Serum biomarkers and risk of hepatocellular carcinoma recurrence after liver transplantation
2019-02-21MariaCitoresJoseLucenaSaradelaFuenteValentinCuervasMons
Maria J Citores, Jose L Lucena, Sara de la Fuente, Valentin Cuervas-Mons
Abstract Liver transplantation (LT) is the only potentially curative treatment for selected patients with cirrhosis and hepatocellular carcinoma (HCC) who are not candidates for resection. When the Milan criteria are strictly applied, 75% to 85%of 3- to 4-year actuarial survival rates are achieved, but up to 20% of the patients experience HCC recurrence after transplantation. The Milan criteria are based on the preoperative tumor macromorphology, tumor size and number on computed tomography or magnetic resonance imaging that neither correlate well with posttransplant histological study of the liver explant nor accurately predict HCC recurrence after LT, since they do not include objective measures of tumor biology. Preoperative biological markers, including alpha-fetoprotein, desgamma-carboxiprothrombin or neutrophil-to-lymphocyte ratio and platelet-tolymphocyte ratio, can predict the risk for HCC recurrence after transplantation.These biomarkers have been proposed as surrogate markers of tumor differentiation and vascular invasion, with varied risk magnitudes depending on the defined cutoffs. Different studies have shown that the combination of one or several biomarkers integrated into prognostic models predict the risk of HCC recurrence after LT more accurately than Milan criteria alone. In this review, we focus on the potential utility of these serum biological markers to improve the performance of Milan criteria to identify patients at high risk of tumoral recurrence after LT.
Key words: Hepatocellular carcinoma; Liver transplantation; Recurrence; Selection criteria; Prognostic score; Biomarker; Alpha-fetoprotein; Systemic inflammatory marker
INTRODUCTION
Liver transplantation (LT) is the best treatment option for selected patients with cirrhosis and small hepatocellular carcinoma (HCC) who are not candidates for resection. Mazzaferro et al[1]proposed the Milan criteria in 1996 for selection of patients with HCC for LT (defined as single lesion ≤ 5 cm, up to three separate lesions with none larger than 3 cm, no evidence of gross vascular invasion, and no regional nodal or distant metastases), and since then they have been applied worldwide.Patients fulfilling these criteria achieve similar survival rates as patients with LT without malignancies, of about 75% to 85% at 3 and 4 years respectively[2]. However,albeit that the Milan criteria are considered too restrictive and limiting for the transplantation option, HCC recurrence develops after LT in up to 20% of the patients[1-3], having adverse negative impact on patient survival. A poor histologic grade of differentiation, presence of vascular invasion, nodule size of > 5 cm, lymph nodes metastases and bilobar tumor involvement are classically associated with an increased risk of HCC recurrence after LT.
The Milan criteria are based on the preoperative tumor macromorphology on computed tomography or magnetic resonance imaging, that neither correlate well with posttransplant histologic study of the liver explant[4,5]nor accurately predict HCC recurrence after LT, since they do not include objective measures of tumor biology. In fact, small HCC may present biological aggressive features with unfavorable post-LT outcome, while other patients with HCC beyond Milan criteria but fulfilling the University of California San Francisco (UCSF) criteria[6]or the Up-to-7 criteria[7]could have a low risk of HCC recurrence in the presence of favorable tumor biology and could benefit from LT.
Liver biopsy is still the gold standard for determining the molecular biology of the tumor, its behavior and invasive characteristics. Some centers deny LT to patients with poorly differentiated tumors on needle biopsy, irrespective of number and size of tumoral nodules, and they have reported an excellent overall survival and low recurrence rates after LT even in patients exceeding Milan criteria[8-10]. However,preoperative biopsy often underestimates poorly differentiated tumors and does not accurately predict microvascular invasion, when compared with the final specimen examination after liver resection or LT[11,12]. Due to these limitations and because of the risk of needle tract tumor seeding, preoperative biopsy is not currently recommended for routine HCC evaluation; although, it is still needed in patients with atypical radiological features and in doubtful cases.
Preoperative biological markers can predict the risk for recurrence after transplantation. Biological markers can be categorized as: (1) serum markers directly related with tumor biology, such as alpha-fetoprotein (AFP) and des-gammacarboxyprothrombin (DCP); or (2) systemic inflammation markers, such as neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR)conditioning tumor progression. In this review, we focus on the utility of these serum biological markers to improve the performance of Milan criteria for predicting recurrence after LT for HCC.
SERUM BIOLOGICAL MARKERS RELATED WITH TUMOR BIOLOGY
AFP
AFP is a 67-kDa glycoprotein that is produced by the liver in early fetal life. In adults,AFP production is restricted to a variety of liver tumors, including HCC, because of the dedifferentiation of hepatocytes. First considered a reliable marker for HCC diagnosis, at present the joined committees of the European Association for the Study of the Liver (commonly known as EASL) and the European Organization for Research and Treatment of Cancer (commonly known as EORTC) consider AFP testing as suboptimal for routine screening of early HCC (2B)[13]. In fact, about 80% of small HCC (< 2 cm) do not show high levels of serum AFP[14,15]. In the other hand, AFP level can be increased in patients with chronic liver disease, with a degree of hepatocytes regeneration in absence of malignancy[16].
Nevertheless, AFP is a surrogate marker of tumor differentiation and vascular invasion[17-20]and has proven a useful biomarker to identify patients at a higher risk for HCC recurrence[21], with varied risk magnitudes depending on the defined AFP cutoffs[22-33]. AFP has been integrated into several prognostic models for predicting recurrence after LT for HCC, by combining AFP level with tumor size and number, at different cutoffs for each variable (Table 1). Integration of AFP into the selection criteria was first proposed for patients receiving living donor (LD) LT in Asian countries, since the fast-track to LDLT may result in inclusion of patients with biologically aggressive HCC.
In the score proposed by Yang et al[34]patients were awarded between 1 and 4 points for each feature, with three different cutoffs: tumor size of 3, 5 and 6.5 cm;tumor number = 1, 3 and 5; and, AFP of 20, 200 and 1000 ng/mL. With a maximum score of 12 points, patients with a score ≥ 7 were considered as nontransplantable patients. In contrast, the Hangzhou criteria[35]consider transplantable patients as those with well or moderately differentiated HCC and having a total tumor diameter of > 8 cm and AFP of < 400 ng/mL. A large study conducted in 6487 patients registered in the Scientific Registry of Transplant Recipients database[36]showed that total tumor volume of ≤ 115 cm3and pretransplant AFP of ≤ 400 ng/mL identified patients at low risk of HCC recurrence after LT more effectively than both the Milan and UCSF criteria. This prognostic score has been validated both retrospectively in Poland[37]and prospectively in a multicenter study carried out in Canada, Switzerland and the United Kingdom[38].
The Liver Transplantation French Study Group developed and validated a prognostic model, known as the AFP model, for predicting recurrence after LT that combines AFP level, tumor size and number, at different cutoffs for each variable[17].Tumor size was assigned: 0, 1 or 4 points when the largest tumor size was ≤ 3 cm,between 3-6 cm or ≥ 6 cm respectively; 0 or 2 points for ≤ 3 nodules or ≥ 4 nodules;and, AFP level added 0, 2 or 3 points for AFP ≤ 100, between 100-1000 or > 1000 ng/mL respectively; with a maximum score of 9 points. A cutoff of 2 points classified patients at low or high risk for HCC recurrence after LT. Thus, AFP > 1000 ng/mL provides enough points for excluding patients from LT whatever the size and number of nodules.
The AFP model better discriminated patients at high and low risk than Milan criteria. This model identifies patients within Milan criteria but with high risk of 5-year HCC recurrence as those having AFP > 1000 ng/mL (37.7% vs 13.3%), while patients beyond Milan criteria but with AFP < 100 have a low risk of HCC recurrence(14.4% vs 47.6%). Indeed, this model has been officially adopted in the liver allocation policy in France since 2013. This score has been validated in a single center from Spain[39]and in two multicenter studies, respectively from Italy[20]and Latin America[40], with similar results. Moreover, the AFP model has also been validated in a cohort of 400 patients with LDLT from Korea, in whom this model showed an improvement in predicting no HCC recurrence but not the occurrence of HCC recurrence[41].
All these models have been proved successful for selecting patients beyond the Milan criteria who will achieve similar outcomes to patients within Milan criteria.Also, in a recent study[42]evaluating the role of AFP as predictor of HCC recurrence with respect to the fulfillment of Milan, UCSF or Up-to-7 criteria, patients beyond Milan criteria but within UCSF or Up-to-7 and with AFP < 100 ng/mL had a minimal risk of HCC recurrence after LT, criteria that have been validated in other studies[43,44].

Table 1 Main selection criteria for liver transplantation including alpha-fetoprotein
Albeit AFP has proved to be a useful biomarker for identifying patients at a higher risk for HCC recurrence, there is no consensus about the best cutoff value to be considered. While different cutoffs have been proposed in several scores[17,34], other criteria include a sole cutoff at 400 ng/mL[35,36,45]or 1000 ng/mL[46]. Also, serial measurements of AFP (accounting for AFP variations) have been considered to better reflect the dynamic variations in the tumor biological behavior than a cutoff value of AFP level in a single assessment. Progression of AFP level while on the waiting list exceeding 15 ng/mL per mo[47,48], 50 ng/mL per mo[49]or 0.1 ng/mL per d[50]have been suggested as strong predictors of HCC recurrence after LT. In contrast, Grąt et al[42]found AFP > 100 ng/mL to better identify patients at risk of HCC recurrence than AFP slope.
DCP
Increased levels of DCP or prothrombin induced by vitamin K absence or antagonist II (PIVKA-II) are found in patients with HCC[51-43]. This abnormal form of prothrombin, produced during malignant transformation of hepatocytes, induces expression of angiogenic factors such as endothelial growth factor receptor and vascular endothelial growth factor (VEGF)[54,55]. Up-regulation of DCP has been found to correlate with the degree of malignancy of HCC, as DCP-positive tumors are characterized by increased likelihoods of intrahepatic metastasis, capsule infiltration,and portal venous invasion[56,57]. Moreover, the DCP-positive and AFP-negative tumors are more aggressive, for high risk of recurrence after treatment, since they are usually larger tumors with a poor grade of differentiation and vascular invasion[58,59].
DCP has been suggested as a stronger predictor of HCC recurrence after LT than AFP[57,60]and some centers from Asia have proposed the combined use of DCP level with tumor number and/or size in selection of candidates for LDLT with or without consideration of the AFP value (Table 2). The Kyoto criteria[61]and the Kyushu criteria[62]have been retrospectively and prospectively validated in the same centers where these scores were proposed[63-65]. Patients beyond Milan criteria but meeting Kyoto criteria had similar recurrence rate as patients within Milan criteria[61], while Kyushu criteria was more powerful than UCSF, Tokyo and Kyoto criteria in predicting HCC recurrence[65].
Other centers have proposed different scores combining AFP and DCP levels with different cutoffs for both serum biomarkers that have improved Milan criteria for selection of patients at higher risk of HCC recurrence after LT. The A-P level criteria[66]included AFP ≤ 200 ng/mL “and” DCP ≤ 100 AU/mol, while the A-P 200 criteria[67]considered AFP ≤ 200 ng/mL “or” DCP ≤ 200 AU/molto identify patients at lower risk of HCC recurrence. Kim et al[68]found AFP > 150 ng/mL and DCP > 100 AU/mol to be associated with a higher risk of HCC recurrence after LT.
Lee et al[69]from Seoul, Korea developed and validated a model to predict recurrence after LDLT for HCC beyond the Milan criteria. Using a multivariate Cox proportional hazard model, the authors derived the model of recurrence after LT(commonly known as MoRAL) score using serum levels of AFP and DCP. Patients with a low MoRAL score (≤ 314.8) and no extrahepatic metastasis, even though their tumors exceeded the Milan criteria, had a lower tumor recurrence risk than patients within the Milan criteria with a high MoRAL score (> 314.8). Finally, the only study carried out in a non-Asiatic center found AFP ≥ 250 ng/mL and DCP ≥ 7.5 ng/mL to be associated with a higher risk of HCC recurrence[70], and added predictive information to the Milan criteria [hazard ratio (HR): 4.5 vs 2.6 with Milan criteria alone].
SYSTEMIC INFLAMMATION MARKERS
NLR and PLR
Two inflammation markers, the NLR and the PLR, have an important role in predicting outcome in several malignancies and have been associated with HCC recurrence after LT. Both the NLR and the PLR measure the proportion of peripheral blood neutrophils or platelets, respectively, to lymphocytes.
The link between NLR and liver malignancies was first demonstrated by Halazun et al[71]in patients who underwent surgery for colorectal liver metastasis. Same authors also reported that patients within Milan criteria and NLR ≥ 5 had a poorer recurrencefree survival than those with NLR < 5 (25% vs 75%) and proposed a pre-LT score for HCC recurrence after LT including NLR and tumor size > 3 cm (C-statistics: 0.74)[72].Since then, NLR has been identified as an independent risk factor for HCC recurrence,along with microvascular invasion and/or tumor size and number in some studies[73-77], but not in others[78-80]. A recent systematic review by Najjar et al[81]and a meta-analysis by Xu et al[82]showed that elevated NLR is associated with a lower recurrence-free survival after LT (pooled HR: 3.77, 95%CI: 2.01-7.06) and with vascular invasion. Because of the different NLR cutoffs considered in the studies included in the meta-analysis (ranging from 2.6 to 6), Xu et al[82]recommend a cutoff NLR value of 4.
The prognostic significance of PLR for HCC recurrence after LT has been less extensively studied than that of NLR, but in a recent systematic review and metaanalysis including 899 patients from five studies, high PLR was associated with a significant increase of HCC recurrence after LT[83]. However, this association must be taken in consideration with great caution since a moderate level of heterogeneity was found among the studies included. In a recent study by Xia et al[84], PLR failed to predict HCC recurrence in patients meeting Milan criteria, but the 5-year recurrencefree survival in patients with HCC beyond Milan criteria but within Hangzhou criteria (total tumor diameter of ≤ 8 cm or > 8 cm, well or moderately differentiated and pretransplant AFP of < 400 ng/mL and PLR < 120) was comparable to the figure for patients within Milan criteria (73.3% vs 72.8%).
Han et al[85]also found that PLR was associated with HCC recurrence after LT, but interestingly a stronger association was found when considering the absolute platelet count. HCC recurrence rate after LT was higher in patients with platelet count of 75 ×109/L or greater at the day before surgery compared to patients with platelet count lower than 75 × 109/L (28.2% vs 13.2%). Moreover, the proportion of poorly differentiated tumors, microvascular invasion and bile duct invasion were higher in patients with platelet count of 75 × 109/L or greater. In the experience of those authors, the incorporation of platelet count at 75×109/L into the Milan criteria significantly increased the predictive power for HCC recurrence, over that of Milan criteria alone.
The molecular mechanisms through which the NLR and PLR are associated with HCC recurrence after LT remain unknown, but several hypotheses have been proposed. Both neutrophils and platelets are involved in vascular invasion and metastatization by increasing the production of proangiogenic factors such asVEGF[86,87]. Moreover, neutrophils, the common inflammatory infiltrate in tumors,have been found to be enriched predominantly in the peritumoral stroma of HCC tissue[75,88], correlating with angiogenesis and disease progression[89]. Within the circulatory system, platelets could help to establish metastatic lesions by blocking tumor cell removal[90,91]. On the other hand, low lymphocyte numbers, which also increase NLR and PLR values, could result in an impaired immunosurveillance against disease development and progression.
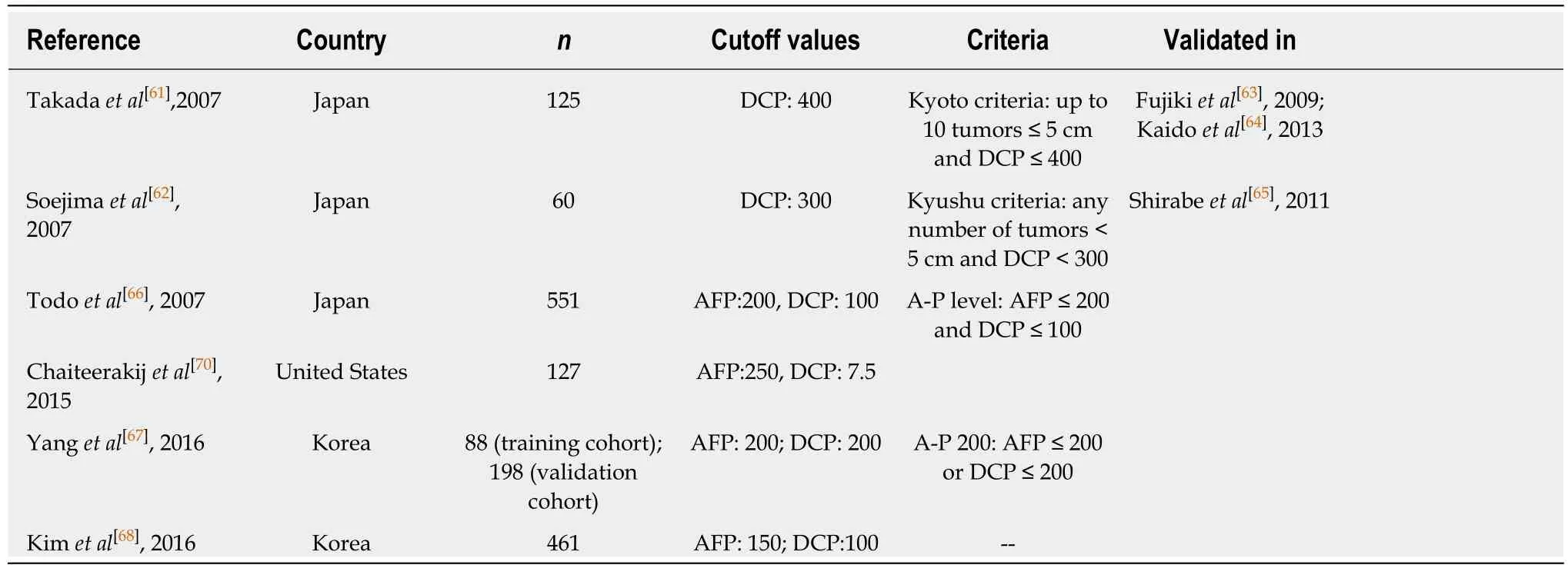
Table 2 Main selection criteria for liver transplantation including des-gamma-carboxyprothrombin
C-reactive protein
The C-reactive protein (CRP) is an acute-phase reactant synthesized by hepatocytes in response to systemic inflammation that has been related with the prognosis of various malignancies, including HCC[92]. Two independent groups from Korea have reported that high CRP level (with cutoff values at 1 mg/dL[93]or 0.3 mg/dL[94]) is an independent risk factor for HCC recurrence after LT, but only in patients beyond Milan criteria.
COMBINATION OF SERUM BIOLOGICAL MARKERS
In recent years, several studies have showed that the combination of several systemic inflammation biomarkers and tumor biomarkers predict the risk of HCC recurrence after LT more accurately (Table 3). In all the nine studies summarized, the relationship among tumor features and HCC recurrence was evidenced, and interestingly all studies analyzing pre-LT AFP level, except for one[95], found AFP to be an independent risk factor for HCC recurrence[80,96-98]. Also, Lai et al[78]found that although AFP and PLR were associated with HCC recurrence in univariate analysis,AFP > 200 ng/mL was the best prognostic factor with an area under the receiver operating characteristic curve (AUC) of 70.6 compared to 66.1 for PLR. Similarly, only two studies[80,99]out of three, found DCP to be an independent factor for HCC recurrence.
Regarding the systemic inflammation markers, NLR was found to be associated with HCC recurrence in six[82,95-99]out of nine studies and CRP in one[96]of two studies,while PLR was not shown as an independent risk factor in any of the four studies in which it was analyzed[78,79,99,100]. The two studies that analyzed inflammation marker sonly, found none of the biomarkers included to be independent risk factors for HCC recurrence[79,100]. Parisi et al[79]analyzed NLR, PLR and the inflammation-based index score (CRP ≥ 10 mg/dL and albumin < 35 gr/L; one point each) in 150 patients within Milan criteria before LT and found that absence of neoadjuvant therapy before LT and exceeding Milan criteria on explant pathology were the only risk factors for HCC recurrence. Fu et al[100]investigated the prognostic role of the systemic inflammation index (SII; absolute platelet count × absolute neutrophil count/absolute lymphocyte count) compared with PLR, NLR and monocyte-to-lymphocyte ratio in patients fulfilling the Hangzhou criteria for LDLT. At a cutoff of 226 × 109/mL, high SSI was associated with larger tumor size, greater total tumor volume, poorer differentiation grade and higher AFP level. Nevertheless, although SII was the best prognostic factor for overall survival, neither SSI nor the other systemic inflammatory markersanalyzed were associated with recurrence-free survival.
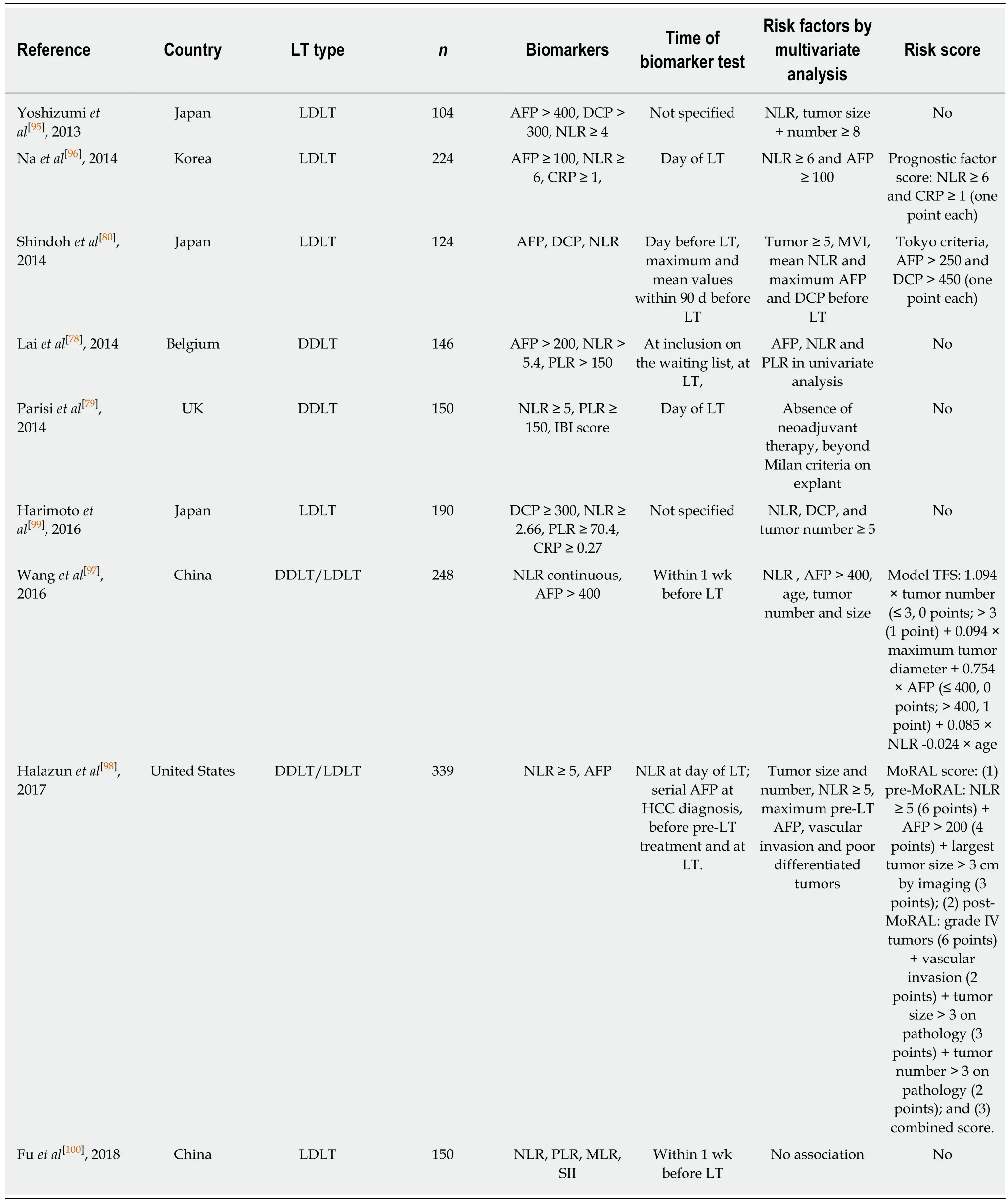
Table 3 Main studies analyzing several pre-liver transplantation systemic inflammation biomarkers and proposed scores
Prognostic scores including inflammatory markers for HCC recurrence after LDLT have been proposed by three different groups from Asia and one group from the United States. Na et al[96]proposed a prognostic factor score assigning 1 point for pre-LT NLR ≥ 6 and CRP ≥ 1 each, and Wang et al[97], who included only males receiving a LDLT, proposed the model tumor free survival, combining tumor morphological features with tumor biological information. Interestingly, both scores were informative only in patients beyond Milan criteria, and not predictive of HCC recurrence in patients within Milan criteria.
The score proposed by Shindoh et al[80]incorporates pre-LT maximum AFP and DCP in the Tokyo criteria (≤ 5 tumors of ≤ 5 cm) to better stratify patients at risk of HCC recurrence after LT. After evaluating three different pre-LT values for NLR, AFP and DCP (the last value before LT, and the maximum and mean values within the 90 d before LDLT), the maximum AFP and DCP values and the mean value of NLR were independently associated with HCC recurrence. However, NLR had a limited prognostic impact (AUC: 0.62) and only maximum AFP and DCP values had sufficient discriminative power (AUC: 0.88 and 0.76 respectively). So, the authors proposed extending the Tokyo criteria by adding AFP > 250 and DCP > 450 (1 point for each variable; maximum score of 3). Patients with a score 0-1 had a 5-year diseasefree survival rate of 97%, opposed to only 20% of patients with a score 2-3.
In 2017, Halazun et al[98]carried out a prospective study of 339 patients to identify predictors of HCC recurrence after LT. Preoperative NLR > 5 (P < 0.0001, HR: 6.2),AFP > 200 (P < 0.0001, HR: 3.8) and tumor size > 3 cm (P < 0.001, HR: 3.2) were found to be independently associated with a worse recurrence-free survival. The authors developed a new MoRAL score for predicting HCC recurrence after LT, mainly in individuals receiving a liver from deceased donors[98]. They constructed three scores:the pre-MoRAL, the post-MoRAL and the combined-MoRAL score, the latter including both pre-MoRAL and post-MoRAL scores. The pre-MoRAL score, included the three preoperative significant variables with a minimum of 0 points (no factors)and a maximum of 13 points (all 3 factors). The highest risk patients in the pre-MoRAL (score > 10) had a 5-year recurrence-free survival of 17.9% compared with 98.6% for the low risk group (P < 0.0001). The post-MoRAL score included four postoperatively available factors related to pathological features in liver explant,namely grade 4 HCCs, vascular invasion, tumor size > 3 cm and tumor number > 3.The pre-MoRAL, post-MoRAL and combo-MoRAL better predicted HCC recurrence after LT than Milan criteria with C-statistics of 0.82, 0.87 and 0.91 respectively.
LIMITATIONS OF PRETRANSPLANT SERUM BIOMARKERS
Most of the studies to date have been retrospective and include a small sample size;moreover, the included patients in the different studies are highly heterogeneous regarding indications for LT, handling of incidental tumors or inclusion of salvage LT.Also, frequent exclusion of patients who died within 1 mo or even 3 mo after LT could have restricted data about the most aggressive tumors. Besides, there is a great variation of time elapsed between the measurement of the markers and LT. Most studies considered these markers from the analytical data of the day before LT, while others considered these values within 1 wk before LT or did not specify it. Also, there is no consensus about the best cutoff value for each biomarker, and it maybe those different cutoffs should be considered in different populations or centers. In addition,comparison of results from multiple laboratories is uncertain because of different laboratory methods and processing techniques for measuring these biomarkers.Another limitation of the different studies reviewed here relies on the analyses of HCC recurrence as a time-dependent variable, such as recurrence or disease-free survival, without accounting for competing risk, such as death. So, patients who died early after LT or whose death was not related to HCC may never have had the chance to experience HCC recurrence.
Albeit the serum markers reviewed here are potential markers to be included in patient selection for LT, their utility is limited and they cannot be universally applied in all patients. Although AFP is considered the most useful pretransplant marker of HCC recurrence after LT, its utility is restricted by the existence of non-AFP secreting HCC. More restricted is the utility of systemic inflammatory markers, for different reasons. Although some meta-analyses have suggested NLR[82]and PLR[83]as useful pretransplant biomarkers for HCC recurrence, they are based on very few retrospective studies (four and five studies respectively), with most having small sample size. However, the most important limitation may be that these inflammatory serum biomarkers can be affected by other conditions, such an acute infection,hematologic disorders, hypersplenism, gastrointestinal tract bleeding or systemic inflammatory diseases, which are frequent in patients with end-stage liver diseases.
OTHER POTENTIAL SERUM BIOMARKERS
In addition to the serum biomarkers reviewed here, some other markers have been proposed as potential risk factors for HCC recurrence after LT.
AFP-L3%, which represents a serum AFP fraction reactive with lens culinaris agglutinin, has been associated with HCC diagnosis[101,102]. In the LT context, an AFPL3% level > 50 ng/mL combined with Milan criteria improved HCC recurrence prediction, when compared with Milan criteria alone[70]. Interestingly, AFP-L3% has been suggested as a highly specific marker of HCC in patients with low AFP level[102],which could overcome the limitation of AFP usefulness as a biomarker of HCC recurrence in patients with AFP-negative HCC. However, more studies are needed for this promising biomarker.
Liquid biopsy has attracted much attention as a feasible and noninvasive tool to identify tumoral markers in peripheral blood for diagnosis, monitoring and prognosis of cancer, overcoming tissue biopsy limitations. Circulating tumoral cells and tumoral cell free nucleic acids in peripheral blood could be advisory of micro metastasis, and their utility has been explored in HCC diagnosis and prognosis[103]. Very few data are available about the potential role of these circulating tumoral components as preoperative predictors of HCC recurrence after LT, and it is still a controversial issue.Although circulating HCC cells have been detected before LT, they have not been associated with HCC recurrence after LT[104]. Regarding circulating nucleic acids, AFP mRNA expression in peripheral blood has been suggested as a surrogate of circulating tumoral cells and has been associated with an increased risk of HCC recurrence after LT[105]. However, their utility is controversial and some authors consider AFP mRNA to be nonspecific for HCC micro metastases.
Some other circulating RNA have been explored, but none of them has been widely recognized as valuable marker of HCC recurrence, probably because none of them are specific for HCC[103]. Circulating tumor DNA has been isolated in patients with HCC,and has been associated with microvascular invasion[106]. However, much effort is still needed in order to consider these circulating tumor components as valuable markers in clinical practice since some limitations still need to be overcome. Although the complex methodology to isolate these tumoral components has improved dramatically, their extremely low frequencies in peripheral blood require more sensitive and cost effective techniques. Also, HCC-specific biomarkers should be validated and evidence of their association with HCC recurrence after LT should be proven.
Finally, different micro (mi) RNA signatures in liver tissue have been associated with HCC recurrence after LT[107,108]. However, the necessity of liver tissue samples limits their application preoperatively, and circulating miRNAs are at present being explored. Several circulating miRNAs have been suggested as potential biomarkers for HCC diagnosis[109], vascular invasion and prognosis[110,111]. To date, to the best of our knowledge, there is no data about the association of miRNAs with HCC recurrence after LT, and future studies are warranted to explore the utility of these promising biomarkers in preoperative prediction of HCC recurrence after LT.
CONCLUSION
Although the Milan criteria have improved survival of patients receiving a LT for small HCC, tumor recurrence after transplantation still develops in about 15% of patients. On the other hand, patients with less aggressive tumors and at lower risk of recurrence have proven benefit of LT. Since the Milan criteria are based on morphological tumor feature sonly, combination of these criteria with other preoperative available biomarkers related with tumor biology could better predict HCC recurrence after LT. Some serum biomarkers have been proposed but there is no consensus about their use, mainly due to the several limitations commented on in this review. In addition, considering that tumor growth patterns are highly variable among individuals, there probably is no perfect single biomarker for HCC prognosis after LT; thus, the combination of biomarkers could be more informative than any single biomarker alone.
For those reasons and taking into account the limitations highlighted here,multicenter prospective studies are demanded and an international consensus is mandatory in order to provide practical recommendations to guide the implementation of serum biomarkers combined with morphological criteria to better stratify patients at high or low risk of HCC recurrence after LT.
杂志排行
World Journal of Hepatology的其它文章
- Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review
- Treatment of primary sclerosing cholangitis in children
- Hepatitis in slaughterhouse workers
- Persistent risk for new, subsequent new and recurrent hepatocellular carcinoma despite successful anti-hepatitis B virus therapy and tumor ablation: The need for hepatitis B virus cure
- Temporal trends of cirrhosis associated conditions
- Clinical factors associated with hepatitis B screening and vaccination in high-risk adults
