Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review
2019-02-21AakashDesaiSoniaSandhuJinPingLaiDalbirSinghSandhu
Aakash Desai, Sonia Sandhu, Jin-Ping Lai, Dalbir Singh Sandhu
Abstract Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, which in turns accounts for the sixth most common cancer worldwide.Despite being the 6th most common cancer it is the second leading cause of cancer related deaths. HCC typically arises in the background of cirrhosis, however,about 20% of cases can develop in a non-cirrhotic liver. This particular subgroup of HCC generally presents at an advanced stage as surveillance is not performed in a non-cirrhotic liver. HCC in non-cirrhotic patients is clinically silent in its early stages because of lack of symptoms and surveillance imaging; and higher hepatic reserve in this population. Interestingly, F3 fibrosis in non-alcoholic fatty liver disease, hepatitis B virus and hepatitis C virus infections are associated with high risk of developing HCC. Even though considerable progress has been made in the management of this entity, there is a dire need for implementation of surveillance strategies in the patient population at risk, to decrease the disease burden at presentation and improve the prognosis of these patients. This comprehensive review details the epidemiology, risk factors, clinical features,diagnosis and management of HCC in non-cirrhotic patients and provides future directions for research.
Key words: Hepatocellular carcinoma; Non-cirrhotic liver; Hepatitis B; Hepatitis C; Risk factors; Clinical features; Diagnostic modalities; Management strategies; Future directions
INTRODUCTION
Primary liver cancer is the sixth most common cancer worldwide, 90% of which are hepatocellular carcinoma (HCC)[1,2]. HCC is the second leading cause of cancer-related deaths worldwide[3]. Although, most of the cases occur in developing countries, its incidence in developed countries has increased recently[4]. HCC typically arises in the setting of cirrhosis however; approximately 20% of HCC’s have been known to develop in a non-cirrhotic liver[5,6]. This sub-group of HCC often presents at advanced stages because surveillance is not performed in a non-cirrhotic liver. Fibrolamellar carcinoma (FLC), a rare variant of HCC also occurs without any background cirrhosis or hepatitis[6,7]. This review discusses the epidemiology, risk factors, clinical features,diagnosis and management of HCC in non-cirrhotic patients as well as provides future direction for research in this population.
LITERATURE SEARCH
A comprehensive literature search was performed and research papers regarding non-cirrhotic HCC and related literature was analyzed to prepare this review article.Special emphasis was placed on research related to the diagnosis and management of non-cirrhotic HCC in the last 5 yr.
EPIDEMIOLOGY
Worldwide, liver cancer is the fifth most common cancer in men and the ninth in women. It is the 2ndleading cause of cancer death in men and the sixth leading among women, with about 745500 deaths in 2012. In the United States, there is expected to be an estimated 42220 new cases and 30200 death cases of liver and intrahepatic bile duct carcinomas in 2018[8,9]. HCC is a little over 2 times more likely in men over women, the incidence being 5.5 per 100000 in male and 2 per 100000 in female in the United States[8]. Non-cirrhotic HCC has a bimodal age distribution, peaking during the 2ndand 7thdecade of life[5]. The FLC variant comprises 1%-9% of all HCC and accounts for less than 1% of HCC in the United States[10,11]. Overall, there is a lack of significant data on HCC that arises in non-cirrhotic liver. Given that HCC is one of the fastest growing cause of cancer-related deaths and has a survival rate of less than 12% in the United States, there is a need for further research to explore the epidemiology of non-cirrhotic HCC[8].
RISK FACTORS
Non-alcoholic fatty liver disease /Non-alcoholic steatohepatitis
Non-alcoholic fatty liver disease (NAFLD) comprises of a spectrum that includes isolated steatosis, non-alcoholic steatohepatitis (NASH, hepatic inflammation and cell death), fibrosis and cirrhosis (Table 1). With the growing obesity epidemic, NAFLD has become the most common liver disorder in the United States. A strong associationhas been reported between fatty liver disease and HCC in non-cirrhotic livers[12,13].NAFLD-related HCC has been acknowledged as a growing burden in this country[14,15]. It has also been recognized as the most common etiology in new HCC cases, likely due to the recent advances in viral hepatitis, especially hepatitis C virus(HCV) treatment[15]. In a study performed by Schütte et al[16]the etiology of majority of HCC in non-cirrhotic liver was related to metabolic syndrome (MS). The causes of non-cirrhotic HCC is shown in Figure 1.

Table 1 Incidence of different risk factors for hepatocellular carcinoma in cirrhotic and non-cirrhotic liver in various studies
NAFLD, with or without NASH is the hepatic manifestation of MS and predisposes to HCC in non-cirrhotic patients[17]. Type 2 diabetes mellitus and obesity that is associated with NAFLD and MS leads to the release of multiple pro-inflammatory cytokines like TNF-alpha, IL-6, leptin and resistin and decreased amounts of adiponectin. These processes favor the development of hepatic steatosis and inflammation within the liver and subsequently precede the development of HCC[12].Even though type 2 diabetes and obesity have both been implicated as independent risk factors for HCC, studies that establish a clear link to HCC in non-cirrhotic livers are scarce[18-20]. Not only MS and obesity but being overweight is also associated with higher risk of HCC. Overweight and obesity increase HCC prevalence in general population and especially in hepatitis B virus (HBV) and HCV patients[21]. Other features of MS like hypertension and dyslipidemia have not been extensively studied for the linkage. A recent Australian study comparing HCC characteristics between cirrhotic and non-cirrhotic NAFLD found that apart from the presence or absence of cirrhosis, the fibrosis stage was the only patient characteristic that conferred a worse prognosis as this was related to the size of the tumors (P = 0.03)[22].
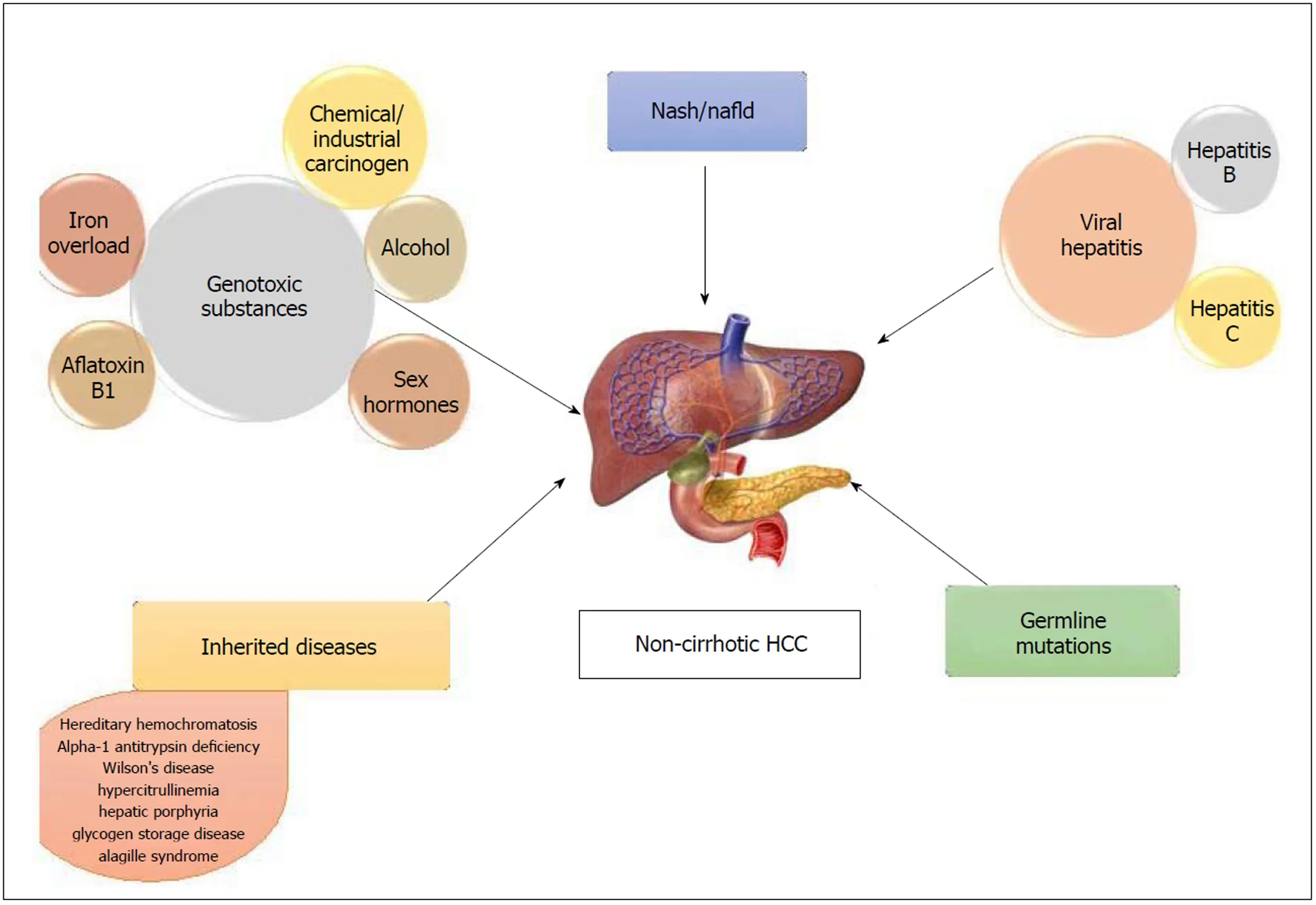
Figure 1 Causes of non-cirrhotic hepatocellular carcinoma. HCC: Hepatocellular carcinoma.
Viral hepatitis
30% of HBV-related HCC occurs in non-cirrhotic patients[23](Figure 2). HBV, a partially double stranded DNA virus is able to integrate into the host cell and acts as a mutagenic agent causing secondary chromosomal rearrangement and increasing genomic instability. In addition, transactivation of genes by the regulatory protein HBx is known to increase cell proliferation, deregulate cell cycle control and interfere with DNA repair and apoptosis[24]. Certain risk factors in chronic hepatitis B patients in turn impart a higher risk for non-cirrhotic HCC. BCP T1762/A1764 mutation and high viral loads have been reported to be strong viral factors and independent predictors of HCC in non-cirrhotic patients who are chronic HBV carriers[25,26]. Older patients with chronic Hepatitis B had a higher annual incidence of non-cirrhotic HCC compared to cirrhotic patients; 1.1% per year in men and 0.3%-0.4% per year in women greater than the age of 55[27]. African American and Asian race has also been associated with a higher incidence of HCC in non-cirrhotic chronic hepatitis B patients[27]. About half of the cryptogenic HCC cases have occult Hepatitis B infection defined by the presence of HBV DNA in the liver or blood without HBsAg[28].
HCC in chronic Hepatitis C patients primarily occurs in the background of cirrhosis that is related to a necroinflammatory state with tissue damage, regeneration, repair and fibrosis[29,30]. HCV being a single stranded RNA virus cannot integrate with the host genome due to the absence of a DNA intermediate. However, it still possesses direct oncogenic potential although lower compared to HBV; with several of its gene products capable of contributing to carcinogenesis[4,31]. The core protein can alter cell regulation via enhanced telomerase activity[31]. Non-structural proteins like NS3, NS4B and NS5A can potentially induce carcinogenesis through interactions with cellular promoters and proteins[32]. The incidence of HCC in non-cirrhotic HCV patients ranges from 4.4%-10.6%[32]. Its role as a major risk factor is suggested by the development of HCC even after eradication of the virus[33]. In treatment naïve HCV patients, male gender, advanced age, persistently elevated aminotransferases, high gamma-glutamyl transferase levels, hepatic steatosis, diabetes and alcohol abuse have all been shown to increase the risk for non-cirrhotic HCC[34,35]. In patients with sustained virologic response after chronic HCV treatment, those who had diabetes mellitus and increased Fibrosis-4 index were at a higher risk. Studies have also shown increased risk of HCC in patients with hepatitis C and F3 fibrosis. Apart from hepatitis C, this increased risk is noted in even patients with hepatitis B and NAFLD who have F3 fibrosis[22,25,27,36]. The exact pathophysiology behind this still remains to be elucidated.
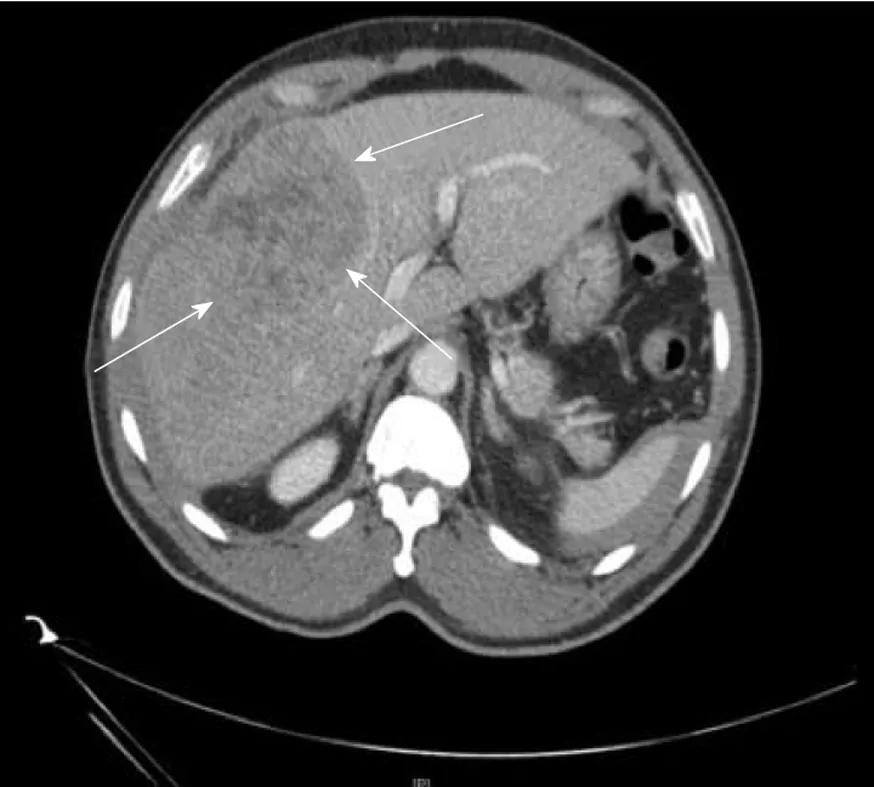
Figure 2 Computed tomography image of a 64-yr-old male with hepatitis B. No cirrhosis found to have large 9 cm mass in right lobe (arrows) on CT abdomen done for abdominal pain.
Genotoxic substances
Alcohol:Several studies have shown heavy alcohol intake in patients with noncirrhotic HCC, however most of these were not statistically significant[4]. Alcohol may not be a major cause in non-cirrhotic patients, but it should still be treated as a serious risk factor for the development of HCC. This is related to its direct genotoxic effect in the development of HCC mediated through endotoxin production, oxidative stress and inflammation[37]. Heavy alcohol intake in the setting of other risk factors like chronic HCV and diabetes mellitus may potentiate the oxidative stress and free radical damage; leading to rapid progression to HCC[38-40].
Aflatoxin B1:Aflatoxin B1 (AFB1) is an extremely potent hepatocarcinogen that is a secondary metabolite produced by fungi, Aspergillus flavus and Aspergillus parasiticus.They are typically found in tropical and sub-tropical regions of the world in which grains such as rice stored in hot humid conditions promote growth of these toxinproducing fungi[41]. Most cases occur in sub-Saharan Africa, Southeast Asia and China where HBV is highly prevalent. However, its incidence in the United States is extremely low; 0.003 in HbsAg negative and 0.08 in HbsAg-positive patients[42]. In addition, its burden in non-cirrhotic individuals is unknown. AFB1 is metabolized by the P450 enzymes in the liver to generate an epoxide, which binds to DNA and induces mutation of the p53 tumor suppressor gene[24,43]. Like cirrhotic patients, noncirrhotic patients with chronic HBV are also at a higher risk for aflatoxin-mediated HCC[44,45].
Iron overload:Secondary iron overload is seen in patients with hematological disease like myelodysplastic syndrome, chronic anemia and polytransfusion[46-48]. The notion that increased liver iron stores in the liver cause HCC in non-cirrhotic patient has been present for at least three decades and there are several case reports that have highlighted the role of excess iron as a potential carcinogen[48,49]. Iron-induced carcinogenesis could be related to the production of reactive oxygen species via Fenton reaction inducing oxidative stress. This in turn promotes protein and DNA modification, blunts immune response by impairing T cell proliferation against tumor transformed cells and induces of p53 mutations[50,51].
Miscellaneous:Chemical and industrial carcinogens like nitrosamines, azo dyes,aromatic amines, vinyl chloride, organic solvents, pesticides and arsenic have been known to induce carcinogenesis in non-cirrhotic liver[52,53]. Studies have shown KRAS mutations in vinyl chloride-induced HCC and HRAS mutations in methylene chloride-induced liver tumors[23]. Polycyclic aromatic hydrocarbons derived from red meat, cigarette smoking, and environmental pollutants also increase the risk of HCC by forming DNA adducts[53-55].
Sex hormones:Several case reports have established a link between chronic anabolic androgen steroid abuse and non-cirrhotic HCC in young professional bodybuilders[56-58]. “Adenoma-carcinoma sequence” or “de novo carcinoma development” are the two proposed mechanisms for carcinogenesis[56]. Oral contraceptive (OCs) is also a known risk factor for the development of non-cirrhotic HCC[59,60]. In a retrospective case series of 26 white women aged under 50 who developed HCC in a non-cirrhotic liver, women who used OCs for 8 or more years had a 4.4-fold increased risk (P < 0.01)[59]. Both anabolic steroids and estrogen undergo significant enterohepatic circulation and slow biliary excretion, which increases intrahepatic concentrations and causes direct toxicity[59].
Inherited diseases
Hereditary hemochromatosis and alpha-1 antitrypsin deficiency are inherited diseases in which HCC occurs frequently without cirrhosis[61-65]. Acute hepatic porphyrias which include 3 autosomal dominant disorders: Acute intermittent porphyria (AIP),variegate porphyria (VP) and hereditary coproporphyria (HCP) has also been considered as a cause of non-cirrhotic HCC[66]. Overproduction of aminolevulinic acid and/or porphobilinogen overproduction and excretion has been implicated as a cause of hepatic carcinogenesis in AIP, but the mechanism is poorly understood in VP and HCP[67].
Hypercitrullinemia, a hereditary urea cycle disorder is associated with hepatocarcinogenesis in non-cirrhotic liver likely via citrulline-mediated promotion effect on hepatocyte proliferation[68].
Wilson’s disease, an autosomal recessive disorder of copper metabolism is also implicated as cause of HCC in scattered case reports. The mechanism is related to copper mediated stimulation of fibroblast growth factor-2 and copper induced stabilization of hypoxia-inducible-factor-1 alpha, which causes expression of genes that promote angiogenesis[69,70]. Even though HCC prevalence in Wilson’s disease is lower than other inherited forms of liver disease, it remains a significant risk factor for development of HCC in non-cirrhotic liver as well.
Glycogen storage disorders (GSD) are a group of inherited metabolic disorders characterized by the accumulation of excessive abnormal glycogen in liver, muscle or both. HCC typically occurs without cirrhosis in glycogen storage disease type 1 (GSD-1) via adenoma-carcinoma sequence[62]. HCC in GSD type III is extremely rare and generally occurs in the background of cirrhosis, however Oterdoom et al[71]reported the first documented case in a non-cirrhotic.
Alagille syndrome, an autosomal dominant disease that causes significant neonatal jaundice and cholestasis in older children has been shown in case reports to cause non-cirrhotic HCC[72,73].
Hepatic vascular disease like Budd-Chiari syndrome and nodular regenerative hyperplasia have been associated with non-cirrhotic HCC in case reports, however the mechanism of hepatocarcinogenesis in the absence of cirrhosis is unknown[74,75].
Germline mutations
Studies establishing associations between germline mutations and non-cirrhotic HCC have been scarce. In a recent study by Donati et al[76], germline mutations in telomerase reverse transcriptase gene (hTERT) were associated with shorter telomere lengths and progression of NAFLD to HCC in non-cirrhotic liver. Future studies may identify such germline mutations, which would allow for closer surveillance in these high-risk individuals.
Hepatic adenoma
Hepatic adenomas (HA) is a benign liver tumor that carries a small risk for the development of HCC[60]. The risk of malignant transformation is controversial and has been heavily debated based on available past research studies. Two studies that analyzed available literature, estimated this risk to be approximately 5%[77,78]. Studies on HA in non-cirrhotic livers have been scarce and those that exist have limitations related to overestimation of the risk and biased reviews of resected cases[79,80].Nonetheless, there is compelling evidence in the literature to reserve resection of adenomas for adenoma diameter > 5 cm with telangiectatic or unclassified subtypes and male sex[77,78,81].
CLINICAL FEATURES
HCC in non-cirrhotic patients is clinically silent in its early stages because of lack of symptoms and surveillance imaging; and higher hepatic reserve in this population[82].The median age of these patients is 69 yr however, the FLC variant commonly occurs in adolescents and young adults, ranging from 10-35 yr at presentation[11,16].Unfortunately, these tumors are often found in advanced stages with approximately 25% of non-cirrhotic HCC presenting with extra-hepatic metastasis[16]. When symptoms do occur, they arise due to large tumor burdens from its insidious progression. The most common presenting symptom is abdominal pain (52%). Other symptoms include abdominal distention, weight loss, malaise, anorexia, fatigue,chronic diarrhea, jaundice, chest pain and fever of unknown origin[4,83,84]. Non-cirrhotic HCC can also present in the form of paraneoplastic syndrome of hypercalcemia or hypoglycemia[82,85]. FLC has been known to present with gynecomastia, recurrent deep vein thrombosis, Budd-Chiari syndrome, non-bacterial thrombotic endocarditis,fulminant liver failure or encephalopathy[10].
DIAGNOSIS
Serum alpha-fetoprotein
Alpha-fetoprotein (AFP), a serum glycoprotein is a commonly used tumor marker for HCC[86]. Due to its poor sensitivity, the American Association for the Study of Liver Disease (AASLD) guidelines suggests surveillance with ultrasound with or without AFP in cirrhotic patients. In non-cirrhotic HCC, the sensitivity of AFP further decreases with elevation less common compared to cirrhotic HCC (31%-67% vs 63%-84%). AFP levels in majority of patients with the FLC-variant HCC are normal[87,88].Levels > 400 ng/mL are essentially diagnostic for non-cirrhotic HCC and are equally prevalent in both groups[89]. This implies that elevated AFP levels may suggest a HCC,but normal levels should never be used to exclude the diagnosis, especially in a patient with high-risk factors. AFP levels however, may have a role in tumor surveillance and prognosis.
Des-gamma-carboxyprothrombin
Des-gamma-carboxyprothrombin (DCP) also known as PIVKA II (protein induced by vitamin K absence) is produced by malignant hepatocytes and its relationship to HCC has been known since 1984[90,91]. DCP has been reported to be more sensitive and specific than AFP for the diagnosis of HCC with a cutoff of > 40 mAU/mL[92].However, its role in the detection of small tumors and early HCC is unclear as various studies have utilized different cutoff values for DCP and AFP[92-94]. Moreover, it has never been studied in non-cirrhotic patients. DCP might be the answer for early HCC diagnosis in non-cirrhotic patients along with monitoring treatment response and recurrence. Perhaps DCP could compliment AFP as evidenced by improved sensitivities emphasized in several studies[91,93]. Future studies should be directed towards establishing a relationship between elevated DCP and non-cirrhotic HCC.
Imaging
The radiological appearance of HCC in cirrhotic and non-cirrhotic patients is very similar, except HCC in non-cirrhotic livers frequently present as a solitary mass with or without satellite lesions, are much larger in tumor size and are often seen with a central scar[95].
Ultrasonography:Ultrasonography (US) is a non-invasive test that allows determining the size, location, morphology and vascular involvement of the lesion.The appearance of HCC on US is variable and non-specific ranging from hypo or hyperechoic lesions with or without heterogeneity or necrotic areas. This imaging modality is limited in the detection of tumors < 2 cm and tumors in a liver base with a heterogeneous diffuse nodular pattern[82,84].
Contrast-enhanced US (CEUS) could be valuable diagnostic tool because the dye allows its use in patients with nephropathies and those with known adverse reactions to other contrast agents[82]. CEUS shows a typical HCC vascular pattern, although inconsistently when compared to computed tomography (CT) and magnetic resonance imaging (MRI) especially for tumors < 2 cm[96-99]. There is a need for such studies in non-cirrhotic HCC, but for now its role in diagnosis is limited. However, it may have a role in guiding biopsies and monitoring tumor response to treatment with anti-angiogenic properties[100,101].
Computed tomography: CT scan can make the diagnosis of HCC with a high degree of confidence, hence proper technique and contrast administration is crucial for an accurate assessment (Figure 3)[82]. The main diagnostic criteria include hypervascularization on the arterial “wash-in” phase and washout during portal phase of enhancement, which is similar in cirrhotic and non-cirrhotic livers[102,103]. It often presents as a single large well-circumscribed encapsulated hypoattenuating lesion on unenhanced CT, with other tumor features like fat involvement, foci of hemorrhage and necrotic areas more common in non-cirrhotic HCC[104]. The FLC variant may show a similar pattern on contrast enhanced imaging as the classic noncirrhotic HCC and often demonstrates internal calcifications, central scars and a discontinuous capsule[105].
Magnetic resonance imaging:MRI is superior to CT for the diagnosis of HCC (Figure 4). Its appearance on T1 sequences varies depending on the degree of fibrosis, necrosis and fat but it more commonly presents as a hypointense lesion. Its appearance on T2 is variable as well but it is commonly a hyperintense lesion. Intracellular fat accumulation, which is present in 10%-17% of non-cirrhotic HCC and 36% of welldifferentiated HCC is easier to detect on MRI compared to CT/US and signifies a better prognosis[82,84]. The gadolinium enhancement shows a similar pattern on MRI as described in contrast enhanced CT[82]. About 50% of non-cirrhotic HCC have a central scar that is detectable by MRI[99]. The FLC variant is hypointense on T1, hyperintense on T2 and heterogenous after gadolinium enhancement[106]. The central scar that is frequently seen in this subtype can be hypo or hyperintense on T2 sequences depending on the presence of necrosis and altered vascularity[107]. The differentiation between HCC and other benign liver lesions (focal nodular hyperplasia and hepatocellular adenoma) on MRI has been challenging in a non-cirrhotic liver often requiring unnecessary interventions for histopathological proof. In conclusion, T1 hypointensity, T2 hypo or hyperintensity, lack of central tumor enhancement and presence of satellite lesions are independent imaging factors, with a combined specificity of 98%, can allow MRI guided diagnosis of HCC in non-cirrhotic liver with a high level of confidence[107].
Percutaneous liver biopsy
Histological diagnosis via liver biopsy may only be necessary if imaging studies are inconclusive for being compatible with HCC[108-110]. The AASLD does not recommend biopsy for lesions > 1 cm if two different imaging studies yield concordant findings[108]. When performed, they are done via transabdominal technique under CT or US guidance with varying degrees of sensitivity (66%-93% based on tumor size)and 100% specificity and positive predictive value[109]. Liver biopsy may be needed in patients who are not candidates for curative resection, to establish diagnosis for the purpose of systemic therapy or transplantation[109].
MANAGEMENT
Surgical Resection
Surgical resection is the treatment of choice for HCC in non-cirrhotic liver and is considered equally safe in non-cirrhotic and cirrhotic patients[111-113]. Since clinical staging systems like Okuda/Barcelona-Clinic Liver Cancer associated with underlying cirrhosis are not relevant in these patients, primary tumor features are utilized for staging and prognosis. Patients that are typically not candidates for surgical resection are those that have extrahepatic spread of their disease or anatomical constraints related to the tumor. Majority of the patients require a major hepatic resection, often with advanced surgical techniques for inferior vena cava or diaphragmatic resection; or total vascular exclusion[114]. These surgeries are feasible due to the preserved liver function and low perioperative mortality when compared to cirrhotic livers[114]. Common complications associated with such resections include intra-abdominal collections and liver insufficiency[115]. Perioperative morbidity and mortality is low when compared to the cirrhotic liver, 29.5% and 2.7% respectively[115].Impressive postoperative survival rates of 96%, 87% and 68% after 1, 3 and 5-yr respectively have been reported in patients with tumors without vascular invasion;factors such as portal vein thrombosis, lymph node involvement and tumor recurrence are associated with poor outcomes[112,113].
Tumor recurrence
Tumor recurrence is the major cause of death in non-cirrhotic livers with HCC since no effective post-operative adjuvant chemotherapy exists[116,117]. The recurrence rate of HCC in non-cirrhotic liver is extremely high after surgical resection. Taking into account the best reported figures, the 1, 3 and 5-yr disease free survival rate is 79%,58% and 54% respectively. Repeat hepatectomy is feasible in these patients due to normal liver function, which allows for good regenerative capacity. A mean time recurrence of 31 mo with a 61% 5-yr survival and 25% 10-yr survival after a first repeat hepatectomy has been reported for non-cirrhotic patients[116]. A good functioning liver also allows for repeat second and third hepatectomies in these patients and can be considered equally safe and effective when compared to the first[84]. However, when surgical resections fail to control recurrent disease or repeat tumors are considered unresectable, patients may need to be evaluated for liver transplantation (LT).
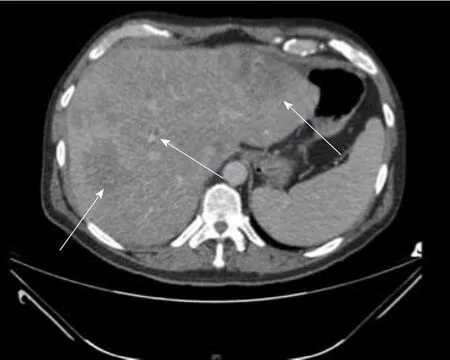
Figure 3 Computed tomography image of a 55-yr-old male with no significant past medical history found to have multifocal hepatocellular carcinoma in the right lobe of liver on imaging done for abdominal pain and jaundice.
LT
Historically, LT was not routinely recommended in non-cirrhotic HCC due to the lack of precise guidelines or selection criteria for this patient population. Very early reports and studies have demonstrated high tumor recurrences and dismal survival outcomes. A systematic review of all reported cases of LT in non-cirrhotic patients from 1966-1988 reported a 5-yr survival rate of 11.2% for HCC and 39.4% for the FLC variant[118]. McPeake et al[119]reported a 40% recurrence rate for lesions 4-8 cm and 78%in lesions > 8 cm. However, Mergental et al[120]reported a 5-yr survival of 50%-70%after analysis of literature and European Liver Transplant Registry with median tumor sizes of 8 cm. This study recommends that Milan criteria should not be used to exclude patients with non-cirrhotic HCC and identified extrahepatic spread, gross vascular or lymph node involvement, multiple tumors and tumor recurrence < 1 yr after resection as predictors of poor outcome after LT. Based on these findings, an international consensus conference report recommended LT in patients with nonresectable HCC or in patients who experience intrahepatic recurrence after surgical resection; provided these patients have no macrovascular invasion or extrahepatic spread[121]. Decaens et al[122]highlighted the need for prospective studies addressing pre-LT imaging for tumor characteristics, response to treatment performed and kinetics of tumor progression during the waiting period and rate of dropout from tumor progression. It will be interesting to note the recurrence and overall survival(OS) of these patients in future studies after improved patient selection and advances in perioperative management and surgical care.
Systemic therapy
Sorafenib is an Food and Drug Administration (FDA) approved oral multi-tyrosine kinase inhibitor, which is the first line therapy for patients with advanced HCC[123,124].It is indicated in patients who are not deemed surgical or transplant candidates with preserved liver function. It inhibits tumor growth and has anti-proliferative, antiangiogenic and pro-apoptotic features[125]. The most common side effects reported include diarrhea and hand-foot skin reactions[126]. Sorafenib has demonstrated survival benefit in cirrhotic HCC, however no studies have formally evaluated outcomes in patients with a non-cirrhotic liver. Given the similarity of angiogenic characteristics between cirrhotic and non-cirrhotic HCC, Sorafenib could have a role in the management of advanced HCC in non-cirrhotic patients[127]. Recently, the FDA approved two new systemic drugs for advanced HCC. Regorafenib, a multikinase inhibitor, and Nivolumab, a PD-1 (programmed cell death protein 1) inhibitor have shown survival benefit in patients who had disease progression despite treatment with Sorafenib[128,129].

Figure 4 Magnetic resonance imaging (e-THRIVE_BH AX 15 min delay) of 61-yr-old male with hepatitis C virus, without cirrhosis showing a 2.8 cm × 3 cm mass lesion in segment 5 consistent with hepatocellular carcinoma.
Systemic chemotherapy with other agents has been ineffective and has resulted in sub-optimal outcomes in cirrhotic advanced HCC. This has been largely due to the underlying cirrhosis with altered drug metabolism, which can lead to serious toxicity requiring either decrease in the dose or discontinuation[130,131]. However, non-cirrhotic HCC patients with a healthy liver may be able to tolerate these agents. Edeline et al[132]in their study had a 52% disease control rate with ECC (epirubicin, cisplatin and 5-flurouracil) or ECF (epirubicin, cisplastin and capecitabine). Romano et al[130]demonstrated partial response to Docetaxel with long-term survival and without severe toxicity in 3 patients. Gras et al[133]reported a complete response with GEMOX chemotherapy without a 5-yr relapse after discontinuation in a patient with advanced FLC-HCC in a non-cirrhotic liver. These reports highlight the need for further trials to explore the use of systemic chemotherapy to improve prognosis of patients with advanced non-cirrhotic HCC. Systemic chemotherapy may result in downsizing of the tumor allowing such patients to be candidates for curative surgical resection or liver transplant.
There have been few studies that have evaluated the combination of Sorafenib with other systemic chemotherapeutic agents in non-cirrhotic advanced HCC. In patients who experience recurrences after LT, combination of Sorafenib and mTOR inhibitor in conjunction with locoregional treatments improved survival, with 1 and 5-year survival rates of 82% and 33% respectively[134]. This could open potential avenues for research for combination chemotherapy and loco-ablative techniques like radiofrequency ablation (RFA), transarterial chemoembolization (TACE) and radioembolization commonly employed in cirrhotic HCC; in the management of noncirrhotic HCC.
Loco-ablative therapies and selective internal radiation therapy
Local ablation therapies like RFA and TACE are considered first line treatment options for unresectable HCC in cirrhotic patients. These techniques along with selective internal radiation therapy (SIRT) have been employed for down staging of tumors and control progression[135,136]. Unfortunately, their role in non-cirrhotic HCC has not been established in studies likely due to the rarity of the disease, benefit of other treatment modalities, poor prognosis and high tumor recurrence. However, for metastatic disease after prior hepatectomy, RFA has been associated with improved progression free survival and OS when compared to transcatheter therapy[135].
A recent case report published by Mafeld et al[136]showed a 94% reduction in tumor size 7 mo after SIRT with Yttrium-90 for unresectable FLC-HCC after which the patient underwent a curative surgical resection. Studies establishing SIRT as a standard of care in regular non-cirrhotic HCC may be difficult given its high 90-d morbidity; complications and lack of long term follow up data in the current case[136].However, SIRT should be considered for the FLC-variant given limited treatment options for unresectable disease and its grave prognosis.
PROGNOSIS AND SURVEILLANCE
Survival of patients with HCC in non-cirrhotic liver mainly depends on tumor related factors such as tumor size, existence of satellite lesions, lack of tumor capsule,vascular invasion, grading, incomplete resection, HBV infection and the amount of intraoperative blood transfusions[117,137-139]. Poor prognostic factors that affected the OS rate in patients undergoing surgical resection include the need for blood transfusion and advanced age > 65. Factors that affected the recurrence free survival rate included the presence of multiple tumors[117]. FLC-HCC has a 70% 5-yr survival following surgical resection whereas for unresectable disease, the 5-yr survival rate is 0-5% with a median survival of 12 mo[140]. The number of tumors and vascular invasion are considered poor prognostic factors in this variant; the 3-yr recurrence free survival rate is 9% in patients with vascular invasion and 35% without[140].
AFP levels could be better suited as prognostic indicators. Burnett et al[141]used AFP staging based on four levels: < 10 ng/mL, 10 to 150 ng/mL, 150 to 500 ng/mL and >500 ng/mL; and found these to be appropriate predictors of prognosis in non-cirrhotic HCC. Witjes et al[89]showed that elevated pre-operative AFP levels were associated with worse outcomes and high recurrence rates. Using an AFP cut off of 9 ng/mL,they demonstrated 1 and 3-yr survival rates of 53% and 21% respectively in patients with high AFP and 86% and 75% in patients with low AFP.
The need for effective surveillance needs to be addressed given the high tumor recurrence rate. The most common and significant issue raised is the delayed presentation of HCC in non-cirrhotic patients. There is a further need to direct research towards alternative cost-effective surveillance strategies and risk factor profiling to identify this high-risk population, decrease the tumor burden upon presentation and improve survival outcomes. Fu et al. reported a high association between high relative telomere length (RTL) and risk of HCC in non-cirrhotic chronic hepatitis B patients. Future studies could expand the role of RTL in serum DNA as a non-invasive biomarker for surveillance in non-cirrhotic HCC[142]. Wang et al[143]developed a prognostic scoring system of HCC after hepatectomy based on 11 independent risk factors and classified patients into low, intermediate and high-risk groups with an 80, 27 and 6-mo recurrence free survival respectively in each group.Such categorization is perhaps what is required in non-cirrhotic patients. Alkaline phosphatase (ALP) has also been reported as an independent risk factor that affects recurrence-free and survival rates[139]. High ALP levels could have a potential role in predicting HCC recurrence in non-cirrhotic patients and could be part of guidelines that establish risk for tumor recurrence. Further studies are necessary however, to validate the use of such scoring systems. This might allow for a more cost-effective and economic surveillance in high and intermediate risk groups.
FUTURE CONSIDERATIONS
microRNA
microRNAs (miRNAs) are endogenous non-coding 21-23 nucleotide RNAs that are involved in post-transcriptional regulation and thus play an important role in almost all main cellular pathways including regulation of major tumor-related genes in carcinogenesis[144,145]. miRNAs are also involved in iron metabolism through regulation of genes that control iron homeostasis in hepatocytes[146]. Dysregulation of miRNAs can lead to iron overload which can lead to the generation of reactive oxygen species and cause oxidative stress which damages DNA, lipids and proteins[147]. miRNA could serve as important diagnostic and prognostic biomarkers in non-cirrhotic HCC. Koh et al[148]identified 16 miRNAs that displayed significant change in expression non-tumor and HCC tissues in non-cirrhotic livers. Analysis of miRNA in the serum is an exciting prospect for the diagnosis and/or prognosis of non-cirrhotic HCC.
Early and unique changes in circulating miRNA in the serum could potentially allow it to be a biomarker for the early detection of non-cirrhotic HCC. In a study performed by Zhang et al[149], a 3 mi-RNA panel comprising of miR-92-3p, miR-107,and miR-3126-5p was equally effective as AFP for diagnosis of early HCC.Furthermore, the combination of miRNA panel and AFP had higher sensitivity and specificity than AFP alone, especially in patients with early HCC or low-level AFP.However, the study did not specify presence or absence of cirrhosis in the patient cohort. miRNA could also serve as an important prognostic marker in non-cirrhotic liver. Dysregulation of certain miRNA has been associated with poor disease-free survival after liver resection of HCC[145]. Another study showed low levels of miRNA(miR-181a-5p) and poor disease control after Sorafenib therapy[150]. Again, these studies did not comment on background liver cirrhosis. In a recent study by Mei et al[151]cirrhotic and non-cirrhotic HCC patients were found to have 41 differentially expressed miRNAs. Specifically, two miRNAs (mir-149 and mir-1296) were strongly associated with non-cirrhotic HCC and influenced the TMN tumor staging.Furthermore, increased mir-149 was associated with increased post-operative survival in non-cirrhotic HCC. Huang et al[152]developed 5-panel mi-RNA that capable of assessing risk in HCC patients. The hope is that future studies could identify a similar miRNA signature for non-cirrhotic HCC patients. Hence, miRNAs are an exciting future research prospect and could perhaps be the solution for improved diagnosis,surveillance and prognosis along with therapeutic management of advanced noncirrhotic HCC.
CONTRIBUTIONS
The current review should help make significant contributions to research progress for HCC in non-cirrhotic liver. It highlights the major risk factors implicated in the development of HCC in a non-cirrhotic liver; especially NAFLD/NASH. It also brings to attention other rare risk factors that would further assist clinicians in decreasing the incidence of cryptogenic HCC. The review has also attempted to drive research towards finding alternative means of diagnosing non-cirrhotic HCC early by exploring other tumor markers like DCP as well as improve surveillance and monitoring of these patients. Finally, the review placed special emphasis on the management of non-cirrhotic HCC by incorporating the latest literature, which would allow researcher to explore other potential avenues especially systematic chemotherapy and loco-ablative techniques.
CONCLUSION
HCC in a non-cirrhotic liver is a complex disease phenomenon with risk factors,pathogenesis, clinical features, management and prognosis that are distinct from the cirrhotic counterpart. Even though considerable progress has been made in the management of this entity, there is a dire need for implementation of surveillance strategies in the patient population at risk, to decrease the disease burden at presentation and improve the prognosis of these patients. The hope is that this review sparks further research to close the knowledge gap and uncover answers to questions that have puzzled experts for decades.
杂志排行
World Journal of Hepatology的其它文章
- Caval replacement with parietal peritoneum tube graft for septic thrombophlebitis after hepatectomy: A case report
- Non-uremic calciphylaxis associated with alcoholic hepatitis: A case report
- Multidisciplinary approach for multifocal, bilobar hepatocellular carcinoma: A case report and literature review
- High prevalence of occult hepatitis C infection in predialysis patients
- Low platelet count: Predictor of death and graft loss after liver transplantation
- Clinical factors associated with hepatitis B screening and vaccination in high-risk adults
