Effects of diabetic ketoacidosis in the respiratory system
2019-02-20AliceGallodeMoraesSalimSurani
Alice Gallo de Moraes, Salim Surani
Abstract Diabetes affects approximately 30 million persons in the United States. Diabetes ketoacidosis is one of the most serious and acute complications of diabetes. At the time of presentation and during treatment of diabetic ketoacidosis (DKA), several metabolic and electrolyte derangements can ultimately result in respiratory compromise. Most commonly, hypokalemia, hypomagnesemia and hypophosphatemia can eventually lead to respiratory muscles failure.Furthermore, tachypnea, hyperpnea and more severely, Kussmaul breathing pattern can develop. Also, hydrostatic and non-hydrostatic pulmonary edema can occur secondary to volume shifts into the extracellular space and secondary to increased permeability of the pulmonary capillaries. The presence of respiratory failure in patients with DKA is associated with higher morbidity and mortality. Being familiar with the causes of respiratory compromise in DKA, and how to treat them, may represent better outcomes for patients with DKA.
Key words: Diabetes ketoacidosis; Respiratory physiology; Mechanical ventilation;metabolic acidosis; Hyperventilation; Kussmaul breathing; Respiratory failure
INTRODUCTION
Diabetes ketoacidosis (DKA) is one of the most serious and acute complications of diabetes. It is characterized by moderate hyperglycemia (blood glucose usually between 250 mg/dL and 800 mg/dL at presentation), metabolic acidosis, and presence of serum ketones with an elevated anion gap[1]. It represents an extreme in the spectrum of hyperglycemia and presentation of complicated diabetes.
Diabetes affects approximately 30 million persons in the United States[2]. Since 2009,there has been an increase of around 6% of hospitalizations due to DKA (from 19.5 to 30.2 per 1000 persons). However, the in-hospital mortality has declined at an annual average rate of 6.8% (from 1.1% to 0.4%)[2].
The presence of DKA is accompanied by several electrolytes, metabolic and acidbase derangements that affect the respiratory system. Depletion of ions, such as potassium and phosphate, affect the respiratory muscles leading to acute respiratory failure[3]. Reduction in colloid osmotic pressure increases lung water content, leading to noncardiogenic pulmonary edema and decrease in lung compliance[4,5]. As a compensatory mechanism, the presence of metabolic acidosis will cause hyperventilation[6].
Respiratory failure in DKA has been associated with increased morbidity and mortality[3,7]. In this review, we analyze the common electrolytes, metabolic and acidbase abnormalities seen in DKA, their association with respiratory failure and its management.
ELECTROLYTE ABNORMALITIES
Potassium, magnesium and phosphorous are intracellular ions which serum concentrations decrease as a direct consequence of hyperglycemia and ketoacidosis(potassium), or as a consequence of the correction of acidosis with insulin(magnesium and phosphorous). A major goal in the treatment of DKA is to closely monitor these ions concentrations as DKA is corrected. Also, replace them on a timely fashion in order to prevent them from reaching critically low values. The clinical significance of their deficit is discussed below.
Potassium
Patients being admitted for DKA usually have a total body potassium deficit that averages 300 to 600 mEq[8]. Osmotic diuresis is provoked by the hyperglycemia resulting from lack of insulin. In an attempt to maintain osmolality, the kidneys will retain sodium ions at the expense of potassium ions[9]. Furthermore, when acidosis is present, hydrogen ions from the bicarbonate nucleus will be reabsorbed at the expenditure of potassium[10].
The gastrointestinal tract is also responsible for potassium loss in DKA. The body will try to maintain osmotic pressure at the cost of tissue and serum electrolytes. An acute hyperkalemia will happen when potassium shifts into the extracellular fluid(ECF), causing gastric cells to preserve hydrogen ions concentration. Consequently,nausea, vomit and diarrhea will occur, promoting even more potassium loss[11,12].However, due to a shift of potassium from intracellular fluid into ECF caused by hyper osmolality and insulin deficiency, only 5% of patients with DKA will present with hypokalemia[8,13].
When potassium levels fall below 2.5 mg/dL, severe ascending muscular weakness can occur[14]. The muscular weakness can affect the respiratory muscles causing acute respiratory failure[15], and requirement of mechanical ventilation[16]. Aggressive potassium replacement should start once serum potassium concentration reaches a value of 3.3 mEq/L[13].
Magnesium
At presentation of DKA, the levels of serum magnesium are usually normal. Excessive amounts of magnesium are excreted during acidosis, secondary to insulin deficiency[17]. As the acidosis gets corrected, magnesium levels fall, reaching their nadir within the first 25 h of acidosis correction[18,19].
Hypomagnesemia, defined as having a serum magnesium concentration below 1.6 mg/dL (0.66 mmol/L), usually doesn't lead to clinically significant symptoms until serum levels fall below 1.2 mg/dL (0.5 mmol/L)[20].
Magnesium regulates intracellular calcium levels, influencing smooth muscle tone[21]. Because of its role in regulating smooth muscle tone, magnesium deficiency has been associated with systemic hypertension, neuromuscular excitability,bronchoconstriction, coronary vasospasm and seizures[22].
Muscular weakness and tetany associated with hypomagnesemia can affect the respiratory muscles, impairing ventilation in patients who are spontaneously breathing and delaying extubation of mechanically ventilated patients[22,23]. Empirical magnesium replacement has been associated with improvement of respiratory muscle power in patients with DKA[23].
When treating patients with DKA, clinicians should aim to keep magnesium levels at normal range, since hypomagnesemia is associated with weakness of the respiratory muscles.
Phosphorous
Acidosis causes potassium shifts into the ECF and hyperglycemia causes phosphaturia by osmotic diuresis, which will ultimately lead to hypophosphatemia.However, DKA patients will present with normal phosphorous concentration due to the shift into the ECF associated with ECF volume concentration[24]. The true state of phosphate equilibrium is revealed with volume expansion[24,25].
Severe hypophosphatemia (< 1 mg/dL) is associated to the depletion of highenergy phosphate compounds in muscles, causing muscular weakness and rhabdomyolysis[3,26].
Acute muscular weakness caused by hypophosphatemia in DKA has been associated with hypercapnic respiratory failure and prolonged mechanical ventilation in critically ill patients[26-28].
Routine replacement of phosphorous in patients who presented with DKA is not beneficial and has been associated with worsening hypomagnesemia and causing hypocalcemia[29,30]. However, if serum phosphate concentration falls below 1 mg/dL,or if hypophosphatemia is associated with cardiac dysfunction or respiratory depression, it should be replaced[28,31,32].
HYPERVENTILATION
The presence of metabolic acidosis will normally generate a respiratory response. The reduction of serum bicarbonate and pH will result in hyperventilation and reduction in carbon dioxide (CO2), partially preventing further fall in pH and bicarbonate concentration. Respiratory compensation for metabolic acidosis will cause the arterial CO2to decrease by 1.2 mmHg for each 1 meq/L fall in the serum bicarbonate[33].
The respiratory response usually begins within 30 min of metabolic acidosis onset,and is generally complete within 12-24 h However, a lag in respiratory compensation can occur when respiratory acidosis develops quickly; more than 4 meq/L of bicarbonate decrease in less than 6-12 h[34,35].
There is a limit to the lungs' ability to compensate for metabolic acidosis. Even with serum bicarbonate concentrations below 6 meq/L, CO2levels cannot fall lower than 8-12 mmHg[34]. Furthermore, the duration of the respiratory compensation is limited by respiratory muscle fatigue[33,36].
Initially, patients will develop tachypnea, which is increased respiratory rate,leading to decrease in CO2concentration. With progression of acidosis, respiratory pattern evolves to hyperpnea, which is increased tidal volume, and ultimately,patients will develop a deep, fast and agonal pattern of breathing, named Kussmaul's respiration (Figure 1A-D)[34,37].
Once patients with DKA develop Kussmaul's respiration, they are reaching the point of respiratory muscles fatigue, and mechanical ventilation should be considered[34,38-40]. Furthermore, patients in DKA are severely “air hungry” prior to intubation, and are at higher risk to develop acute respiratory distress syndrome(ARDS)[3,41]due to hyperpnea. Mechanical ventilation in these patients is particularly delicate, since a lung protective strategy, with low tidal volumes and controlled transpulmonary pressures, should be maintained, while attempting to increase minute-ventilation until metabolic acidosis is completely corrected[42,43].
PULMONARY EDEMA
There are two types of pulmonary edema that have been described in patients with DKA: One associated with elevated pulmonary venous pressure and another associated with increased pulmonary capillary permeability. The diagnosis is made based on clinical findings of dyspnea, an A-a gradient on arterial blood gas and chest image showing bilateral pulmonary infiltrates.
Pulmonary edema due to elevated pulmonary venous pressure
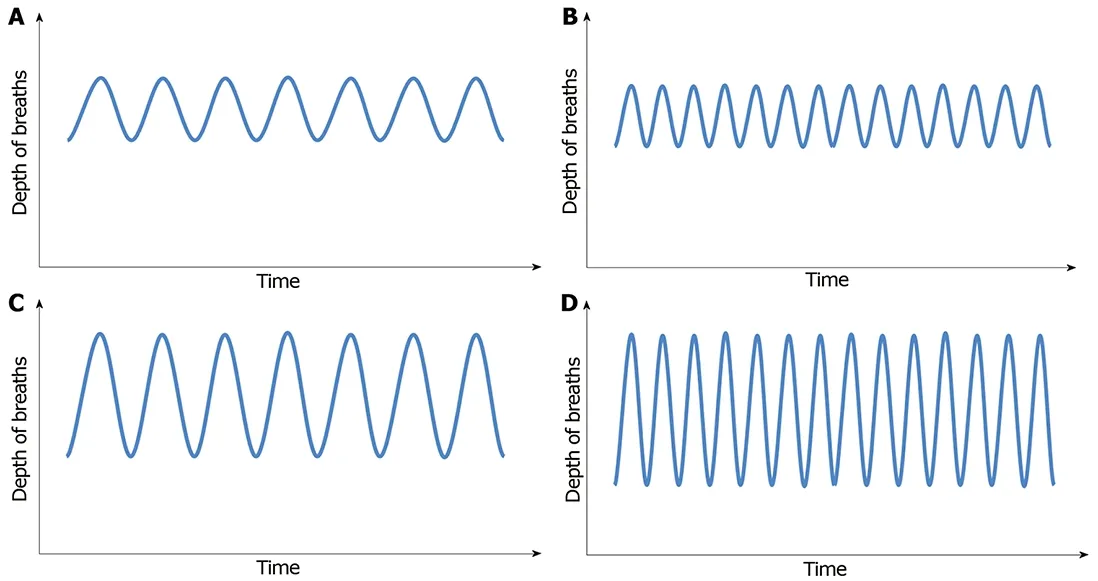
Figure 1 Depth of breaths. A: Normal (eupnea); B: Tachypnea - increased respiratory rate; C: Hyperpnea - normal rate, deep inspirations; D: Kussmaul's -tachypnea and hyperpnea.
Also known as hydrostatic pulmonary edema, it is usually existent at presentation of DKA, is corrected during the treatment of DKA and is more common in patients with concomitant renal failure[44-47]. The occurrence of circulatory overload and pulmonary edema with elevated pulmonary venous pressure is a result of the acute shift of an abundant volume of fluid into the extracellular compartment. This fluid shift happens as a consequence of solute accumulation in the extracellular compartment secondary to hyperglycemia[44]. Therefore, correction of hyperglycemia shifts fluid back into cells,also correcting hydrostatic pulmonary edema. However, some patients might require hemodialysis and mechanical ventilation.
The degree of fluid shift and, consequently, the likelihood of developing hydrostatic pulmonary edema during a DKA episode are determined by the severity of hyperglycemia and by the volume status prior to the development of DKA[47]. The amount of fluid transferred from the cells into the extracellular space is directly proportional to the changes in serum glucose concentration[48]. The patients' volume status at the time of hyperglycemia onset is also a determinant of the volume that will shift into the extracellular space. Patients with baseline low extremity edema and/or anasarca have been shown to shift larger amounts of fluid and have a higher incidence of pulmonary edema, than those patients who are euvolemic when becoming hyperglycemic[49].
Even though hydrostatic pulmonary edema has been described more commonly in patients with advanced renal disease, there are several cases reported in patients with DKA who developed pulmonary edema without having renal dysfunction. Several cases have been reported of takotsubo cardiomyopathy happening in the setting of DKA and causing pulmonary edema[50,51]. There are also reports of myocardial dysfunction secondary to severe acidosis and electrolyte abnormalities[52].
Pulmonary edema due to increased pulmonary capillary permeability
Also known as non-hydrostatic pulmonary edema, this type of pulmonary edema is caused by changes at the histological level of the alveolar epithelium. In diabetic patients, there is thickening of the alveolar epithelium and pulmonary capillary basal membrane, corroborating the presence of pulmonary microangiopathy[53,54].
ARDS can develop during the course of DKA or during its treatment[3], and it is more frequent and severe than hydrostatic pulmonary edema[54,55]. The mechanism of ARDS in DKA is not completely understood. The most accepted explanation is activation of lymphocytes and release of cytokines, especially interleukin-1, which serum levels are much higher during treatment of DKA[56-58].
The treatment of non-hydrostatic pulmonary edema in DKA is supportive. Focus should be on treating DKA and its exacerbating factor, early intubation and protective lung ventilation.
CONCLUSION
In DKA, respiratory failure is caused by several electrolytes, metabolic and cardiac and lung end-organ damage. Developing respiratory failure during DKA onset or treatment is associated with high mortality. Early recognition and treatment of the risk factors for the development of respiratory failure have the potential to decrease morbi-mortality of patients with DKA.
杂志排行
World Journal of Diabetes的其它文章
- Exploratory metabolomics of metabolic syndrome: A status report
- Early vs late oral nutrition in patients with diabetic ketoacidosis admitted to a medical intensive care unit
- Relationship between sonographically measured median nerve cross-sectional area and presence of peripheral neuropathy in diabetic subjects
- Diabetes in the Kokan region of India
- Update on biomarkers of glycemic control
