Bariatric surgery in patients with non-alcoholic fatty liver disease -from pathophysiology to clinical effects
2019-03-21TeaLaursenChristofferHagemannChunshanWeiKonstantinKazankovKarenThomsenFilipKnopHenningGrnb
Tea L Laursen, Christoffer A Hagemann, Chunshan Wei, Konstantin Kazankov, Karen L Thomsen,Filip K Knop, Henning Grønbæk
Abstract Non-alcoholic fatty liver disease (NAFLD) is increasingly recognized as a significant liver disease, and it covers the disease spectrum from simple steatosis with a risk of development of non-alcoholic steatohepatitis (NASH) to fibrosis,subsequent cirrhosis, end-stage liver failure, and liver cancer with a potential need for liver transplantation. NAFLD and NASH are closely related to obesity,metabolic syndrome, and type 2 diabetes (T2D). The role of gut hormones,especially glucagon-like peptide 1 (GLP-1), is important in NAFLD. Bariatric surgery has the potential for inducing great weight loss and may improve the symptoms of metabolic syndrome and T2D. Recent data demonstrated significant effects of bariatric surgery on GLP-1 and other gut hormones and important lipid metabolic and inflammatory abnormalities in the pathophysiology of NAFLD.Therefore, bariatric surgery may reverse the pathological liver changes in NAFLD and NASH patients. In the present review, we describe NAFLD and NASH pathophysiology and the primary effects of bariatric surgery on metabolic pathways. We performed a systematic review of the beneficial and harmful effects and focused on changes in liver disease severity in NAFLD and NASH patients. The specific focus was liver histopathology as assessed by the invasive liver biopsy. Additionally, we reviewed several non-invasive methods used for the assessment of liver disease severity following bariatric surgery.
Key words: Non-alcoholic fatty liver disease; Non-alcoholic steatohepatitis; Bariatric surgery; Insulin resistance, Gut hormones; Glucagon-like peptide 1; Steatosis;Inflammation; Fibrosis
INTRODUCTION
Alarming increases in obesity and diabetes coupled with changes towards unhealthy lifestyles and dietary habits have contributed to a dramatic increase in non-alcoholic fatty liver disease (NAFLD), which affects 25%-30% of the general population[1].NAFLD is most often asymptomatic and consists of a disease spectrum ranging from simple steatosis (NAFL) and steatohepatitis (NASH) to fibrosis and cirrhosis, with significant clinical consequences, including but not limited to ascites, varices, hepatic encephalopathy, liver cancer and liver transplantation or early death[2]. NASH may develop with hepatic inflammation, hepatocellular injury, macrophage and hepatic stellate cell activation in patients with simple steatosis. If untreated, NASH may progress to cirrhosis. NASH-induced cirrhosis is fast becoming the most common indication for liver transplantation, which is strongly associated with poor quality of life[3].
NAFLD is closely related to obesity and type 2 diabetes mellitus (T2D), and it is often termed the hepatic manifestation of metabolic syndrome[4,5]. The prevalence of NASH increases with components of the metabolic syndrome in T2D[6]. Available epidemiological data suggest a prevalence of NAFLD of 40%-70% in European T2D patients[1]. Insulin resistance is more prevalent in NASH patients than patients with simple steatosis[7], and patients with NAFLD without T2D exhibit decreased insulin sensitivity[8].
Bariatric surgery is an efficient treatment of obesity and causes sustained weight loss with potential reductions in hepatic fat, inflammation and fibrosis[9,10]. Roux-en-Y gastric bypass surgery (RYGB) is the most effective treatment for obesity[11]. This procedure improves glycaemic control, and T2D patients experience a reduced need for antidiabetic medication within days after surgery[9]. Sleeve gastrectomy (SG) and adjustable gastric banding (AGB) are alternative surgical approaches that significantly reduce gastric volume without changing the upper gastrointestinal tract anatomy. SG has gained popularity in recent years and been established as a comparable method to RYGB. In contrast, AGB is associated with less weight loss than RYGB surgery. The indication for bariatric surgery is severe obesity with or without T2D and/or other comorbidities[9].
LITERATURE SEARCH
We searched the following databases: MEDLINE Ovid (1946 to June 2018), Science Citation Index Expanded (Web of Science; 1900 to June 2018), and PubMed [Bethesda(MD): National Library of Medicine (US) 1966 to June 2018]. The following search terms were used: “Non-alcoholic fatty liver disease” (MeSH, all fields) or “Nonalcoholic steatohepatitis” (all fields) and “bariatric surgery (MeSH, all fields). Only English language articles were selected, and case reports were excluded. Full-text evaluation was performed, and references from relevant manuscripts were reviewed manually for additional manuscripts. This search strategy identified 404 studies at the end of June 2018. Studies were included in our comprehensive review if they were published between January 2010 and June 2018, were prospective or retrospective observational studies and if they evaluated the effects of bariatric surgery on histopathological NAFLD. In total, we ended up with 13 studies.
PATHOGENESIS OF NAFLD
The pathogenic mechanisms for the development and progression of NAFLD are complex and multifactorial[12](Figure 1). Genetic and epigenetic factors affect the development of NAFLD and NASH progression and potentially influence or modify risk factors[13-16]. Dietary sugars, fat, adipose tissue lipolysis, and de novo lipogenesis contribute to increased hepatic fat influx and accumulation in obese patients[17]. Obese patients exhibit increased adipose tissue mass, which leads to adipocyte dysfunction,including insulin resistance, increased lipolysis and apoptosis, and results in local inflammation and cytokine release. Insulin resistance reduces insulin-induced inhibition of lipolysis, and negatively affects the ability of the adipose tissue to store fat, which results in increased free fatty acids in the blood. Insulin resistance induces further insulin secretion, which instigates high blood insulin levels[18,19].
Hepatic de novo lipogenesis is also augmented in obese patients, partially due to enzyme upregulation induced by hyperinsulinaemia, elevated plasma glucose levels and endoplasmic reticulum (ER) stress[20-22]. Lipid accumulation in the liver primarily consists of triglycerides, which may not be hepatotoxic per se, but reflects the general inability of hepatocytes to handle fatty acids and leads to the concurrent accumulation of toxic lipid metabolites[23-25]. Long-chain saturated fatty acids resulting from de novo lipogenesis specifically harm liver cells via triggering the formation of reactive oxygen species, which highly contribute to hepatic lipotoxicity[19].
Activation of death receptors and their ligands, induction of ER stress, the production of reactive oxygen species and mitochondrial stress and dysfunction lead to hepatocyte injury and death with subsequent release of proteins, debris, etc., which are collectively defined as damage-associated molecular patterns (DAMPs)[26,27]. Fatty acids, DAMPs and pathogen-associated molecular patterns (PAMPs), e.g., bacteria and endotoxins, likely originating from a leaky gut, are the primary inducers of hepatic inflammation, which involves activation of resident and recruited macrophages in the liver[28,29]. Macrophage activation results in pro-inflammatory cytokine secretion and the activation of hepatic stellate cells into myofibroblasts,which secrete the collagen that contributes to extracellular matrix formation.Myofibroblasts are also directly responsive to cytokines, DAMPs and PAMPs, thus further propagating fibrosis formation[30].
METABOLIC EFFECTS OF BARIATRIC SURGERY
Bariatric surgery has tremendous effects on metabolic functions. Buchwald et al[31]performed a meta-analysis of 136 studies that assessed the impact of bariatric surgery on metabolic outcomes and reported a complete resolution of T2D in more than 75%of diabetic patients and an excessive weight loss of almost 60%. A review of key results from the Swedish Obese Subjects study reported a 72% remission rate of T2D two years post-bariatric surgery[32]. Several other studies demonstrated that RYGB and SG were superior to conventional pharmacological therapy in achieving glycaemic control in T2D patients[33-35].
NAFLD is closely associated with obesity and T2D, and the mechanisms implicated in improving obesity and T2D following bariatric surgery likely play important roles in the resolution of NAFLD. Several mechanisms independent of weight loss are involved in the initial metabolic responses to RYGB and SG procedures and maintaining these improvements over the long term, despite the obvious causality between weight loss and improvements in T2D and NAFLD.

Figure 1 Risk factors and mechanisms associated with non-alcoholic fatty liver disease development and progression. NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis; ER: Endoplasmic reticulum; ROS: Reactive oxygen species; HCC: Hepatocellular carcinoma.
The almost immediate metabolic benefits of bariatric surgery, independent of any significant weight loss, have been known for decades[36]but are striking nonetheless[31,37,38]. Three primary mechanisms are involved in the improved glycaemic control associated with the RYGB and SG procedures: (1) early improved hepatic insulin sensitivity due to the post-surgery calorie restriction; (2) late improved peripheral insulin sensitivity due to weight loss; and (3) improved post-prandial insulin secretion due to a rise in glucagon-like peptide 1 (GLP-1) secretion (Figure 2).Several studies investigated the post-surgical metabolic changes, and whether the change in the release of gut hormones or surgery-induced restriction of food intake provides the essential effects on glycaemic control remains controversial. Jørgensen et al[38,39]found an increase in post-prandial GLP-1, insulin secretion and hepatic insulin sensitivity within days after RYGB, and this increase was sustained for at least 1 year in diabetic and non-diabetic-matched subjects, which is consistent with other RYGB and SG studies[37,40,41]. The GLP-1 increase represents a powerful beta-cell stimulus and is explained by the accelerated entry of nutrients into the small intestine after RYGB[42,43]and SG[44], which increases the glucose absorption rate in the L cells responsible for the GLP-1 secretion[45]. The accelerated transport of nutrients into more distal parts of the small intestine may further explain the exaggerated GLP-1 response because a higher density of L cells are found in this area[46]. Notably, GLP-1 may have beneficial gene-regulatory effects on fatty acid oxidation and insulin sensitivity in hepatocytes[47], but these findings require confirmation in humans. Postprandial glucagon responses also increase post-operatively[38,48]despite the inhibitory effects of GLP-1 on glucagon secretion[39]. This paradoxical effect (in the context of improved glucose metabolism) may represent gut-derived glucagon[42]and may exert an attenuating effect on glycaemic control post-surgery.
Steven et al[49], among others[50], demonstrated that the reduced liver fat content from calorie restriction explained the early improvement in hepatic insulin sensitivity,as illustrated using magnetic resonance imaging. These data suggest that significant caloric restriction explains the almost immediate metabolic benefits from bariatric surgery due to improved liver function[49,51]. Vetter et al[48]demonstrated that the improvements in liver insulin sensitivity from RYGB exceeded lifestyle modifications.However, blockade of the GLP-1 receptor using the antagonist exendin9-39 consistently lowered insulin secretion after RYGB and SG[39,52], and it reversed the postprandial hyperinsulinaemic hypoglycaemia observed post-operatively[38], which confirmed the causative role of GLP-1 in beta-cell stimulation[53-55]. Calorie restriction is the leading mechanism of the early metabolic changes after bariatric surgery, but the gut hormones, particular GLP-1, remain crucial for the fine-tuning of the glycaemic control and post-prandial insulin secretion[56].
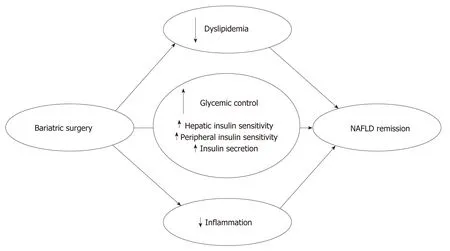
Figure 2 Main metabolic effects of bariatric surgery on remission. NAFLD: Non-alcoholic fatty liver disease.
A hyperinsulinaemic clamp study of Bojsen-Møller et al[37]demonstrated that peripheral insulin sensitivity, as assessed using glucose disposal and suppression of fatty acids, increased after three months in relation to the surgery-induced weight loss. Lifestyle modifications exert the same powerful effects on glycaemic control and NAFLD[48,57], but this intervention generally fails to sustain the short-term weight loss[32]. Notably, bariatric surgery is superior in maintaining calorie restriction and long-term weight loss, which is facilitated at least partially by the reduced appetite observed post-operatively[58,59]. The long-term reduced appetite may be attributable to a favourable shift in the anorectic gut hormones GLP-1 and peptide-YY (PYY)[58,60]. An antagonist study using exendin9-39 and a dipeptidyl peptidase 4 (DPP-4) inhibitor(blocking the DPP-4-mediated formation of active PYY from its precursor) in RYGB-operated subjects demonstrated a 20% increase in food intake[61]. Several other factors are candidates for the long-term metabolic improvements of bariatric surgery,including ghrelin[62], adiponectin[63], increased plasma bile acids[64]and changed intestinal microbiota[65], but proof of causality for these factors remains to be established in humans.
HEPATIC EFFECTS OF BARIATRIC SURGERY
Bariatric surgery affects NAFLD not only through a rapid and substantial weight loss but also via simultaneous effects on important lipid metabolic and inflammatory pathways involved in NAFLD pathophysiology[66,67]. Bariatric surgery promotes changes in three crucial metabolic areas influencing NAFLD: improved glucose homeostasis, improved lipid metabolism and reduced inflammatory activity (Figure 2). These effects are followed by significant effects on liver abnormalities in NAFLD and NASH patients.
Liver histology
A systematic review and meta-analysis[68]in 2008 assessed the histological effects of bariatric surgery in NAFLD patients. This review concluded that the features of steatosis, steatohepatitis, and fibrosis improve or resolve in most patients following weight loss after bariatric surgery. A Cochrane review in 2010 reported more discrete conclusions[69]. These authors were not able to identify any randomized clinical trials at that time and advised caution even though several reports demonstrated potential favourable effects of bariatric surgery. Most studies reported beneficial effects on steatosis, and more than half of the studies demonstrated significant improvements in histological inflammation. Six studies demonstrated improvement in fibrosis scores,but 4 studies[70-73]reported some worsening of fibrosis.
Our literature search did not identify any randomized controlled trials that assessed the hepatic histological effects of bariatric surgery in NAFLD patients. We performed a comprehensive review of prospective and retrospective observational studies published since 2010 to evaluate the effects of bariatric surgery on histopathological NAFLD (Table 1). We identified 13 studies: Eight studies with prospective designs, two studies with retrospective designs and three studies in which the design was not obvious. The types of surgery included RYGB, AGB and SG,and most studies assessed the effect of RYGB. The sample size ranged from 9 to 578 patients. The studies clustered into three categories based on participant numbers:Two large studies with more than 150 participants[74,75], three studies with 50-150 participants[10,76,77]and eight small studies with less than 50 participants[78-85].
In the largest study by Caiazzo et al[74]including more than 500 patients, the effects of RYGB and AGB were compared. Improvement and resolution of steatosis,inflammation and fibrosis were observed one and five years after both types of surgery. Biopsies at all three time points (before and 1 and 5 years post-surgery) were available in 315 patients, and the authors did not describe any cases of worsening. The best effects on weight loss and liver histology were achieved in the RYGB patients,and the primary effect derived from a greater weight loss but additionally explained by a more positive influence on glucose and lipid metabolism.
The second largest study of 160 patients undergoing RYGB or ABG with a mean follow-up of 31 mo demonstrated resolution or improvement of steatosis and inflammation in most patients[75]. Fibrosis resolved or improved in more than half of the patients. However, 8% of the patients progressed or developed steatosis de novo after surgery. Portal inflammation worsened in 10% and developed de novo in 27% of the patients, and 16% developed lobular inflammation after surgery. Fibrosis progressed in 12% of the patients with pre-surgery fibrosis and another 21%developed de novo fibrosis. Three patients developed NASH de novo after surgery.
In general, all of the smaller studies reported improvements in steatosis,inflammation and fibrosis. However, three of these studies found a worsening of some histological features, e.g., inflammation[79]and fibrosis[77,81], and three other studies reported no worsening[76,78,80]. The remaining studies did not describe worsening in any patients. The histological liver changes were accompanied with beneficial effects on metabolic syndrome[78,79], hypertension, dyslipidaemia and obstructive sleep apnoea[84]. Most studies performed follow-up biopsies after one year or later after surgery, but two studies performed follow-up at three[83]and six[80]months. Notably, the effects of surgery were visible at these time points, even for fibrosis.
Lassailly et al[10]investigated differences in patients with resolution of NASH one year after surgery and patients with persistent NASH and found that these patients had lost significantly less weight and were more frequently classified with a refractory IR profile, which suggests that the weight loss was of primary importance.
Non-invasive methods
Other studies used diverse non-invasive methods to examine the hepatic effects of bariatric surgery and found improvements in general. Several studies investigated how bariatric surgery affected the levels of circulating liver transaminases, in general reporting favourable effects, as summarized in a meta-analysis[86]. However,transaminases exhibit limited accuracy for the prediction of NASH severity.
Several studies used non-invasive fibrosis scores. One study demonstrated decreases in NAFLD fibrosis scores, ratio of aminotransferase (AST) to alanine aminotransferase (ALT), AST-to-platelet ratio index (APRI), and BARD score (BMI,ASAT/ALAT ratio, and the presence of T2D) one year after surgery[87]. Decreases in the NAFLD fibrosis score were confirmed in several other studies. Two studies of RYGB found a significant decrease 12 mo[88]and 36 mo after surgery[89], but higher levels in patients who regained weight after the initial weight loss. One study of 56 adolescents described a decrease in NAFLD fibrosis score one and two years after AGB[90]. However, Simo et al[91]questioned the feasibility of the NAFLD fibrosis score in relation to RYGB because several patients were wrongly classified or ended in a group of indeterminate classification. None of these studies included pre- or postsurgery biopsies as golden standards for treatment effects.
Forty-two patients who underwent diverse types of surgery were followed up with liver stiffness measurements and controlled attenuation parameter (CAP) using transient elastography. Liver stiffness declined from 8.6 to 6.0 kPa one year after surgery. CAP values declined from 322 dB/m at baseline to 251 dB/m at one year.These changes paralleled the histological changes in 32 of the patients[85]. The decrease in liver stiffness was consistent with a decrease from 6.95 to 5.37 kPa in another study of 38 patients after bariatric surgery[92]. Another study of 100 prospectively included bariatric patients reported a decrease from 12.9 to 7.1 kPa[87].
Magnetic resonance imaging (MRI) is increasingly used in the assessment of liver pathology. A prospective study of 31 obese patients undergoing RYGB demonstrated a significant reduction in hepatic fat content using MRI spectroscopy 12 mo after surgery[93]. A decrease in the liver fat fraction using MRI was also observed six[94]and 12 mo[95]after surgery in other studies. These results were supported by a decreased or complete resolution of liver steatosis/NAFLD on ultrasonography one to five yearsafter SG[96,97]. A decrease in hepatic left lobe volume on ultrasound was also observed after two years in 75 women who underwent laparoscopic AGB[98]. Two small studies found improvements in liver damage and NAFLD using ultrasound imaging[99]and CT[100].
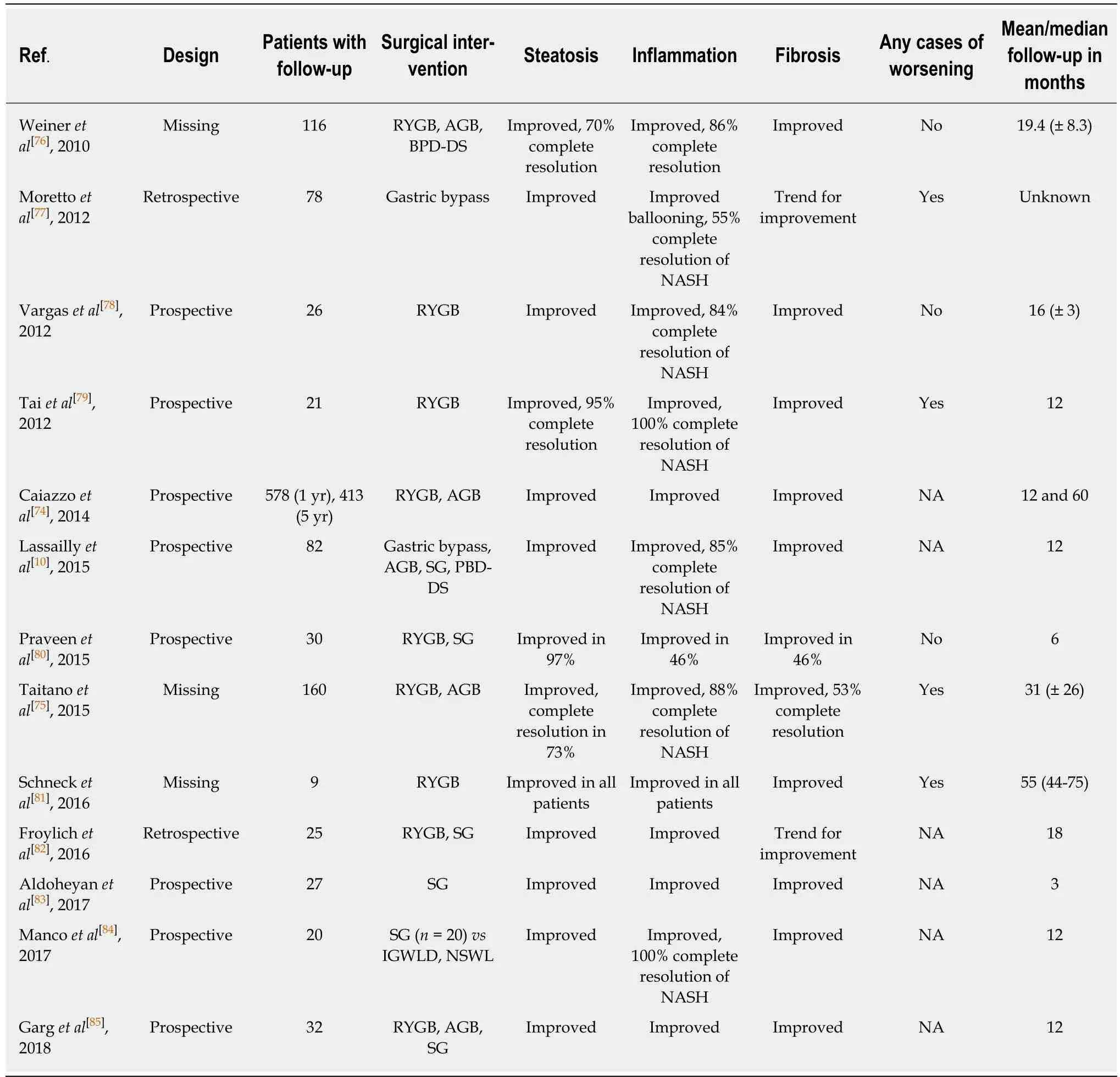
Table 1 Recent studies (2010-) with histological assessment of liver biopsies at follow-up
Additional exploratory results include decreased serum levels of the hepatocyte apoptosis marker cytokeratin (CK)-18 one year after surgery in nine patients, which was maintained approximately four years later with corresponding improvements in liver histology[81]. Significant improvements in metabolic liver function capacity using LiMAx, which is based on hepatic13C-methacetin metabolism by cytochrome P450 1A2, were also observed six and 12 mo after surgery[101].
Liver-related mortality
Overall, current studies reported no significant effects on liver-related mortality after bariatric surgery, but larger long-term follow-up studies are necessary to firmly establish the effect of bariatric surgery on liver-related mortality. A recent nation-wide study of patients after bariatric surgery observed no increase in all-cause mortality compared with the general population. However, there was an increased mortality rate ratio 2.01 (95%CI: 1.06-3.84) for gastrointestinal and liver diseases, including peritonitis and intestinal obstruction[102]. Mortality was significantly reduced in patients undergoing bariatric surgery compared to a propensity-score matched cohort of obese patients, but there was no difference in survival when the analysis was restricted to include NASH patients only[103]. Liver cirrhosis is a relative contraindication to bariatric surgery. However, no increased risks of postoperative complications or cirrhosis-related complications were observed in 13 cirrhosis patients undergoing SG with a follow-up of 18 mo. Weight loss in the cirrhosis patients was comparable to the non-cirrhotic patients[104].
CONCLUSION
In conclusion, bariatric surgery has the potential to induce great weight loss and improve the features of metabolic syndrome and T2D. Recent data demonstrate significant effects of bariatric surgery on GLP-1 and other gut hormones and important lipid metabolic and inflammatory abnormalities involved in the pathophysiology of NAFLD. Therefore, bariatric surgery may reverse the pathological liver changes in NAFLD and NASH patients. Several cohort studies demonstrated improvements in NASH histology, but some studies reported worsened liver histology after bariatric surgery. No studies demonstrated reduced liver-related mortality.
Large randomized clinical trials with long-term follow-up are needed to demonstrate the beneficial effects of bariatric surgery and identify a definitive role of bariatric surgery in NASH patients.
杂志排行
World Journal of Hepatology的其它文章
- Hepatic encephalopathy: Lessons from preclinical studies
- Comprehensive analysis of HFE gene in hereditary hemochromatosis and in diseases associated with acquired iron overload
- Clinical outcomes after major hepatectomy are acceptable in lowvolume centers in the Caribbean
- Central line-associated bloodstream infection among children with biliary atresia listed for liver transplantation
- Parallel transjugular intrahepatic portosystemic shunt with Viatorr®stents for primary TlPS insufficiency: Case series and review of literature
- Necrolytic acral erythema in a human immunodeficiency virus/hepatitis C virus coinfected patient: A case report
