Pseudothrombus deposition accompanied with minimal change nephrotic syndrome and chronic kidney disease in a patient with Waldenström's macroglobulinemia:A case report
2019-03-14MercyJulianMwamunyiHongYanZhuChunZhangYaPeiYuanLiJunYao
Mercy Julian Mwamunyi, Hong-Yan Zhu, Chun Zhang, Ya-Pei Yuan, Li-Jun Yao
Abstract
Key words: Waldenström's macroglobulinemia; Pseudothrombi; Nephrotic syndrome;Chronic kidney disease; Case report
INTRODUCTION
Waldenström's macroglobulinemia (WM) firstly described by J Waldenström in 1944,accounting for 2% of all hematological malignancies.As a rare lymphoid neoplasia,WM was interpreted primarily by bone marrow infiltration and immunoglobulin M(IgM) monoclonal gammopathy secreted in serum[1,2].It affects three per million people per year and more prevalent in Caucasian males with an average age of 64 years at diagnosis[3].The World Health Organization (WHO) classified WM as lymphoplasmacytic lymphoma (LPL) secreting IgM proteins, belonging to the non-Hodgkin B lymphomas (NHL)[4].
Clinical manifestations are linked to the deposition of IgM in various organs, which may result in hepatomegaly, splenomegaly, and lymphadenopathy.High titers of IgM results in hyperviscosity syndrome with associated complications[5].Renal complications rarely occur, but the most common renal manifestations are mild proteinuria and microhematuria[5,6].Among all patients, less than 3% develop endstage renal disease.Moreover, nephrotic syndrome is seldom seen in WM.Few reports have described that the occurrence of nephrotic syndrome is less than 7% of patients with WM[7].
We herein present an unusual case of WM that presented with pseudothrombi depositing in capillaries associated with minimal change nephrotic syndrome and chronic kidney disease (CKD).
CASE PRESENTATION
Chief complaints and history of present illness
A 52-year-old Chinese man presented with a sudden onset of edema affecting the face and bilateral lower limbs, abdominal distension after eating, and weight gain (7 kg in 10 d).
History of present illness
His medical history was notable for nephrotic syndrome.
History of past illness
The patient denied a history of trauma.However, he had a kidney stone diagnosed in 2005.
Physical examination
The physical examination showed bilateral lower extremity edema in the absence of hepatosplenomegaly and lymphadenopathy.His blood pressure was 147/94 mmHg.
Laboratory examinations
Hemoglobin 14.2 g/dL [Normal (N) 13.5-17.5 g/dL], white blood cells (WBC) 7.76 G/L (N 4.5-11.0 G/L), red blood cells (RBC) 4.44 T/L (N 4.7-6.1T/L), lymphocytes 18.2% (N 20%-40%), neutrophils 74.6% (N 40%-60%), monocytes 5.9% (N), eosinophils 1% (N), platelets count 376 g/L 9 (N), urea 8.82 mmol/L (N 2.5-7.1 mmol/L),creatinine 211 μmol/L (N 60-110 μmol/L), glomerular filtration rate (GFR) 34.8 mL/min, calcium 2.12 mmol/L (N 2.2-2.7 mmol/L), uric acid 253.2 μmol/L (N),chloride 111.9 mmol/L (N 98-106 mmol/L), albumin 19.9 g/L (N 35-55 g/L), globulin 59.0g/L (N 20-35 g/L), urine glucose 1+, urine occult blood 3+, urine protein 3+, urine WBC 167/μL (N < 11/μL), urine RBC 147/μL(N < 14/μL), urine epithelial cells 19/μL(N < 6/μL), urine kappa 1140 mg/L (N < 19 mg/L), urine lambda 367 mg/L (N < 50 mg/L), and urine nitrates were negative.Blood glucose, liver function tests, lipids,coagulation, hepatitis B virus, and hepatitis C virus markers were normal.
Thyroid function parameters were:FT3 1.8 pmol/L (N 2.6-5.7 pmol/L), FT4 6.6 pmol/L (N 9-19.18 pmol/L), and thyroid stimulating hormone 10.78 μIU/mL (N 0.35-4.94 μIU/mL).Elevated serum cytokines included vascular endothelial growth factor(VEGF)-A 93.92 pg/mL (N 1.09-39.23 pg/mL), interleukin-6 (IL-6) 23.52 pg/mL (N 1.32-4.72 pg/mL), MIF 24.65 pg/mL (N 3.99-7.93 pg/mL), and MIP-1a 109.72 pg/mL(N 6.93-18.57 pg/mL); the rest were normal.Serum β-2 microglobulins 4.8 mg/L (N 1.0-3.0 mg/L), C3 0.739 g/L (N 0.790-1.520 g/L), and C4 were normal.Serum immunoglobulin and light chains were as follows:IgG 1.81 g/L (N 7.51-15.60 g/L), IgA 0.79 g/L (N 0.82-4.53 g/L), IgM 41.9 g/L (N 0.460-3.040 g/L), serum kappa 4.89 g/L (N 1.70-3.70 g/L), and serum lambda 0.32 g/L (N 0.90-2.10 g/L).Bence-Jones proteinuria was negative.
Imaging examinations
Renal imaging revealed that the left kidney was about 9 cm x 5.4 cm in size and right kidney about 9.5 cm x 4.8 cm.Renal perfusion decreased with no obstruction, and GFR decreased to 34.8 mL/min.
Echocardiographic findings suggested decreased left ventricular diastolic function in the ascending aorta.
Chest computed tomography (CT) showed left ventricular failure, mild edema in both lungs, and mild bilateral pleural effusion.
Whole body bone single photon emission CT showed a slight increase in the concentration of the bone-imaging agent in the upper and lower jaw.The left posterior sixth rib-imaging agent was less and evenly distributed.The other parts of the bone-imaging agent were evenly distributed, and no abnormal changes were noted in the double kidneys and bladder.These findings reflected enhanced bone metabolism limited to the mandible; bone metabolism in the sixth ribs was slightly decreased and no apparent changes were observed in the metabolism of other bones in the body.
Bone marrow histology showed active hematopoietic tissue proliferation with an increasing focal ratio of lymphocytes and plasmacytes.
Bone marrow fluorescencein situhybridization test was normal.Bone marrow immunohistochemical staining results were:CD34 (-), CD117 (-), TDT (-), MP0 (+),CD3 (-) CD10 (-), CD20 (±), CD61 (+), κ (±), and λ (-).
Bone marrow immunophenotyping test did not detect any monoclonal lymphocytes or monoclonal plasmacytes with abnormal phenotype.
Renal biopsy
On light microscopy, the most extensive section showed 28 glomeruli that were nonlobulated and non-sclerotic, with one glomerular capillary loop shrunken, and their walls were slightly thickened with a small number of layers.The volume of residual glomeruli increased; generally, the number of cells was 80-120 per glomerulus,mesangial cells and mesangial matrix were slightly increased, capillary loops were open, and the number of infiltrating cells was < 3/glomeruli, mainly mononuclear cells.Red cells and “pseudothrombi” were seen in several capillary loops.One capillary loop was embedded into the urinary pole (Figure 1).
The periodic Schiff-Methenamine (PASM) and Masson staining showed that a large number of fuchsinophilic depositions were found in the basement membrane and under the endothelium.The tubulointerstitium presented moderate lesions.Diffuse turbidity, granular degeneration, and partial small and fine vacuolar degeneration were found in the tubular epithelial cells.Some small vessels were atrophic, and the basement membrane of tubules was thicker (Figure 2).
Alkaline Congo red staining was negative.Electron microscopy revealed diffused effacement of podocyte foot processes, and only mild mesangial hyperplasia and a few electron dense deposits (Figure 3).
There was no clear immunoglobulin or deposition of complement components under the immunofluorescence microscope.

Figure 1 Light microscopy.
FINAL DIAGNOSIS
A diagnosis of WM associated with minimal change nephrotic syndrome was made.Furthermore, the decreased GFR caused by capillary occlusion was diagnosed as CKD.
TREATMENT
The patient was treated with atorvastatin, human albumin, torsemide, alprostadil,levothyroxine, bortezomib, thalidomide, and dexamethasone.At the time of this report, the patient responded to the above therapy and has stabilization of renal function.Table 1 shows the chemotherapy treatment regimen used.
OUTCOME AND FOLLOW-UP
He is being on our required regular clinic follow-up since diagnosis.He has remained stable.
DISCUSSION
Renal involvement associated with WM is scarce compared to multiple myeloma, due to rare hypercalcemia and less Bence-Jones proteinuria.As few as 3.8%-7.4% of the patients advanced to renal failure in autopsies of WM patients.Its etiology is still unclear; however, the most recent theory is autoimmune sensitivity to self-antigens.Although proteinuria and microhematuria are not limited, the frequency of nephrotic syndrome is reported to be less than 7%.Minimal change nephrotic syndrome is a common complication of Hodgkin’s lymphoma but rarely seen in patients with WM.To date, only four WM cases have been previously reported in the literature[3,8-10].In WM, a T lymphocyte disorder that leads to a decreased CD4/CD8 ratio and abnormal secretion of lymphokines has been published and may be associated with the occurrence of minimal change nephrotic syndrome[11].
In this case, light microscopy showed a large number of pseudothrombi deposited in the capillaries of the patient’s kidney, together with diffused effacement of podocyte foot processes observed by electron microscopy.In addition, our patient also presented with proteinuria, indicating that this was minimal change nephrotic syndrome accompanied with pseudothrombus deposition.We also believe that proteinuria was a result of pseudothrombus deposition in the glomeruli capillary.The pathophysiology of renal pseudothrombi associated with WM is unknown, but there are some mechanisms suggesting that WM patients are known to have an elevated risk of venous thrombosis[12].Complications of WM include hyperviscosity and raised von Willebrand factor[13].Additionally, the paraprotein may act as an antiphospholipid antibody; interactions of the paraprotein with the local renal microvasculature or the complement system could all contribute to a prothrombotic state, thus damaging the endothelial cells, disrupting the glomerular microcirculation,and hence lowering GFR[14], whereby our patient’s GFR was 34.8 mL/min, BUN was 8.82 μmol/L, and creatinine was 211 μmol/L.Besides, the patient had mild interstitial fibrosis, and we could not find monocyte, eosinophil, or plasmacyte infiltration in the interstitial area as compared with the results of other reports[15].Thus, the declined GFR seen on this patient might have resulted from WM induced hyperviscosity.

Figure 2 Light microscopy.
One intriguing finding of our case was that serum IgM level correlates with proteinuria and serum creatinine.After the first circle of bortezomib, thalidomide,and dexamethasone therapy, the plasma IgM level decreased by 55%, plasma creatinine decreased by 29%, and plasma albumin increased by 23%.Thus, high levels of serum IgM are not only a diagnostic criterion for WM, but also a clinical parameter of WM associated kidney damage.Unlike the normal pathophysiology of podocyte injury, coagulation can also cause podocyte effacement and proteinuria.Immunofluorescence microscopy did not show immunoglobulin or deposition of complement components even though the patient’s serum IgM was ten times more,which was 41.9 g/L.Some reports suggest that the absence of IgM deposition in the renal biopsy together with high serum levels of monoclonal IgM can be due to glomerular basement membrane being repaired as a result of the combined treatment,so renal IgM deposition will disappear[16].In our case, the patient did not receive any treatment before his diagnosis, which suggested that kidney dysfunction was not a prerequisite for IgM deposition.
Electron microscopy only showed mesangial hyperplasia and a few electron dense deposits; however, others have shown significant proliferation in the mesangial,epithelial, and endothelial cells of the matrix, and a lot of dense depositions in the GBM, Bowman's capsule, and tubular basement wall[17].
We have shown here, for the first time, that a WM patient had elevated serum IL-6 and VEGF-A, and recent studies have shown that IL-6 and VEGF-A levels in the sera of WM patients were abnormal compared with nonmalignant subjects.Malignant B cells secrete detectable levels of these cytokines[18].Nonetheless, it is important also to note that elevated plasma cytokines can be observed in CKD as well and mostly caused by increased production derived from oxidative stress, chronic inflammation,and fluid overload.Concurrently, decreased clearance of IL-6 due to impaired renal function also contributes to its accumulation[19].Furthermore, elevated serum VEGF-A was linked to diabetic nephropathy and glomerular diseases in humans, and proteinuria, glomerulomegaly, glomerular basement membrane thickening,mesangial expansion, loss of slit diaphragms, and podocyte effacement were associated with increased kidney VEGF-A content in adult mice overexpressing VEGF164[20].This appears to be the first case to report elevated serum levels of IL-6 and VEGF in a patient with WM and CKD associated with pseudothrombi.Therefore,it remains unclear whether IL-6 and VEGF elevation is caused by CKD or WM in this patient.To our knowledge, no study has yielded any suggestions regarding this association, so this question remains to be further determined.

Figure 3 Electron microscopy.
Regarding the treatment of WM, chemotherapy with a combination of bortezomib,thalidomide, and dexamethasone as shown in Table 1 was a success, and complete remission was achieved in case of minimal change nephrotic syndrome occurring in the setting of WM.This treatment achieved hematological remission, thus suppressing serum IgM, hyperviscosity, and nephrotic range proteinuria, as observed in our case.Renal complications disappeared, podocyte efficiency was recovered, and proteinuria remission was ameliorated.
CONCLUSION
We present a rare case of WM with accumulation of lots of pseudothrombi deposited in the capillaries, associated with minimal change nephrotic syndrome and CKD due to capillary occlusion.
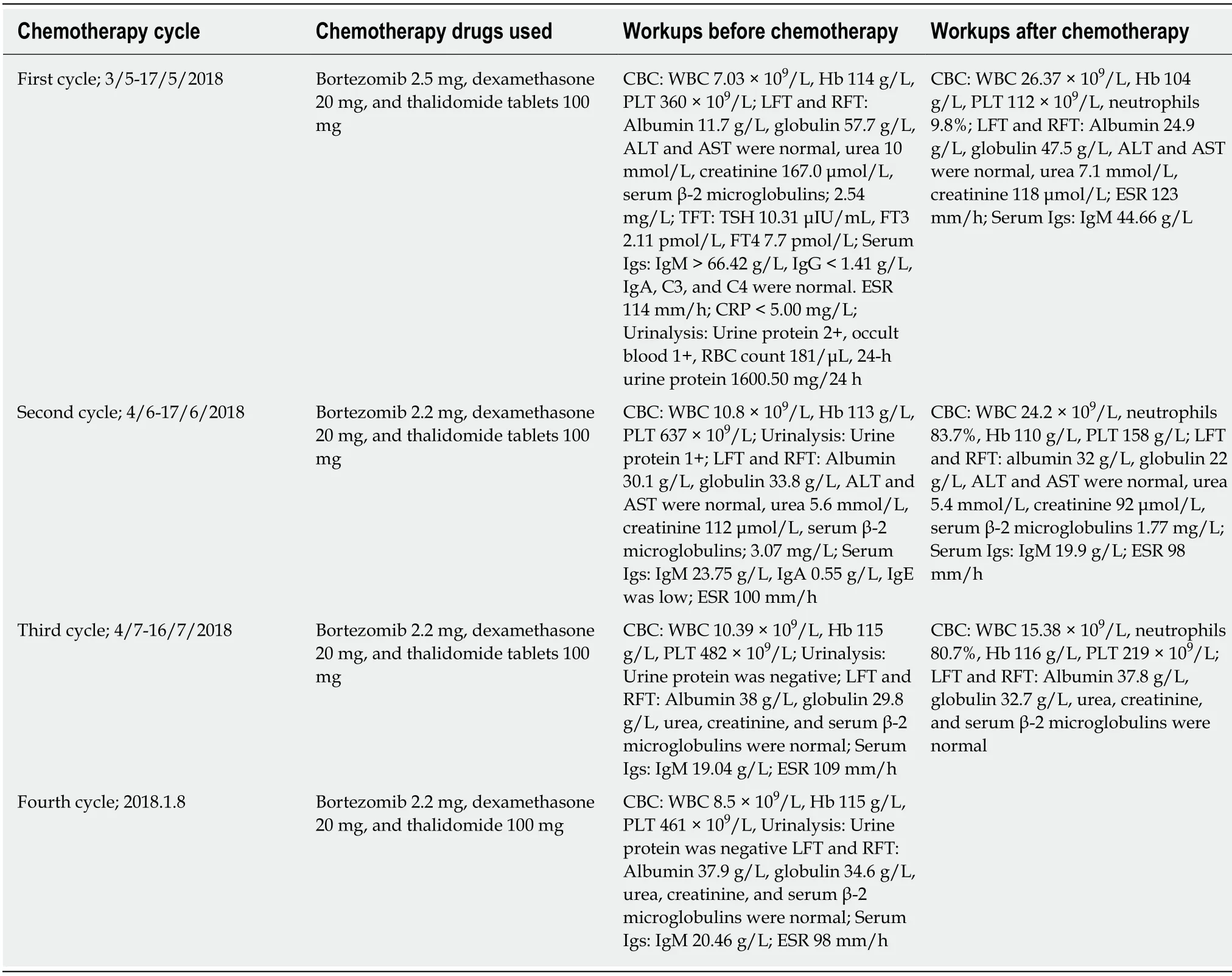
Table 1 Chemotherapy regimen
杂志排行
World Journal of Clinical Cases的其它文章
- Malignant syphilis accompanied with neurosyphilis in a malnourished patient:A case report
- Ex vivo revascularization of renal artery aneurysms in a patient with solitary kidney:A case report
- Hepatocellular carcinoma successfully treated with ALPPS and apatinib:A case report
- Treatment of invasive fungal disease:A case report
- Acute pancreatitis connected with hypercalcemia crisis in hyperparathyroidism:A case report
- Ulcerative colitis complicated with colonic necrosis, septic shock and venous thromboembolism:A case report
