转录因子BES1/BZR1调控植物生长发育及抗逆性
2019-03-19于好强孙福艾冯文奇路风中李晚忱付凤玲
于好强,孙福艾,冯文奇,路风中,李晚忱,付凤玲
转录因子BES1/BZR1调控植物生长发育及抗逆性
于好强,孙福艾,冯文奇,路风中,李晚忱,付凤玲
四川农业大学玉米研究所,农业部西南玉米生物学与遗传育种重点实验室,温江 611130
油菜素内酯(brassinosteroid, BR)是植物特有的甾体激素,在植物生长发育及逆境应答过程中起重要作用。转录因子BES1/BZR1(BRI1 EMS SUPPRESSOR 1/BRASSINAZOLE RESISTANT 1)是BR信号转导的核心成员,被BR信号激活后,结合到下游靶基因启动子区的E框(CANNTG)或BRRE元件(CGTGT/CG),调节靶基因表达。除介导BR信号,BES1/BZR1还参与脱落酸、赤霉素及光等信号转导途径,协同调控植物的生长发育。最新研究发现,BES1/BZR1还参与调控植物的抗逆性。本文对转录因子BES1/BZR1通过信号转导调控植物生长发育和抗逆性分子机制的新近研究进展进行了综述,以期为相关研究提供参考。
油菜素内酯;生长发育;信号转导;抗逆性;BES1/BZR1转录因子
油菜素内酯(brassinosteroid, BR)是植物特有的甾体激素,在生长发育及环境胁迫应答中起重要作用,其生理活性远高于生长素(auxin, IAA)、赤霉素(gibberellins, GA)、细胞分裂素(cytokinin, CTK)、脱落酸(abscisic acid, ABA)和乙烯(ethylene, ET)[1,2]。BR合成基因过量表达或缺失对植物生长发育及产量、品质等农艺性状育均产生严重影响[3~6]。BR信号转导被阻断的植物则显现矮化、开花延迟、早衰等缺陷表型[7,8]。
BR被细胞膜上BRASSINOSTEROID INSENSITIVE 1 (BRI1)及BRI1-ASSOCIATED RECEPTOR KINASE1 (BAK1)等激酶接受后,通过信号转导激活转录因子BRI1 EMS SUPPRESSOR 1 (BES1)及其同源蛋白BRASSINAZOLE RESISTANT 1 (BZR1)的活性[9,10]。BES1与BZR1氨基酸序列相似性达88%,N端结构域相似性高达97%[11],编码基因以家族形式存在,本课题组在前期研究中将其统一命名为BES1/BZR1[12]。被BR信号激活后,BES1/BZR1直接或与其他转录因子一起结合到生长发育相关基因启动子的E框(CANNTG)或BRRE元件(CGTGT/ CG),调节这些基因的表达[13~15]。例如,BES1/BZR1抑制叶腋分生组织发育基因表达,可促进小穗发育,增加水稻产量[16]。BES1/BZR1调节根尖分生组织发育相关基因表达,进而调控根发育[4,17~19]。除介导BR信号,BES1/BZR1还参与ABA、GA及光等信号转导途径,调控植物的生长发育以及抗冻、耐旱、抗病等抗逆性。
本文对转录因子BES1/BZR1通过信号转导调控植物生长发育和抗逆性分子机制的新近研究进展进行了综述,以期为相关研究提供参考。
1 BES1/BZR1介导BR信号转导
2002年,Wang等[20]利用EMS诱变筛选到一个BR合成抑制突变体(),图位克隆获得基因,该基因编码核蛋白且受BR诱导。同年,Yin等[21]利用EMS诱变筛选到BR受体抑制因子BES1,受BR诱导并在细胞核中积累。后经证实BES1是一个BZR1类蛋白(BZR1- like protein),二者具有高度的序列相似性,N端均有一个核定位信号(NLS),C端均有22~24个丝氨酸或苏氨酸残基(S/TXXXS/T),该残基是BIN2、GSK-3等激酶磷酸化位点,磷酸化后进入细胞质被14-3-4蛋白降解[16,20,21]。直至2005年,Yin等[10]进一步证实BES1/BZR1是植物中特有的新一类转录因子,也是BR信号转导途径的唯一转录因子。细胞膜上的BRI1、BKI1和BAK1等激酶接受BR信号后,自身磷酸化并催化BRASSINOSTEROID-SIGNALLING KINASE1 (BSK)和CONSTITUTIVE DIFFERENTIAL GROWTH1 (CDG1)磷酸化,BSK与CDG1进一步磷酸化BRI1-SUPPRESSOR1 (BSU1),BSU1催化BRASSINOSTEROID INSENSITIVE2 (BIN2)去磷酸化,导致其自身被蛋白酶体降解,削弱BIN2对BES1/BZR1的磷酸化从而使其活性增加[9,10,21~23],BES1/BZR1通过调节下游靶基因的表达,调控植物的生长发育(图1A)。
BES1/BZR1成员N端均有一个bHLH结构域,可特异性结合到靶基因启动子区的E框或BRRE元件[10,24~26]。此外,多数BES1/BZR1成员均含有能被BIN2等激酶磷酸化的丝氨酸(serine, S)富集位点,个别成员包含一个与蛋白稳定性紧密相关的脯氨酸(proline, P)、谷氨酸(glutamic acid, E)、丝氨酸(serine, S)和苏氨酸(threonine, T)富集区(PEST基序)[10]。目前,拟南芥()和水稻()BES1/BZR1基因家族已被全部鉴定:拟南芥AtBES1/BZR1基因家族有6个成员,且功能存在部分冗余[10,20,21];水稻OsBES1/BZR1基因家族有4个成员[27]。玉米()ZmBES1/BZR1基因家族有11个成员[23,28]。此外,从白菜(ssp)、棉花()、油菜()和桉树()中均鉴定出多个BES1/ BZR1基因家族成员[29~33](表1)。进一步研究证实,BES1/BZR1基因家族成员通过不同信号途径调控植物生理代谢过程,进而调控植物生长发育及逆境响应。

:未知过程;:相互作用;:促进作用;:抑制作用
A:BES1/BZR1介导的BR信号转导;B:BES1/BZR1参与的ABA信号途径;C:BES1/BZR1参与的GA信号途径;D:BES1/BZR1参与的光信号途径;E:BES1/BZR1调控逆境应答途径;F:BES1/BZR1参与的生长素、乙烯及其他信号途径。BR:油菜素内酯;BES1/BZR1:转录因子;BKI1、BRI1、BAK1、BSK1及CDG1:蛋白激酶;BSU1:BRI1抑制因子;ABA:脱落酸;GA:赤霉素;PP2C:2C型丝氨酸苏//氨酸蛋白激酶;PP2A:2A型丝氨酸/苏氨酸蛋白激酶;PYL:ABA受体;BIN2:磷酸激酶;ABI3与ABI5:ABA响应的bZIP转录因子;DELLA:赤霉素负调控转录因子;SINAT与COP1:E3泛素连接酶;GATA2与HY5:光形态建成相关转录因子;UVR8:紫外光受体;PIF4:光敏色素互作因子;CRY:隐花色素。RD26与WRKY26:干旱相关转录因子;REF:乙烯应答因子;MEK6:促细胞分裂原活化蛋白激酶;P:磷。
2 BES1/BZR1参与ABA信号途径
ABA是植物体内重要激素之一,通过其直接受体PYL (pyrabactin resistance 1-like protein)、第二信使2C型蛋白磷酸酶(PP2C)及第三信使蔗糖非酵解型蛋白激酶(SnRK)向下游进行信号传递,在植物生长发育及抗逆过程中扮演重要角色,如衰老、抗旱、耐盐等[34~36]。研究发现,在突变体中,BZR1结合到ABA诱导型转录因子ABA INSENSITIVE 5 (ABI5)编码基因的启动子,抑制其表达,因而抑制突变体对ABA诱导的应答[37]。同时,BES1抑制ABA调节的转录因子ABI3编码基因的表达,进而抑制ABI3对下游ABI5转录因子的激活,致使ABA信号转导受阻,表现为苗期发育迟缓[38,39]。
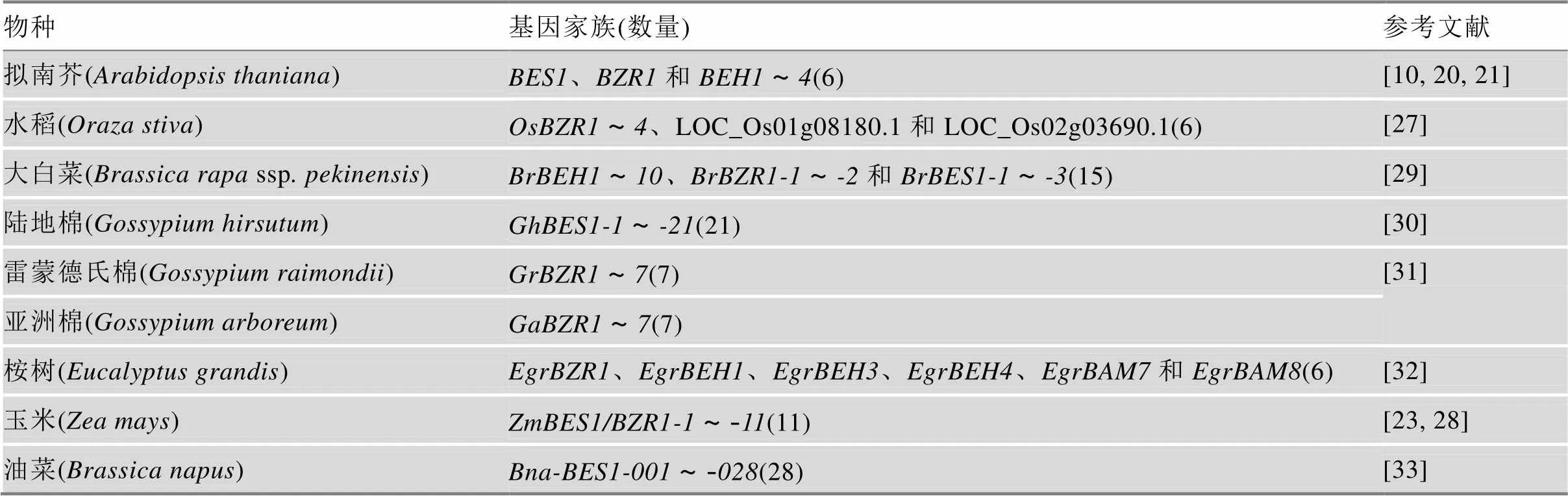
表1 已鉴定的不同植物BES1/BZR1基因家族成员
此外,外源ABA不仅诱导基因表达,而且诱导BES1蛋白磷酸化,使其稳定性降低,从而抑制BR信号转导,此过程依赖于ABA第二信使PP2C成员ABI1和ABI2[12,29,40,41]。最新研究表明,ABI1、ABI2与BIN2激酶互作后催化BIN2去磷酸化,从而调控BES1活性。ABA还可促进BIN2磷酸化并抑制ABI2的活性[42]。在ABA存在时,BIN2磷酸化ABI5使其稳定性增强,调控种子发育过程[43]。在大豆()中,PP2C-1与GmBZR1直接互作,催化GmBZR1去磷酸化以增强GmBZR1活性,促进种子大小相关基因()、()和()等表达,调控种子的大小与重量[44,45]。BZR1也可结合到ABA受体PYL6编码基因的启动子区,上调表达,从而参与PYL6介导的ABA信号转导[46]。研究还发现,BZR1的PEST结构域与蛋白磷酸酶2A(PP2A)的B亚基直接互作,使BZR1被PP2A去磷酸化,激活BZR1介导的BR信号途径,调控植物的生长发育[47](图1B)。
3 BES1/BZR1参与GA信号途径
作为植物体内重要激素之一,GA在种子萌发、细胞分裂、胚珠形成等生长发育过程中起关键作用[48,49]。研究发现,在BR缺失的突变体中,GA合成关键基因表达显著下调,而在突变体中,基因表达显著上调。同时,在和突变体中,基因均受BR诱导,表达显著上调。有研究表明,基因启动子区不含BES1/BZR1转录因子的结合位点。研究人员通过电泳迁移实验(electrophoretic mobility shift assays, EMSA)和染色质免疫共沉淀(chromatin immunoprecipitation, Chip)实验证实,BES1/BZR1可结合基因启动子区一个非E-Box且长度为12 bp的基序(Motif)[50,51]。此外,GA信号负调控因子DELLA家族蛋白(RGA、GAI、RGL1、RGL2和RGL3)可以和BES1/BZR1结合,阻止BES1/BZR1与靶基因的结合[50,52~55]。这些研究结果证实,DELLA蛋白降解可促使BES1/BZR1活性增强,BES1/BZR1结合GA合成相关基因启动子,使其表达上调,促进GA积累。此外,GA可通过PP2A促进BES1/BZR1的去磷酸化[54]。
在水稻中,BR诱导GA合成基因表达,促使GA积累。外源GA又抑制BR合成及其信号转导。进一步研究表明,GA合成关键基因、、和的启动子均包含CATGTG、BRRE或G-box元件。BES1/BZR1与这类元件直接结合,调节下游基因的表达,进而影响GA合成[56]。在番茄()中过表达,GA合成关键酶之一的酮戊二酸脱氢酶2 (2-ODD2)蛋白水平在果实成熟期显著增加,致使GA显著积累促进果实成熟[57]。水稻OsBZR1能够促进miR396d的积累,调控其靶基因()的表达,通过参与的GA合成及信号转导途径,调控水稻株高及叶夹角等形态建成[58](图1C)。
4 BES1/BZR1参与光信号途经
光是植物光合作用的能量之源,在调控植物生长发育中起关键作用,如光信号参与调控种子萌发、光形态建成和开花等[59]。转录因子GATA2、HY5正向调控植物光形态建成并受光诱导积累,黑暗促使其降解。研究发现,被BR激活的BZR1直接与GATA2互作,抑制转录,调控拟南芥幼苗下胚轴伸长[60,61]。黑暗条件下,HY5能特异地结合BZR1,抑制BZR1与子叶开闭相关基因的结合能力,调控光形态建成[61]。光敏色素互作因子(phytochrome interacting factor,PIF)是一类bHLH转录因子,在黑暗条件下,PIF大量积累,促进植物暗形态建成,但在光照条件下,PIF发生磷酸化后降解,促进植物光形态建成[62,63]。研究发现,BES1/BZR1与PIF 4相互作用,形成异源二聚体后作用于共同靶基因,其中80%靶基因受光诱导参与光形态建成[11]。此外,BZR1与PIF4共同作用的靶基因还受GA诱导,GA促进细胞伸长的过程依赖于BZR1和PIF4。DELLA- BZR1-PIF4复合体调控下游靶基因paclobutrazol resistance家族(PREs)表达,促进细胞伸长,调控光形态建成[11,53]。在高温条件下,BZR1和PIF4相互作用,调控植物热形态建成[64]。
最近研究发现,去磷酸化的BES1可与紫外光受体UVR8 (UV RESISTANCE LOCUS 8)互作,二者的复合体受紫外光(UV-B)诱导,并在细胞核大量积累。同时,UV-B不仅抑制BES1靶基因表达,其受体UVR8又抑制BES1与DNA的结合作用,最终控制植物光形态建成过程[65]。在蓝光条件下,其受体隐花色素(cryptochrome, CRY) CRY1和CRY2特异性与去磷酸化的BES1互作,抑制BES1与DNA结合活性及其靶基因表达,最终抑制下胚轴伸长[66]。
综上所述,BES1/BZR1参与光信号途径调控植物的形态建成过程。此外,还有研究发现,植物体内BES1/BZR1的磷酸化状态及稳定性也受光信号调控。黑暗条件促进BES1/BZR1去磷酸化以增强活性,而光照条件下,大多数BZR1被BIN2磷酸化以保持失活状态[61,67,68]。Kim等[68]研究发现,黑暗条件下,E3泛素连接酶COP1催化磷酸化后的BZR1降解,去磷酸化的BZR1积累。同时,光照条件可诱导E3泛素连接酶SINAT积累,SINAT泛素化BES1促使其降解。相反,黑暗条件抑制SINAT的积累,从而阻止BES1降解[69](图1D)。
5 BES1/BZR1调控植物抗逆性
BES1/BZR1除调控植物生长发育外,在响应生物和非生物逆境胁迫过程中也起重要作用。Guo等[70]研究发现,BES1/BZR1调控硫代糖苷合酶基因的表达,促进硫代糖苷合成,而硫代糖苷在植物与食草动物或与微生物互作中起重要作用。随后,Miyaji等[71]发现BZR1可能参与茉莉酸信号途径,增强植物抗虫能力。此外,研究表明,病原物相关分子模式(pathogen-associated molecular pattern, PAMP)感知可促进BES1磷酸化,在PAMP诱导的免疫反应(PAMP-triggered immunity, PTI)过程中,BES1作为病原菌诱导的MITOGEN-ACTIVATED PROTEIN KINASE 6 (MPK6)的直接底物被其磷酸化,参与调控植物对病菌的免疫反应[72]。
Singh等[73]研究发现,低磷胁迫促进BES1/ BZR1由细胞核向细胞质转移,低磷条件下,BES1/ BZR1显著积累,维持根系正常生长,赋予拟南芥对低磷胁迫的耐受性。研究表明,BES1/BZR1可促进转录因子CBF (C-repeat binding factor)、WRKY6以及ABA受体PYL6等编码基因的表达,并与WRKY54转录因子直接互作,正调控拟南芥耐寒性,但负调控其耐旱性[46,74]。研究还发现,BES1/BZR1与NAC转录因子家族的RD26存在拮抗关系,BES1/ BZR1结合到基因启动子,抑制表达,而RD26蛋白又与BES1/BZR1蛋白结合,抑制RD26干旱应答调节功能[75]。同时,在干旱和低碳胁迫下,BES1与泛素受体DSK2互作而被降解,参与胁迫诱导的自噬反应过程,调控植物适应逆境[76]。
此外,在拟南芥、油菜和桉树中,BES1/BZR1基因的表达受盐、干旱、热和冷等胁迫的诱导或抑制[29,32,33],表明该基因家族参与这些逆境胁迫响应过程(图1E)。
6 BES1/BZR1参与的信号转导网络
BES1/BZR1是BR信号转导途径特异的转录因子,通过介导BR信号调控植物生长发育。但近年来研究发现,BES1/BZR1在ABA、GA、光及逆境信号中也发挥着重要作用,并且还参与IAA、ET等信号途径。如BZR1直接与生长素诱导基因、的启动子结合而抑制其表达,从而影响生长素的合成,调控植物生长发育[77]。BES1/BZR1通过下调乙烯合成关键酶基因、和表达,抑制乙烯合成,而且与乙烯响应因子ERF72互作调控其下游基因表达,最终影响植物生长发育过程[4,78](图1F)。综上所述,BES1/BZR1参与多种信号途径,调控植物生长发育及逆境应答,其功能和作用机制表现出多样性。但是,BES1/BZR1调控植物响应逆境胁迫方面的研究还不够深入,除拟南芥外,在作物及其他植物中BES1/BZR1抗逆功能研究尚未见报道。因此,本文将BES1/BZR1参与的信号转导网络进行了归纳总结(图1),以期为后续相关研究提供参考。
[1] Nolan T, Chen J, Yin Y. Cross-talk of brassinosteroid signaling in controlling growth and stress responses., 2017, 474(16): 2641–2661.
[2] Song XJ. Crop seed size: BR matters., 2017, 10(5): 668–669.
[3] Li XJ, Chen XJ, Guo X, Yin LL, Ahammed GJ, Xu CJ, Chen KS, Liu CC, Xia XJ, Shi K, Zhou J, Zhou YH, Yu JQ.overexpression induces alteration in phytohormone homeostasis, development, architecture and carotenoid accumulation in tomato., 2016, 14: 1021–1033.
[4] Lv B, Tian H, Zhang F, Liu J, Lu S, Bai M, Li C, Ding Z. Brassinosteroids regulate root growth by controlling reactive oxygen species homeostasis and dual effect on ethylene synthesis in., 2018, 14(1): e1007144.
[5] Sahni S, Prasad BD, Liu Q, Grbic V, Sharpe A, Singh SP, Krishna P. Overexpression of the brassinosteroid biosynthetic geneinsimultaneously increases seed yield and stress tolerance., 2016, 6: 28298.
[6] Yang J, Thames S, Best NB, Jiang H, Huang P, Dilkes BP, Eveland AL. Brassinosteroids modulate meristem fate and differentiation of unique inflorescence morphology in., 2018, 30(1): 48–66.
[7] Corvalán C, Choe S. Identification of brassinosteroid genes in., 2017, 17(1): 5.
[8] Kir G, Ye H, Nelissen H, Neelakandan AK, Kusnandar AS, Luo A, Inzé D, Sylvester AW, Yin Y, Becraft PW. RNA interference knockdown of BRASSINOSTEROID INSENSITIVE1 in maize reveals novel functions for brassinosteroid signaling in controlling plant architecture., 2015, 169(1): 826–839.
[9] Belkhadir Y, Jaillais Y. The molecular circuitry of brassinosteroid signaling., 2015, 206(2): 522–540.
[10] Yin Y, Vafeados D, Tao Y, Yoshida S, Asami T, Chory J. A new class of transcription factors mediates brassinosteroid- regulated gene expression in., 2005, 120(2): 249–259.
[11] Wang ZY, Bai MY, Oh E, Zhu JY. Brassinosteroid signaling network and regulation of photomorphogenesis., 2012, 46: 701–724.
[12] Yu H, Feng W, Sun F, Zhang YY, Qu JT, Liu B, Lu F, Yang L, Fu F, Li W. Cloning and characterization of BES1/ BZR1 transcription factor genes in maize., 2018, 86(2): 235–249.
[13] Oh E, Zhu JY, Wang ZY. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses., 2012, 14(8): 802–809.
[14] Qiao S, Sun S, Wang L, Wu Z, Li C, Li X, Wang T, Leng L, Tian W, Lu T, Wang X. The RLA1/SMOS1 transcription factor functions with OsBZR1 to regulate brassinosteroid signaling and rice architecture., 2017, 29(2): 292–309.
[15] Ye H, Li L, Guo H, Yin Y. MYBL2 is a substrate of GSK3-like kinase BIN2 and acts as a corepressor of BES1 in brassinosteroid signaling pathway in., 2012, 109(49): 20142–20147.
[16] Bai XF, Huang Y, Hu Y, Liu HY, Zhang B, Smaczniak C, Hu G, Han ZM, Xing YZ. Duplication of an upstream silencer of FZP increases grain yield in rice., 2017, 3(11): 885–893.
[17] Jiang J, Zhang C, Wang X. A recently evolved isoform of the transcription factor BES1 promotes brassinosteroid signaling and development in., 2015, 27(2): 361–374.
[18] Martins S, Montiel-Jorda A, Cayrel A, Huguet S, Roux CP, Ljung K, Vert G. Brassinosteroid signaling-dependent root responses to prolonged elevated ambient temperature., 2017, 8(1): 309.
[19] Salazar-Henao JE, Lehner R, Betegón-Putze I, Vilarrasa- Blasi J, Caño-Delgado AI. BES1 regulates the localization of the brassinosteroid receptor BRL3 within the provascular tissue of theprimary root., 2016, 67(17): 4951–4961.
[20] Wang ZY, Nakano T, Gendron J, He J, Chen M, Vafeados D, Yang Y, Fujioka S, Yoshida S, Asami T, Chory J. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis., 2002, 2(4): 505–513.
[21] Yin Y, Wang ZY, Mora-Garcia S, Li J, Yoshida S, Asami T, Chory J. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation., 2002, 109(2): 181–191.
[22] Kim TW, Guan S, Sun Y, Deng Z, Tang W, Shang J X, Sun Y, Burlingame A L, Wang ZY. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors., 2009, 11(10): 1254–1260.
[23] Kim TW, Guan S, Burlingame AL, Wang ZY. The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2., 2011, 43(4): 561–571.
[24] He JX, Gendron JM, Sun Y, Gampala SSL, Gendron N, Sun CQ, Wang ZY. BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses., 2005, 307(5715): 1634–1638.
[25] Sun Y, Fan XY, Cao DM, Tang W, He K, Zhu JY, He JX, Bai MY, Zhu S, Oh E, Patil S, Kim TW, Ji H, Wong WH, Rhee SY, Wang ZY. Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in., 2010, 19(5): 765–777.
[26] Yu X, Li L, Zola J, Aluru M, Ye H, Foudree A, Guo H, Anderson S, Aluru S, Liu P, Rodermel S, Yin Y. A brassinosteroid transcriptional network revealed by genome-wide identification of BES1 target genes in., 2011, 65(4): 634–646
[27] Bai MY, Zhang LY, Gampala SS, Zhu SW, Song WY, Chong K, Wang ZY. Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice., 2007, 104(34): 13839–13844.
[28] Manoli A, Trevisan S, Quaggiotti S, Varotto S. Identification and characterization of the BZR transcription factor family and its expression in response to abiotic stresses inL., 2018, 84(3): 423–436.
[29] Saha G, Park JI, Jung HJ, Ahmed NU, Kayum MA, Kang JG, Nou IS. Molecular characterization of BZR transcriptionfactor family and abiotic stress induced expression profiling in., 2015, 92: 92–104.
[30] Shu HM, Guo SQ, Gong YY, Jiang L, Zhu JW, Ni WC. Identification and expression analysis of the Brassinosteroid signal gene GhBES1 family in upland cotton., 2017, 32(4): 7–12.束红梅, 郭书巧, 巩元勇, 蒋璐, 朱静雯, 倪万潮. 陆地棉油菜素内酯信号基因GhBES1家族的鉴定及表达分析. 华北农学报, 2017, 32(4): 7–12.
[31] Guo XL, Lu P, Wang YY, Cai XY, Wang XX, Zhou ZL, Wang YH, Wang CY, Wang KB, Liu F. Genome-wide identification and expression analysis of the gene family encoding Brassinazole resistant transcription factors in cotton., 2017, 29(5): 415–427.郭新磊, 路普, 王园园, 蔡小彦, 王星星, 周忠丽, 王玉红, 王春英, 王坤波, 刘方. 棉花BZR基因家族的全基因组鉴定及表达分析. 棉花学报, 2017, 29(5): 415–427.
[32] Fan C, Guo G, Yan H, Qiu Z, Liu Q, Zeng B. Characterization of Brassinazole resistant (BZR) gene family and stress induced expression in., 2018, 24(5): 821–831.
[33] Song X, Ma X, Li C, Hu J, Yang Q, Wang T, Wang L, Wang J, Guo D, Ge W, Wang Z, Li M, Wang Q, Ren T, Feng S, Wang L, Zhang W, Wang X. Comprehensive analyses of the BES1 gene family inand examination of their evolutionary pattern in representative species., 2018, 19: 346.
[34] Tao Y, Wang YG, Li HJ, Li WC, Fu FL. Upstream messengers of abscisic acid signaling pathway in plant., 2016, 30(9): 1722–1730.陶怡, 王盈阁, 李鸿杰, 李晚忱, 付凤玲. 植物脱落酸信号转导途径的上游信使. 核农学报, 2016, 30(9): 1722–1730.
[35] Raghavendra AS, Gonugunta VK, Christmann A, Grill E. ABA perception and signalling., 2010, 15(7): 395–401.
[36] Li HQ, Chen C, Chen RR, Song XW, Li JN, Zhu YM, Ding XD. Preliminary analysis of the role of.1 andin the ABA and alkaline stress response of the soybean using the CRISPR/Cas9-based gene double-knockout system., 2018, 40(6): 496–507.李慧卿, 陈超, 陈冉冉, 宋雪薇, 李佶娜, 朱延明, 丁晓东. 利用CRISPR/Cas9双基因敲除系统初步解析大豆和对ABA及碱胁迫的响应.遗传, 2018, 40(6): 496–507.
[37] Yang X, Bai Y, Shang J, Xin R, Tang W. The antagonistic regulation of abscisic acid-inhibited root growth by brassinosteroids is partially mediated via direct suppression of ABSCISIC ACID INSENSITIVE 5 expression by BRASSINAZOLE RESISTANT 1.,, 2016, 39(9): 1994–2003.
[38] Lopez-Molina L, Mongrand S, McLachlin DT, Chait BT, Chua NH. ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination., 2002, 32(3): 317–328.
[39] Ryu H, Cho H, Bae W, Hwang I. Control of early seedling development by BES1/TPL/HDA19-mediated epigenetic regulation of ABI3., 2014, 5: 4138.
[40] He JX, Gendron JM, Yang Y, Li J, Wang ZY. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in., 2002, 99(15): 10185–10190.
[41] Zhang S, Cai Z, Wang X. The primary signaling outputs of brassinosteroids are regulated by abscisic acid signaling., 2009, 106(11): 4543–4548.
[42] Wang H, Tang J, Liu J, Hu J, Liu J, Chen Y, Cai Z, Wang X. Abscisic acid signaling inhibits Brassinosteroid signaling through dampening the dephosphorylation of BIN2 by ABI1 and ABI2.2017, 11(2): 315–325.
[43] Hu Y, Yu D. BRASSINOSTEROID INSENSITIVE2 interacts with ABSCISIC ACID INSENSITIVE5 to mediate the antagonism of brassinosteroids to abscisic acid during seed germination in., 2014, 26(11): 4394–4408.
[44] Jiang WB, Huang HY, Hu YW, Zhu SW, Wang ZY, Lin WH. Brassinosteroid regulates seed size and shape in., 2013, 162(4): 1965–1977.
[45] Lu X, Xiong Q, Cheng T, Li QT, Liu XL, Bi YD, Li W, Zhang WK, Ma B, Lai YC, Du WG, Man WQ, Chen SY, Zhang JS. A PP2C-1 allele underlying a quantitative trait locus enhances soybean 100-seed weight., 2017, 10(5): 670–684.
[46] Li H, Ye K, Shi Y, Cheng J, Zhang X, Yang S. BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in, 2017, 10(4): 545–559.
[47] Tang W, Yuan M, Wang R, Yang Y, Wang C, Oses-Prieto JA, Kim TW, Zhou HW, Deng Z, Gampala SS, Gendron JM, Jonassen EM, Lillo C, DeLong A, Burlingame AL, Sun Y, Wang ZY. PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1., 2011, 13(2): 124–131.
[48] Gomez MD, Barro-Trastoy D, Escoms E, Saura-Sánchez M, Sánchez I, Briones-Moreno A, Vera-Sirera F, Carrera E, Ripoll JJ, Yanofsky MF, Lopez-Diaz I, Alonso JM, Perez-Amador MA. Gibberellins negatively modulate ovule number in plants., 2018, 145(13), doi: 10.1242/dev.163865.
[49] Vishal B, Kumar PP. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid., 2018, 9: 838.
[50] Gallego-Bartolomé J, Minguet EG, Grau-Enguix F, Abbas M, Locascio A, Thomas SG, Alabadí D, Blázquez MA. Molecular mechanism for the interaction between gibberellin and brassinosteroid signaling pathways in., 2012, 109(33): 13446–13451.
[51] Unterholzner SJ, Rozhon W, Papacek M, Ciomas J, Lange T, Kugler KG, Mayer KF, Sieberer T, Poppenberger B. Brassinosteroids are master regulators of gibberellin biosynthesis in., 2015, 27(8): 2261–2272.
[52] Allen HR, Ptashnyk M. Mathematical modelling and analysis of the brassinosteroid and gibberellin signalling pathways and their interactions., 2017, 432: 109–131.
[53] Bai MY, Shang JX, Oh E, Fan M, Bai Y, Zentella R, Sun TP, Wang ZY. Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in., 2012, 14(8): 810–817.
[54] Li QF, Wang C, Jiang L, Li S, Sun SS, He JX. An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in., 2012, 5(244): ra72.
[55] Li QF, He JX. Mechanisms of signaling crosstalk between brassinosteroids and gibberellins., 2013, 8(7): e24686.
[56] Tong H, Xiao Y, Liu D, Gao S, Liu L, Yin Y, Jin Y, Qian Q, Chu C. Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice., 2014, 26(11): 4376–4393.
[57] Liu L, Liu H, Li S, Zhang X, Zhang M, Zhu N, Dufresne CP, Chen S, Wang Q. Regulation of BZR1 in fruit ripening revealed by iTRAQ proteomics analysis., 2016, 6: 33635.
[58] Tang Y, Liu H, Guo S, Wang B, Li Z, Chong K, Xu Y. OsmiR396d affects gibberellin and brassinosteroid signaling to regulate plant architecture., 2018, 176(1): 946–959.
[59] Kami C, Lorrain S, Hornitschek P, Fankhauser C. Light-regulated plant growth and development., 2010, 91: 29–66.
[60] Luo XM, Lin WH, Zhu S, Zhu JY, Sun Y, Fan XY, Cheng M, Hao Y, Oh E, Tian M, Liu L, Zhang M, Xie Q, Chong K, Wang ZY. Integration of light- and brassinosteroid- signaling pathways by a GATA transcription factor in., 2010, 19(6): 872–883.
[61] Li QF, He JX. BZR1 interacts with HY5 to mediate Brassinosteroid- and light-regulated cotyledon opening inin darkness., 2015, 9(1): 113–125.
[62] Chen M, Chory J. Phytochrome signaling mechanisms and the control of plant development., 2011, 21(11): 664–671.
[63] Leivar P, Quail PH. PIFs: pivotal components in a cellular signaling hub., 2011, 16(1): 19–28.
[64] Ibanez C, Delker C, Martinez C, Bürstenbinder K, Janitza P, Lippmann R, Ludwig W, Sun H, James GV, Klecker M, Grossjohann A, Schneeberger K, Prat S, Quint M. Brassinosteroids dominate hormonal regulation of plant thermosmorphogenesis via BZR1., 2018, 28(2): 303–310.
[65] Liang T, Mei S, Shi C, Yang Y, Peng Y, Ma L, Wang F, Li X, Huang X, Yin Y, Liu H. UVR8 interacts with BES1 and BIM1 to regulate transcription and photomorphogenesis in., 2018, 44(4): 512–523.e5.
[66] Wang W, Lu X, Li L, Lian HL, Mao ZL, Xu PB, Guo T, Xu F, Du SS, Cao XL, Wang S, Shen H, Yang HQ. Photoexcited CRYPTOCHROME1 interacts with dephosphorylated BES1 to regulate Brassinosteroid signaling and photomorphogenesis in., 2018, doi: 10.1105/tpc.17.00994.
[67] Li QF, Huang LC, Wei K, Yu JW, Zhang CQ, Liu QQ. Light involved regulation of BZR1 stability and phosphorylation status to coordinate plant growth in., 2017, 37(2), doi: 10.1042/ BSR20170069.
[68] Kim B, Jeong YJ, Corvalán C, Fujioka S, Cho S, Park T, Choe S. Darkness and gulliver2/phyB mutation decrease the abundance of phosphorylated BZR1 to activate brassinosteroid signaling in., 2014, 77(5): 737–747.
[69] Yang M, Li C, Cai Z, Hu Y, Nolan T, Yu F, Yin Y, Xie Q, Tang G, Wang X. SINAT E3 ligases control the light-mediated stability of the Brassinosteroid-activated transcription factor BES1 in., 2017, 41(1): 47–58.e4.
[70] Guo R, Qian H, Shen W, Liu L, Zhang M, Cai C, Zhao Y, Qiao J, Wang Q. BZR1 and BES1 participate in regulation of glucosinolate biosynthesis by brassinosteroids in., 2013, 64(8): 2401–2412.
[71] Miyaji T, Yamagami A, Kume N, Sakuta M, Osada H, Asami T, Arimoto Y, Nakano T. Brassinosteroid-related transcription factor BIL1/BZR1 increases plant resistance to insect feeding., 2014, 78(6): 960–968.
[72] Kang S, Yang F, Li L, Chen H, Chen S, Zhang J. Thetranscription factor BES1 is a direct substrate of MPK6 and regulates immunity., 2015, 167(3): 1076–1086.
[73] Singh AP, Fridman Y, Friedlander-Shani L, Tarkowska D, Strnad M, Savaldi-Goldstein S. Activity of the brassinosteroid transcription factors BRASSINAZOLE RESISTANT1 and BRASSINOSTEROID INSENSITIVE1-ETHYL METHANESULFONATE-SUPPRESSOR1/BRASSINAZOLE RESISTANT2 blocks developmental reprogramming in response to low phosphate availability., 2014, 166(2): 678–688.
[74] Chen J, Nolan TM, Ye H, Zhang M, Tong H, Xin P, Chu J, Chu C, Li Z, Yin Y.WRKY46, WRKY54, and WRKY70 Transcription factors are involved in brassinosteroid-regulated plant growth and drought responses., 2017, 29(6): 1425–1439.
[75] Ye H, Liu S, Tang B, Chen J, Xie Z, Nolan TM, Jiang H, Guo H, Lin HY, Li L, Wang Y, Tong H, Zhang M, Chu C, Li Z, Aluru M, Aluru S, Schnable PS, Yin Y. RD26 mediates crosstalk between drought and brassinosteroid signalling pathways., 2017, 8: 14573.
[76] Nolan TM, Brennan B, Yang M, Chen J, Zhang M, Li Z, Wang X, Bassham DC, Walley J, Yin Y. Selective autophagy of BES1 mediated by DSK2 balances plant growth and survival., 2017, 41(1): 33–46.e7.
[77] Oh E, Zhu JY, Bai MY, Arenhart RA, Sun Y, Wang ZY. Cell elongation is regulated through a central circuit of interacting transcription factors in thehypocotyl., 2014, 3, doi: 10.7554/eLife.03031.
[78] Liu K, Li Y, Chen X, Li L, Liu K, Zhao H, Wang Y, Han S. ERF72 interacts with ARF6 and BZR1 to regulate hypocotyl elongation in., 2018, 69(16): 3933–3947.
The BES1/BZR1 transcription factors regulate growth, development and stress resistance in plants
Haoqiang Yu, Fuai Sun, Wenqi Feng, Fengzhong Lu, Wanchen Li, Fengling Fu
Brassinosteroid (BR) is a class of plant-specific steroidal hormone and plays vital roles in plant growth, developmental and stress response. As the core component of BR signaling, the BES1/BZR1 transcription factors are activated by the BR signal, bind to the E-box (CANNTG) or BRRE element (CGTGT/CG) enriched in the promoter of downstream target genes and regulate their expression. Besides BR signal transduction, BES1/BZR1s are also involved in other signaling pathways such as abscisic acid, gibberellin and light to co-regulate plant growth and development. Recently, BES1/BZR1s were found to be related to stress resistance. In this review, we summarize recent advances of molecular mechanism of the BES1/BZR1 transcription factors regulating plant growth, development and stress resistance through signal transduction to provide a reference for related researches.
brassinosteroid; growth and development; signal transduction; stress resistance; BES1/BZR1 transcription factors
2018-09-07;
2019-02-20
四川省科技计划应用基础项目(编号:2018JY0470)资助[Supported by the Sichuan Science and Technology Program (No.2018JY0470)]
于好强,博士,讲师,研究方向:植物分子生物学。E-mail: yhq1801@sicau.edu.cn
付凤玲,博士,教授,研究方向:玉米遗传育种与生物技术。E-mail: ffl@sicau.edu.cn
10.16288/j.yczz.18-253
2019/2/25 15:23:43
URI: http://kns.cnki.net/kcms/detail/11.1913.R.20190225.1523.004.html
(责任编委: 张宪省)
