric cancer
