Central line-associated bloodstream infection among children with biliary atresia listed for liver transplantation
2019-03-21NicoleTriggsStaceyBeerSonamMokhaKatHosekDanielleGuffeyCharlesMinardFlorMunozRyanHimes
Nicole D Triggs, Stacey Beer, Sonam Mokha, Kat Hosek, Danielle Guffey, Charles G Minard, Flor M Munoz,Ryan W Himes
Abstract BACKGROUND Pre-transplant nutrition is a key driver of outcomes following liver transplantation in children. Patients with biliary atresia (BA) may have difficulty achieving satisfactory weight gain with enteral nutrition alone, and parenteral nutrition (PN) may be indicated. While PN has been shown to improve anthropometric parameters of children with BA listed for liver transplantation,less is known about the risks, particularly infectious, associated with this therapy among this specific group of patients.AIM To describe the incidence, microbiology, and risk factors of central line-associated bloodstream infection (CLABSI) among children with BA listed for liver transplantation.METHODS Retrospective review of children aged ≤ 2-years of age with BA who were listed for primary liver transplantation at Texas Children’s Hospital from 2008 through 2015 (n = 96). Patients with a central line for administration of PN (n = 63) were identified and details of each CLABSI event were abstracted. We compared the group of patients who experienced CLABSI to the group who did not, to determine whether demographic, clinical, or laboratory factors correlated with development of CLABSI.RESULTS Nineteen of 63 patients (30%, 95%CI: 19, 43) experienced 29 episodes of CLABSI during 4800 line days (6.04 CLABSI per 1000 line days). CLABSI was predominantly associated with Gram-negative organisms (14/29 episodes, 48%)including Klebsiella spp., Enterobacter spp., and Escherichia coli. The sole polymicrobial infection grew Enterobacter cloacae and Klebsiella pneumoniae. Grampositive organisms (all Staphylococcus spp.) and fungus (all Candida spp.)comprised 9/29 (31%) and 6/29 (21%) episodes, respectively. No demographic,clinical, or laboratory factors were significantly associated with an increased risk for the first CLABSI event in Cox proportional hazards regression analysis CONCLUSION There is substantial risk for CLABSI among children with BA listed for liver transplantation. No clinical, demographic, or laboratory factor we tested emerged as an independent predictor of CLABSI. While our data did not show an impact of CLABSI on the short-term clinical outcome, it would seem prudent to implement CLABSI reduction strategies in this population to the extent that each CLABSI event represents potentially preventable hospitalization, unnecessary healthcare dollar expenditures, and may exact an opportunity cost, in terms of missed allograft offers.
Key words: Parenteral nutrition; Central line-associated bloodstream infection; Pediatric;Microbiology; Central venous catheter
INTRODUCTION
Biliary atresia (BA) is a progressive obliterative cholangiopathy which presents in the first months of life and it is the most common indication for liver transplantation in children. While a patient’s pre-operative nutritional status is an important driver of transplant outcomes[1-3], malnutrition is prevalent among patients with BA[3-5].Common nutritional rehabilitation strategies include provision of calorically dense enteral formulas, enrichment in medium chain triglycerides, and use of nasogastric feeding tubes. In spite of these efforts, many children with BA will fail to achieve the desired catch-up growth, and will ultimately be prescribed parenteral nutrition (PN).Although PN has been shown to improve the nutritional status of children with BA on the liver transplant waitlist[5,6], PN delivered though a central venous catheter(CVC) introduces a new set of risks to the patient, including mechanical, infectious,and metabolic complications. We sought to characterize infectious complications of PN, specifically central line-associated bloodstream infection (CLABSI) among children with BA on the liver transplant waitlist. The aim of our investigation was to describe the incidence and microbiology of CLABSI among this cohort of patients and to elucidate potential risk factors which might be leveraged for prevention efforts.
MATERIALS AND METHODS
We identified children aged ≤ 2-years of age with BA who were listed for primary liver transplantation at Texas Children’s Hospital from 2008 through 2015. Utilization of a CVC for administration of PN was ascertained through pharmacy records,radiology reports, and clinical documentation. Demographic, anthropometric,laboratory, and clinical data were collected retrospectively through review of electronic medical records. The decision to use PN and its specific prescription was at the clinical discretion of the treating hepatologist, working together with a registered dietitian. Weight-for-length z-scores at the time of listing were calculated according to the World Health Organization standards using an online calculator available at https://peditools.org/.
Central line days accrued from the time of line insertion until line removal, liver transplantation, or removal of the patient from the transplant waitlist for a reason other than transplantation (i.e., death, clinical deterioration, or clinical improvement),whichever came first.
Our operational definition of CLABSI is based on the Center for Disease Control and Prevention/National Healthcare Safety Network (CDC/NHSN) definition:laboratory-confirmed bloodstream infections, not secondary to infection at another body site, among patients with a CVC[7]. In contrast to the CDC/NHSN definition,however, which is designed to capture healthcare facility acquired infections, we regarded all CLABSI events as relevant, even if the place of origin was in the community. To that end, central line days accrued, and CLABSI events were recorded,for children while admitted in a healthcare facility and while at home.
According to our clinical practice, febrile patients with a CVC and no other localizing source were admitted to hospital. Broad-spectrum antibiotics were initiated,guided by sensitivities of prior blood cultures, when applicable. Antibiotics were tailored to the specific isolate when sensitivities were available. Daily blood cultures were obtained until two consecutive cultures were sterile. CVCs were removed if sterility was not achieved, or sooner, if clinical circumstances warranted. Seven to 14 d of parenteral antibiotic therapy was completed, starting from the first sterile day.
Data are presented as frequency with percent, means with standard deviations, or medians with 25thand 75thcentiles. Chi-squared, Wilcoxon rank sum, or t-tests were used to compare groups, as appropriate. Kaplan-Meier survival analysis and Cox proportional hazards regression were used to analyze the time-to-the-first CLABSI event and Cox proportional hazards regression was used to evaluate risk factors for CLABSI. This study was approved by the Baylor College of Medicine institutional review board.
RESULTS
Ninety-six patients with BA, ≤ 2-years of age, were listed for liver transplantation between 2008 and 2015 at our center. Sixty-three (66%) patients had a CVC placed for administration of PN. Nineteen of 63 patients (30%, 95%CI: 19, 43) experienced 29 episodes of CLABSI during 4800 line days (6.04 CLABSI per 1000 line days) (Figure 1).
Baseline demographic and clinical data
Clinical and demographic data at the time of listing for liver transplantation are shown in Table 1. In univariate analyses, there was a higher proportion of patients with public or no insurance in the CLABSI group, compared to the no CLABSI group(84.2% vs 56.8%, P = 0.036). There were uniform, but non-significant trends, towards greater growth retardation in the CLABSI group, as mean length-for-age z-scores,weight-for-age z-scores, and weight-for-length z-scores were lower. Among all 63 patients with a CVC for administration of PN, the median age at the time of line placement was 6.6 mo (IQR 5.4, 9) and the median number of line days were 58 (IQR 30, 96), with a range of 4-255 d (data not shown).
Characteristics of CLABSI events

Figure 1 Patient flowchart. BA: Biliary atresia; LT: Liver transplant; CVC: Central venous catheter; PN: Parenteral nutrition; CLABSI: Central line-associated bloodstream infection.
Among patients who developed CLABSI, the median time to first event was 28 d (IQR 12, 53). The earliest CLABSI occurred on line day 3 and the latest event on line day 80.Kaplan-Meier analysis revealed that 75% of patients remained free of CLABSI after 43 line days (95%CI: 21, 62, Figure 2). No demographic, clinical, or laboratory factors were significantly associated with an increased risk for the first CLABSI event in Cox proportional hazards regression analysis (Table 2).
Fourteen of 19 (74%) affected patients experienced a single episode of CLABSI.There were 3 patients with two episodes, and 1 patient experienced four and five episodes each. Only the patient with five episodes had the same organism isolated on more than one occasion, in this case Klebsiella pneumoniae. Overall, CLABSI was predominantly associated with Gram-negative organisms (14/29 episodes, 48%)including Klebsiella spp., Enterobacter spp., and Escherichia coli. The sole polymicrobial infection grew Enterobacter cloacae and Klebsiella pneumoniae. Gram-positive organisms(all Staphylococcus spp.) and fungus (all Candida spp.) comprised 9/29 (31%) and 6/29(21%) episodes, respectively (Figure 3).
Clinical outcomes
While the short-term clinical outcomes did not significantly differ between the two groups (Table 1), there were trends towards a higher rate of transplantation and a lower rate of clinical deterioration in the CLABSI group. The single waitlist death in the CLABSI group was not directly attributable to the CLABSI event.
DISCUSSION
Nutritional rehabilitation is a cornerstone in the management of pediatric liver transplant candidates. Those ≤ 2-years of age with BA comprise a large and fairly homogenous group of patients whose nutritional deficits have been welldocumented[2,4,8]. In spite of calorically dense enteral formulas and modular supplements, often delivered via nasogastric tubes, it is not uncommon for patients to still not achieve satisfactory growth. On the other hand, two studies have now demonstrated that PN improves the nutritional status (i.e., mid-arm circumference and triceps skinfold thickness) of malnourished patients with BA on the transplant waitlist[5,6], underscoring the important role this therapy plays in the nutritional optimization of liver transplant candidates.
In our large pediatric liver transplant program, about 2/3 of liver transplant candidates, ≤ 2-years of age, with BA received PN. This is higher than the 41%reported by Wendel et al[6], and to the 53% reported by Sullivan et al[5], though inclusion criteria were not identical. On a per patient basis, we observed that 30% of patients with a CVC for PN met rigorous criteria for CLABSI, similar to the 33%reported by Wendel et al[6]. While Sullivan et al[5]found 52% of their cohort with a CVC for PN had a positive blood culture, it is not known how many of these represented CLABSI, as opposed to secondary bacteremia or contaminants, making direct comparison impossible. Our CLABSI rate, 6.04/1000 line days, is greater than that ofWendel et al[6](3.8/1000 line days), though it is not clear whether the same definition of CLABSI was used in their study. While these methodological differences may be barriers to generalizing CLABSI rates from center-to-center, and population-topopulation, collectively our data calls attention to a facet of the care of transplant candidates which may be underappreciated. We would suggest that calculation and internal benchmarking of an individual program’s CLABSI rate may be a good balancing measure on initiatives to improve the nutritional status of pediatric transplant candidates.

Table 1 Clinical and demographic data at time of liver transplant listing and short-term clinical outcome of patients with and without central line-associated bloodstream infection
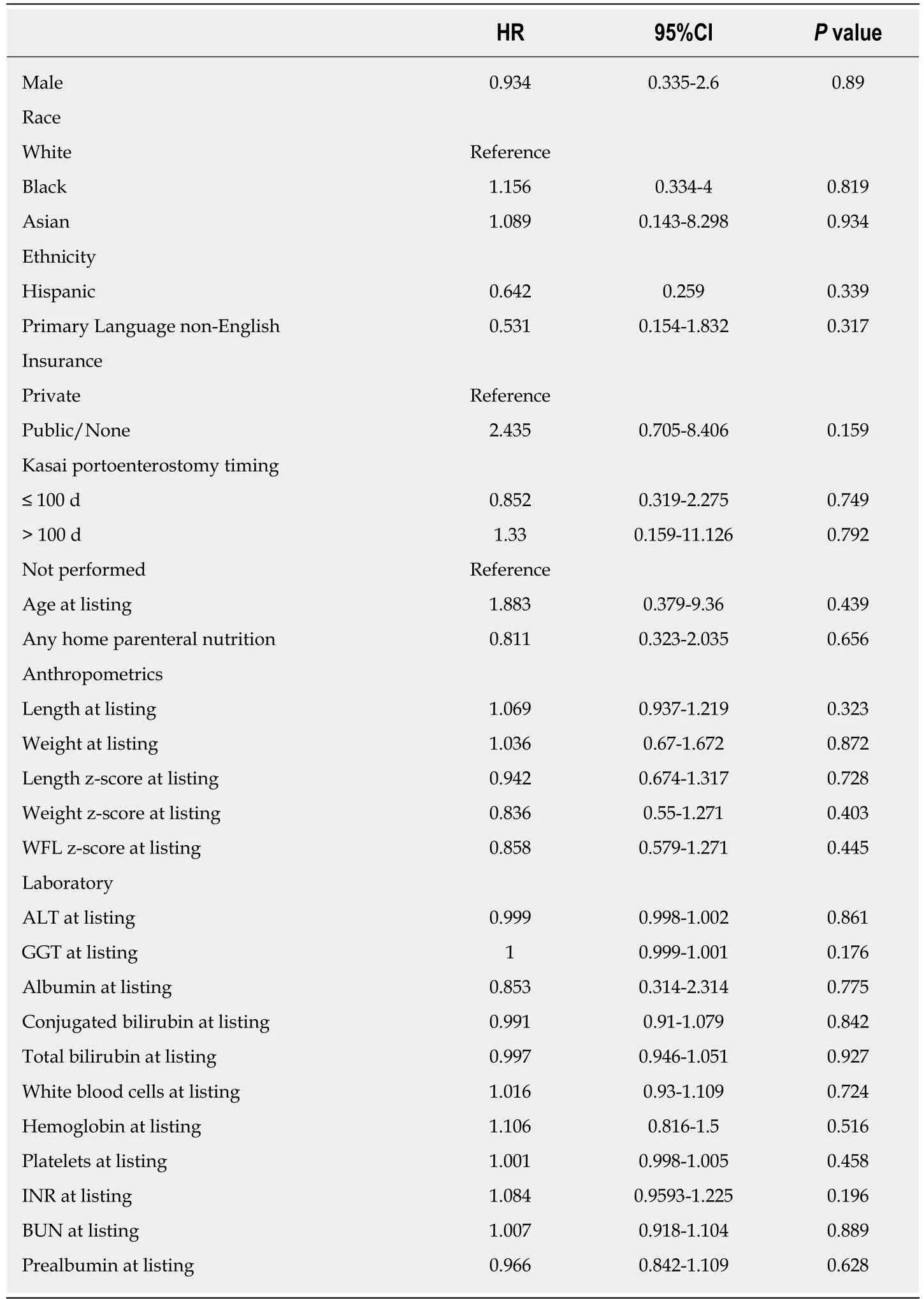
Table 2 Demographic, clinical, and laboratory factors assessed as potential risk factors for central line-associated bloodstream infection
In our univariate analyses, children with public/no insurance were overrepresented in the CLABSI group. While our data do not offer potential explanations for this finding, it is congruent with an evolving body of literature which documents disparities in access to, or outcomes of, pediatric liver transplantation[9-11]. In agreement with Sullivan et al[5], we did not find that CLABSI impacted short-term clinical outcomes, namely whether the patient was removed from the waitlist for transplantation, deterioration, or improvement. The single waitlist death in our CLABSI cohort was unrelated, however, Sullivan et al[5]reported 3 deaths among the 25 patients with BA who received PN, one of which was due to fungal sepsis,highlighting that this threat is tangible. Due to the relatively small number of individuals in each group, these findings should be interpreted with caution,however.
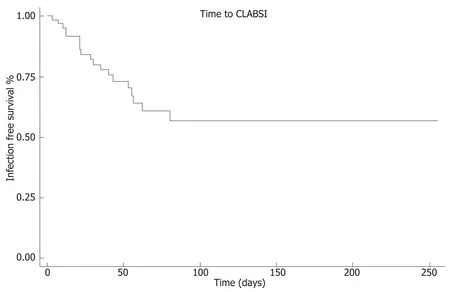
Figure 2 Time to central line-associated bloodstream infection survival analysis. Kaplan-Meier curve of time to CLABSI indicates that 75% of patients remained free of CLABSI on line day 43. CLABSI: Central line-associated bloodstream infection.
The microbiology of our CLABSI was predominantly Gram-negative, enteric organisms. Among children with BA, secondary bacteremia from ascending cholangitis is also a diagnostic consideration. However, care was taken in adjudicating CLABSI to exclude positive blood cultures when a concurrent diagnosis of cholangitis was made. Given the difficulties in diagnosing cholangitis in young children though, the possibility remains that some cholangitis episodes were not recognized, which may have led to an overestimation of CLABSI. Candida comprised about 20% of our CLABSI events, therefore it may also be prudent to consider a fungal etiology for CLABSI among young transplant candidates with BA receiving PN.
As expected, time with a CVC is related to incident CLABSI; according to our survival analysis, 75% of our patients remained free of CLABSI on line day 43. While interventions to reduce total line days may reduce CLABSI, this strategy is difficult to implement in the transplant candidate. Uncertainty with regard to timing of transplantation and the lack of data to support specific clinical or laboratory thresholds for nutritional intervention breeds an environment in which PN is prescribed from the time enteral nutrition is deemed to be insufficient until transplantation. A better understanding of anthropometric or nutritional thresholds associated with specific clinical outcomes may help to more precisely utilize PN. In the meantime, strategies for CLABSI reduction which have been studied in other patient populations should be explored in transplant candidates as well. CVC insertion bundles and parental line care training[12], ethanol locks[13], and taurolidine locks[14]have been suggested for CLABSI reduction in children with other conditions receiving PN.
Strengths of our data include the comparatively large size of the cohort and the application of a CLABSI definition which captures both the events occurring within a healthcare environment and in the community. A principal limitation of our data is its retrospective nature; this precluded standardized data collection and led to nonuniform utilization criteria and prescriptions for PN. And while our sample size is large for study of a very specific sub-group of pediatric patients, in absolute terms, its size limits statistical power. Post-hoc power calculations suggest that the sample sizes observed in this study has about 67% power to detect a hazards ratio of 2.0 between two groups, assuming 30% of patients in one group have CLABSI and 9% in the second group, and alpha = 0.05 (two-sided). It is likely that only a concerted multicenter effort, like Studies in Pediatric Liver Transplantation, would be able to overcome the power problem, however information on CVC use is not currently collected in this registry.
In conclusion, our series calls attention to the substantial risk for CLABSI among children with BA listed for liver transplantation. No clinical, demographic, or laboratory factor we tested emerged as an independent predictor of CLABSI, but time with a CVC was directly related to incident CLABSI. While our data did not show an impact of CLABSI on the short-term clinical outcome, it would seem prudent to implement CLABSI reduction strategies in this population to the extent that each CLABSI event represents potentially preventable hospitalization, unnecessary healthcare dollar expenditures, and may exact an opportunity cost, in terms of missed allograft offers.
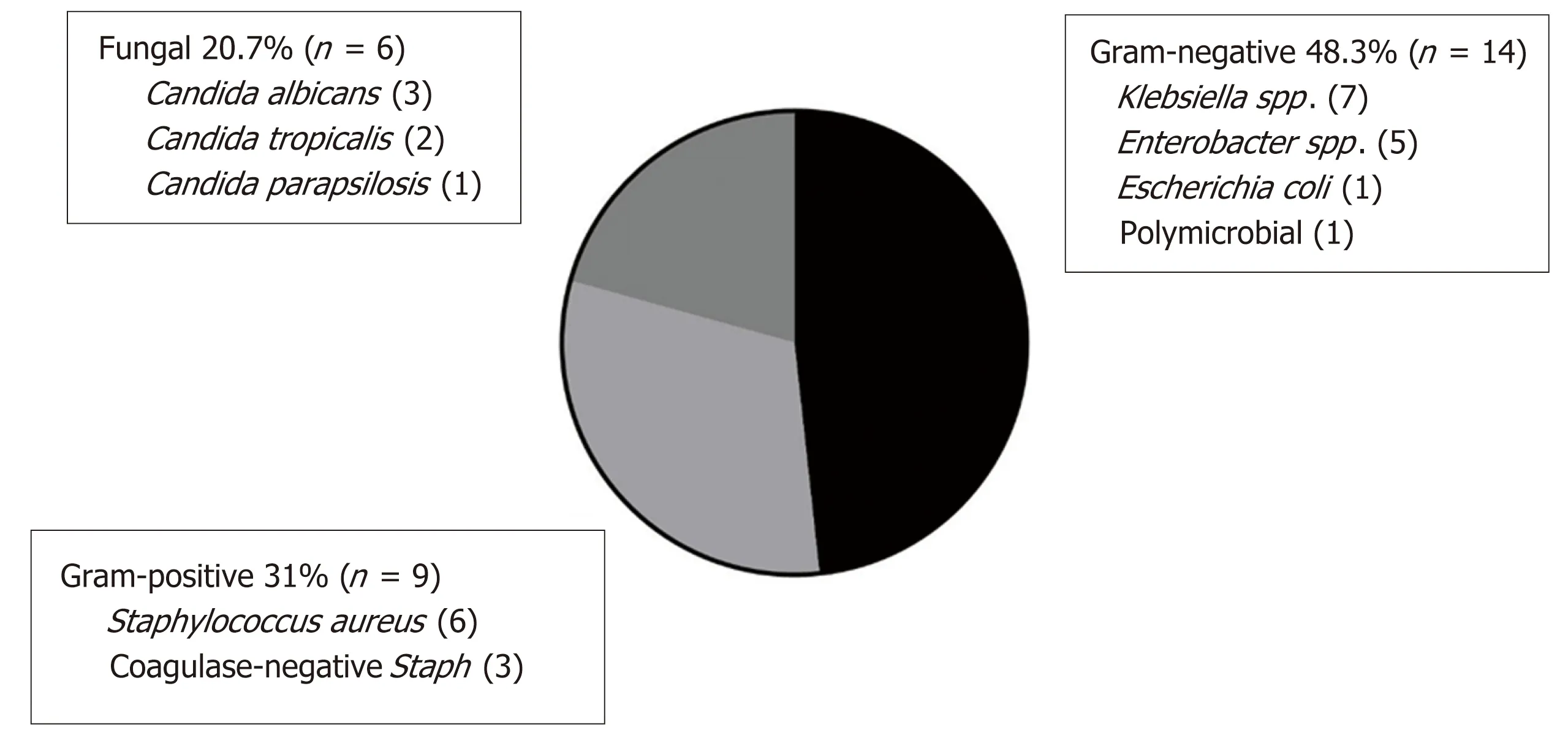
Figure 3 Microbiology of central line-associated bloodstream infection events.
ARTICLE HIGHLIGHTS
Research background
Children with biliary atresia (BA) undergoing liver transplantation benefit from pre-operative optimization of their nutritional status. When feeding enterally is insufficient to rehabilitate these patients, parenteral nutrition (PN) may be a useful adjunct. While this modality has been shown to improve the growth of children with BA listed for liver transplantation, it is also associated with distinct risks, chief among them the risk of infection associated with an indwelling central venous catheter.
Research motivation
Our group was motivated to pursue this project so that the field might have a better understanding of the infectious risks of PN given to children with BA on the liver transplant waitlist, and thus make informed decisions regarding risk and benefit to the patient.
Research objectives
The objective of our study was to describe the incidence, microbiology, and risk factors of central line-associated bloodstream infection (CLABSI) among children with BA listed for liver transplantation.
Research methods
Retrospective, single-center review.
Research results
Nineteen of 63 patients (30%) experienced 29 episodes of CLABSI during 4800 line days (6.04 CLABSI per 1000 line days). CLABSI were predominantly associated with Gram-negative organisms (14/29 episodes, 48%) including Klebsiella spp., Enterobacter spp., and Escherichia coli.The sole polymicrobial infection grew Enterobacter cloacae and Klebsiella pneumoniae. Grampositive organisms (all Staphylococcus spp.) and fungus (all Candida spp.) comprised 9/29 (31%)and 6/29 (21%) episodes, respectively. There were no demographic, laboratory, or clinical features associated with CLABSI risk in our model.
Research conclusions
CLABSI events are not rare among children with BA, receiving PN, while listed for liver transplantation. In spite of the frequency of events, CLABSI were not associated with mortality,or removal from the transplant waitlist due to becoming too ill to transplant. Since none of the factors tested in our model were associated with CLABSI risk, we propose meticulous application of known CLABSI-reducing strategies, such as line insertion bundles.
Research perspectives
Owing to the relatively small volume of pediatric liver transplants performed, even at the largest centers, future efforts should consider leveraging existing databases, such as Studies in Pediatric Liver Transplantation, to address these questions.
杂志排行
World Journal of Hepatology的其它文章
- Bariatric surgery in patients with non-alcoholic fatty liver disease -from pathophysiology to clinical effects
- Hepatic encephalopathy: Lessons from preclinical studies
- Comprehensive analysis of HFE gene in hereditary hemochromatosis and in diseases associated with acquired iron overload
- Clinical outcomes after major hepatectomy are acceptable in lowvolume centers in the Caribbean
- Parallel transjugular intrahepatic portosystemic shunt with Viatorr®stents for primary TlPS insufficiency: Case series and review of literature
- Necrolytic acral erythema in a human immunodeficiency virus/hepatitis C virus coinfected patient: A case report
