Ethanol exposed maturing rat cerebellar granule cells show impaired energy metabolism and increased cell death after oxygen-glucose deprivation
2019-02-13AnaSpataruDianaLeDucLeonZagreanAnaMariaZagrean
Ana Spataru , Diana Le Duc , Leon Zagrean Ana-Maria Zagrean
1 Division of Physiology and Neuroscience, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania
2 King's College Hospital, London, UK
3 Institute of Human Genetics, University of Leipzig Hospitals and Clinics, Leipzig, Germany; Department of Evolutionary Genetics, Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany
Abstract Alcohol, a widely abused drug, has deleterious effects on the immature nervous system. This study investigates the effect of chronic in vitro ethanol exposure on the metabolism of immature rat cerebellar granular cells (CGCs) and on their response to oxygen-glucose deprivation (OGD). Primary CGC cultures were exposed to ethanol (100 mM in culture medium) or to control ethanol-free medium starting day one in vitro(DIV1). At DIV8, the expression of ATP synthase gene ATP5g3 was quantified using real-time PCR, then cultures were exposed to 3 hours of OGD or normoxic conditions. Subsequently, cellular metabolism was assessed by a resazurin assay and by ATP level measurement. ATP5g3 expression was reduced by 12-fold (P= 0.03) and resazurin metabolism and ATP level were decreased to 74.4 ± 4.6% and 55.5 ± 6.9%, respectively after chronic ethanol treatment compared to control values (P < 0.01). Additionally, after OGD exposure of ethanol-treated cultures, resazurin metabolism and ATP level were decreased to 12.7 ± 1.0% and 9.0 ± 2.0%from control values (P < 0.01). These results suggest that chronic ethanol exposure reduces the cellular ATP level, possibly through a gene expression down-regulation mechanism, and increases the vulnerability to oxygen-glucose deprivation. Thus, interventions which improve metabolic function and sustain ATP-levels could attenuate ethanol-induced neuronal dysfunction and should be addressed in future studies.
Key Words: cell culture; chronic ethanol exposure; oxygen-glucose deprivation; cerebellar granule cells; toxicity;gene expression; cellular ATP; cellular metabolism; metabolic impairment; cell death
Introduction
Alcohol is a widely abused drug and a recognized teratogenic factor. In humans, intrauterine exposure causes fetal alcohol syndrome, a disorder characterized by cranio-facial malformations, growth retardation, structural brain abnormalities, and behavioral and cognitive impairment (Famy et al., 1998; Sulik, 2005).
Alcohol can damage fetal tissues in several ways. In the immature brain, gamma-aminobutyric acid and N-methyl-D-aspartate receptors are important targets involved in cell death associated with ethanol exposure (Ikonomidou et al., 2000;Prendergast et al., 2004). Disruption in intracellular calcium homeostasis and mitochondrial dysfunction resulting in generation of highly reactive oxygen species and DNA oxidative damage have been described after alcohol exposure (González et al., 2007; Kouzoukas et al., 2013). Chronic ethanol toxicity has been associated with low ATP levels and alteration in the mitochondrial membrane substrate transport and respiratory chain function (Videla et al., 1973; Xu et al., 2005; Haorah et al., 2013). Also, the expression of the genes coding for mitochondrial complexes II, IV, and V is markedly decreased in animals exposed in utero to ethanol (Chu et al., 2007).
Not all offspring exposed to ethanol develop the same degree of functional impairment, suggesting that additional factors are involved in the genesis of alcohol-related fetal anomalies (Hoyme et al., 2005). In this respect increased susceptibility to metabolic stressors in utero or postnatally could account for some of the features classically associated with ethanol-mediated injury. Perinatal ischemia is a well-characterized and relatively common occurrence (Kurinczuk et al., 2010) and ischemic injury shares common intracellular mechanisms with ethanol-induced damage, thus alterations in cerebrovascular and metabolic function have been suggested as potential culprits. Mice exposed to alcohol during development exhibit a persistent and long-term loss of cranial blood flow and a decreased capacity to recover after an ischemic injury (Bake et al., 2017). The inhibition of angiogenesis during embryonic development through the increased production of reactive oxygen species was also described after ethanol exposure (Wang et al., 2016). In addition, alcohol impairs insulin-mediated actions in the immature brain, leading to alterations in glucose transport in nervous cells and reduction in neuronal oxygen and glucose uptake (De La Monte et al., 2005; Abdul Muneer et al., 2011).
Chronic gestational exposure to ethanol was previously shown to aggravate the cellular injury after a hypoxic/hypoglycemic episode in the immature brain (Le Duc et al.,2015). In the present study, we investigate the molecular mechanisms that lead to ethanol-mediated impairment in the developing brain. To this end we use an in vitro para-digm and assess cerebellar granular cells' (CGCs) metabolic and energetic status after chronic ethanol exposure, followed or not by an episode of oxygen-glucose deprivation.
Materials and Methods
Ethics statement
All animal procedures were carried out with the approval of the local ethics committee for animal research from Carol Davila University (approval from April 2015), in accordance with the European Communities Council Directive 86/609/EEC on the protection of animals used for scientific purposes.
Primary cerebellar granular cell culture
All reagents were purchased from Sigma-Aldrich (St. Louis,MO, USA), unless stated otherwise in the text.
Primary CGC cultures were obtained from Wistar rat pups aged three days, using an enzymatic protocol, as described elsewhere (Zagrean et al., 2014). Cells were plated at a density of 2.8 × 105/well in 4- and 24-well plates (Nunclon Delta Si, Thermo Fisher Scientific, New York, NY, USA)treated with Poly-D-Lysine. Cultures were maintained in supplemented Basal Medium Eagle culture medium (CM)containing horse serum (10%), glucose (32 mM), glutamine(2 mM), antibiotic/antimycotic (Gibco®, Thermo Fisher Scientific, New York, NY, USA), and KCl (25 mM), in a humidified incubator (5% CO2in air) at 37°C. After 24 hours cytosine arabinoside (10 μM) was added to CM to inhibit proliferation of non-neuronal cells and to obtain a homogenous glutamatergic CGCs culture. CM was replaced every other day with fresh CM, as described further.
Experimental groups
Chronic ethanol treatment and exposure to OGD resulted in four experimental groups: cultures treated with ethanol(EtOH group), cultures treated with ethanol and exposed to OGD (EtOH OGD group), non-treated cultures exposed to OGD (OGD group), and non-treated cultures kept in control normoxic conditions (control group).
All cultures were assessed at DIV8, when CGCs developed the characteristics of mature neurons, e.g. glutamate receptors and intercellular connections.
Chronic exposure to ethanol
Alcohol blood concentrations in humans vary widely after a binge drinking episode and are correlated with the body weight, the quantity and timing of ethanol consumption.While a blood alcohol level of at least 80 mg/dL (17.4 mM) is required to define an episode of binge drinking, much higher concentrations (up to 100 mM) were reported amongst intoxicated alcohol consumers (Adachi et al., 1991; Brennan et al., 1995). Concentrations close to 100 mM were previously used in the in vitro toxicology studies and were shown to induce a severe and quantifiable injury in CGC populations(Kouzoukas et al., 2013).
To guarantee a high ethanol concentration in culture and to ensure a cyclic exposure, mimicking the human binge drinking pattern (Maier and West, 2001), we opted for the following exposure paradigm:
After one day in vitro (DIV1), the CM of the cultures from the EtOH group was replaced with CM containing 100 mM ethanol. The CM was renewed every other day (DIV3, DIV5,and DIV7) with a fresh CM containing 100 mM ethanol.The other cultures were used as control and had the CM replaced with fresh ethanol-free CM using the same paradigm.
Oxygen-glucose deprivation exposure
At DIV8, cultures were selected randomly from the EtOH and control groups and exposed to OGD or to control conditions (normoxia and glucose containing experimental medium) as described elsewhere (Le Duc et al., 2015).
For OGD exposure, an experimental medium lacking glucose (EM-G) and containing NaCl (120 mM), KCl (25 mM), MgSO4 (0.62 mM), CaCl2(1.8 mM), HEPES (10 mM), adjusted to pH 7.4 was used. Cultures were washed three times with EM-G and deoxygenated EM-G was added in each well. Immediately afterwards, cultures were placed in a humidified hypoxic chamber (Billups-Rotthenberg, Del Mar, San Diego, CA, USA) and exposed for ten minutes to a continuous flush of anoxic gas (5% CO2in 95% N2) to obtain an anoxic atmosphere. The chamber was then sealed and placed in a thermostat at 37°C for three hours. Control cultures were transferred in experimental medium with glucose (11 mM) (EM + G) and kept in an incubator for the same period, in a humidified 5% CO2atmosphere. After 3 hours of OGD the cultures were transferred in EM + G and reoxygenated in a 5% CO2atmosphere.
Cell viability assessment
Cell viability and metabolism was assessed using resazurin,a compound that can be reduced to resorufin by mitochondrial and cytosolic dehydrogenases. Resorufin is a red dye, whose concentration in the culture medium is directly proportional to cell viability and can be fluorometrically assessed (White et al., 1996).
Immediately after OGD or control conditions, cultures were incubated in a resazurin solution (100 μM in EM + G)for 3 hours to allow resazurin to metabolize. Resorufin fluorescence was measured with a spectrophotometer (DTX 880,Beckman Coulter, Brea, CA, USA), using an excitation filter set at 535 nm and an emission filter set at 595 nm, immediately after adding the resazurin solution (the initial reading)and 3 hours later (the final reading). Results are expressed in relative units of fluorescence as difference between the final and the initial readings and are reported as percentage from the values in the control group.
Cellular ATP measurement
Cellular ATP level was measured using an enzymatic assay (Promega, Fitchburg, WI, USA) based on luciferin oxidation to oxyluciferin under the action of luciferase in the presence of ATP. Immediately after OGD or control conditions, cultures were incubated in a solution of ATP reagent, according to manufacturer's instructions (one to one ratio in EM). The cell lysate was homogenized on an orbital shaker for two minutes, transferred in a dark 96-well plate(Greiner Bio One, Monroe, NC, USA) and left to equilibrate for ten minutes in dark. The luminescence was measured using a multimodal detector (DTX 880, Beckman Coulter,Brea, CA, USA). A calibration curve was constructed using ATP solutions of different concentrations (10-4M to 10-9M). A reduction in cell viability can result in lower protein content. Thus, to account for a possible viability reduction in cells exposed to ethanol, ATP values were normalized to the protein content and results are expressed as μg ATP/mg protein/well relative to the control cultures' values.
Protein measurement
The protein content in cultures was measured using the bicinchoninic acid (BCA) method (Smith et al., 1985). In the presence of protein, in basic medium, Cu2+is reduced to Cu1+.The resulting Cu-BCA colorimetric complex has a maximal absorbing capacity in the range of 540-600 nm. Cell lysis was induced by incubating cultures with a compatible lytic reagent(Cell Titer Glo, Promega, Madison, WI, USA). The lysate was transferred in a clear 96-well plate (Nunclon Delta Si, Thermo Fisher Scientific, New York, NY, USA) and incubated in BCA reagent for 30 minutes at 37°C. Absorbance was measured with a spectrophotometer (DTX 880, Beckman Coulter, Brea,CA, USA) using a filter set at 595 nm and a calibration curve was obtained using protein solutions (bovine serum albumin)of different concentrations (0 to 1000 μg/mL).
Microscopic examination
Immediately after OGD or control conditions, cultures were evaluated microscopically for morphology, maturation, and viability in phase-contrast and fluorescence using a Zeiss Axiovert25 inversion microscope (Zeiss, Oberkochen, Germany) at 400× magnification. Three different dyes were used for cell staining: Calcein-AM, Hoechst 33342, and propidium iodide. Calcein-AM is a hydrophobic, non-fluorescent compound hydrolyzed by intracellular esterases to Calcein,a fluorescent compound that distributes into the cytoplasm(Bratosin et al., 2005). Hoechst 33342 is a cell-permeable fluorescent dye that binds nucleic acids, marking the nucleus of all cells, irrespective of their viability and indicating the degree of chromatin clumping (Crowley et al., 2016). Propidium iodide is a fluorescent agent that stains the nuclei of dead cells, as it is not incorporated by the cells with an intact cytoplasmic membrane (Jiajia et al., 2017).
Cultures were incubated in Calcein-AM (10 μM) medium for 20 minutes, Hoechst 33342 (10 μM) medium for 10 minutes, and propidium iodide (50 μg/mL) medium for 5 minutes in dark, according to manufacturer's instructions. After incubation, the cultures were examined microscopically using special filters: 350 nm (Hoechst 33342), 500 nm (Calcein),and 600 nm (propidium iodide), and microphotographs were taken using a Sony digital incorporated camera.
Gene expression quantification of ATP5g3
Four separate CGC cultures were used for the control(non-treated and normoxic) and EtOH groups. RNA isolation, reverse transcription, and quantitative polymerase chain reaction (qPCR) measurements were performed as previously described (Le Duc et al., 2015). Briefly, total RNA was isolated using 500 μL TRI REAGENT™and RNA was reverse transcribed (Superscript, Invitrogen, Waltham, MA, USA)with oligo(dT) primer in a total reaction volume of 20 μL.cDNA (1 μL) was further subjected to real-time PCR using Platinum-SYBR Green qPCR Supermix (Invitrogen), forward and reverse primers (0.9 μM), and rhodamine X (100 nM,5-carboxy-X-rhodamine, passive reference dye). Primers for ATP5g3-gene were designed with the Primer3 software (Thermo Fisher Scientific, New York, NY, USA): forward primer 5′-TGC ATC AGT ATT ATC TCG ACC AG-3′ and reverse primer 5′-GCA CCT GCA CCA ATG AAT TT-3′. PCR was performed in an MX 3000P instrument (Stratagene, La Jolla,CA, USA) with the protocol: five minutes 50°C, two minutes 95°C, and 40 cycles of 15 seconds 95°C, 30 seconds 60°C.The presence of a single amplicon was confirmed using the product melting curve and the amplicon size was evaluated by agarose gel electrophoresis. The housekeeping gene B2m(β2-microglobulin) was used for normalization ΔCT= CT(gene)- CT(B2m)(Smith et al., 1985). The relative expression levels are given as the difference between the ΔCTcorresponding to the control and EtOH groups, respectively (ΔΔCT(gene)=ΔCT(geneNormalGroup)- ΔCT(geneEtOHGroup)). Gene regulation ratios between the EtOH and Control groups are given as 2-ΔΔCTvalues (Livak and Schmittgen, 2001).
Statistical analysis
Data were analyzed using GraphPad Prism8 (La Jolla, CA,USA) and are represented as percentage mean values ± the mean error (SEM) normalized to control conditions for each experimental group. Statistical significance between groups was calculated using a two-sided, unpaired t-test. Values of P ≤ 0.05 were considered statistically significant.
Results
Ethanol effects on cellular morphology, metabolism, and ATP levels
Cultures treated with ethanol displayed morphological changes suggestive of chronic injury: reduced cellular density and increased cellular waste. When assessed using fluorescence microscopy, cultures from the EtOH group demonstrated an increased number of propidium iodide-positive cells and condensed pyknotic nuclei compared with the control group (Figure 1A).
Chronic ethanol treatment significantly lowered the CGC metabolic activity compared to control cultures (P < 0.001)(Figure 1B). This change was accompanied by a decrease in the cellular ATP level in ethanol treated cultures compared to control cultures (P < 0.01) (Figure 1C).
Ethanol effect on ATP5g3 gene expression
Gene expression levels of ATP5g3 (ATP synthase, H+transporting, mitochondrial Fo complex, subunit C3 (subunit 9))were assessed by qPCR in both control and ethanol treated CGCs cultures. The results showed on average a 12-fold reduction in the expression of ATP5g3 gene in CGCs after ethanol exposure (P = 0.03; fold change calculated as 2-ΔΔCT(Livak and Schmittgen, 2001)) (Figure 2).
Ethanol and OGD effects on cellular morphology,metabolism, and ATP levels
Microscopic examination revealed that OGD exposure caused significant morphological changes in both ethanol-treated and non-treated cultures, with morphological evidence of cytoplasmic swelling, particularly prevalent in areas of high cellular density, with a loss of phase-bright cellular contours and deficient intercellular connections. Fluorescence microscopy showed low Calcein staining, more propidium iodide positive cells, and an increased number of condensed nuclei in ethanol treated cultures subjected to OGD, when compared to OGD exposed, non-treated cultures (Figure 3A).
Cellular metabolism in OGD group was significantly reduced compared to control cultures and diminished further in EtOH OGD group (P < 0.0001) (Figure 3B). OGD exposure alone significantly reduced the ATP level compared to control cultures, while the combination of ethanol exposure and OGD further decreased ATP level (P < 0.0001) (Figure 3C).
Discussion
Exposure to ethanol in utero causes structural alterations in brain development, impaired cognition, behavioral anomalies, and growth retardation. The extreme susceptibility of the immature brain to alcohol could be related to its direct effects on membrane receptors, the activation of intracellular apoptotic pathways, mitochondrial impairment, the toxicity of alcohol metabolites or to the indirect actions on maternal metabolism and nutritional status (Goodlett and Horn, 2001). However, the exact pathophysiological mechanism remains largely unknown.
The present study used an in vitro chronic intermittent exposure paradigm of immature cerebellar granular neurons to high concentrations of ethanol. This model was chosen for several reasons. Cerebellar granular neurons constitute a glutamatergic population highly susceptible to the alcohol-induced injury (Hauser et al., 2003). Ethanol administration began at DIV1 in culture, on neurons obtained from 3-day-old rat pups, a developmental period associated with maximal susceptibility to deleterious ethanol-mediated effects (Siler-Marsiglio et al., 2006). Additionally, the in vitro environment allowed an intermittent repeated exposure to a known concentration of alcohol to mimic the binge drinking behavior relevant to human consumption. Under these conditions the direct effects of ethanol can be separated from those of its extra neuronal metabolites or indirect effects (Goodlett and Horn, 2001). Finally, this in vitro model could be potentially useful in future studies exploring neuroprotective strategies.
Consistent with previous reports (Videla et al., 1973;Guadagnoli et al., 2016), our results show that the ATP level is markedly reduced in cultures treated with ethanol. The cellular ATP level can be decreased by ethanol through direct and indirect mechanisms. Alcohol can impair the mitochondrial function by decreasing the production of proteins implicated in ATP synthesis and altering the electron chain transport (Cahill et al., 1999; Xu et al., 2005). Swart and colleagues demonstrated that in the dorsal hippocampus of juvenile rats exposed to ethanol in the early postnatal period,the ATP synthase is down-regulated more than 2-fold (Swart et al., 2018). In our study, chronic ethanol-exposure of immature CGCs was also associated with a reduction of the mitochondrial ATP synthase, isoform 3 gene (Atp5g3) expression, a gene responsible for mitochondrial ATP synthesis during oxidative phosphorylation. Under these circumstances, the down-regulation of Atp5g3 could explain the reduced ATP levels that we measured in the ethanol-treated CGCs. In an attempt to understand whether ATP-synthase dysregulation occurs in an in vivo fetal alcohol syndrome model, we analyzed gene expression data available on a public-domain microarray dataset (GSE9545, GPL1261 using the GEO2R function from Gene Expression Omnibus Database) (Wang, 2008). None of the ATP-synthase subunits were significantly differentially expressed after performing stringent multiple testing correction. However, Atp5s, Atp5o, and Atp5a1 were down-regulated, while Atp5g2 was up-regulated in the ethanol-exposed group. Surprisingly,multiple subunits of ATPases were also down-regulated after ethanol exposure (Atp2, Atp6, Atp8). While the statistical significance of these results is questionable, they also hint towards a disturbed ATP metabolism. Thus, further research should be granted to test whether multiple subunits of ATP synthases, as well as ATPases are dysregulated by ethanol.
Cellular metabolic activity, evaluated by resazurin reducing capacity, was markedly decreased in cultures exposed to ethanol. Resazurin metabolism can take place under the action of mitochondrial, cytosolic, and microsomal enzymes (Gonzalez and Tarloff, 2001). Hence, resazurin reduction is an overall reflection of cellular metabolism and is not specific for electron transport and mitochondrial function only (Rampersad,2012). In aerobic conditions, ATP is formed exclusively in the mitochondria. The higher reduction in the ATP levels compared to the overall metabolic activity, could imply a greater insult on the mitochondrial activity compared to that of other cellular compartments. This is further supported by the marked reduction of the mitochondrial ATP synthase Atp5g3.
In the present study, an OGD-induced insult was shown to aggravate the cellular lesion induced by a chronic intermittent exposure to ethanol in vitro. The present results support a previous study showing that ethanol and OGD have synergistic deleterious effects on immature CGCs (Le Duc et al., 2015) and suggest that the deleterious effects of ethanol are mediated through metabolic impairment and could be aggravated by an OGD episode. While overall metabolic activity is significantly decreased in the EtOH group after OGD exposure, the difference in ATP level between OGD and EtOH groups fails to reach significance. This may be explained by the severe mitochondrial injury after OGD,which alone leads to significant ATP depletion (Almeida et al., 2002). Our findings suggest that the higher metabolic susceptibility to stressors can at least partially be explained by a basal decrease in the ATP level that is associated and could be caused by a down-regulation in Atp5g3 gene expression. This observation has important implications, as the co-occurrence of developmental metabolic stressors, such as ischemia and alcohol exposure may arise more frequently in vivo than previously thought. Therefore, interventions which improve metabolic function or increase ATP production could be useful in limiting ethanol-related insults and should be addressed in future studies.
Figure 1 Ethanol exposure leads to reduced metabolism of cerebellar granule neurons.
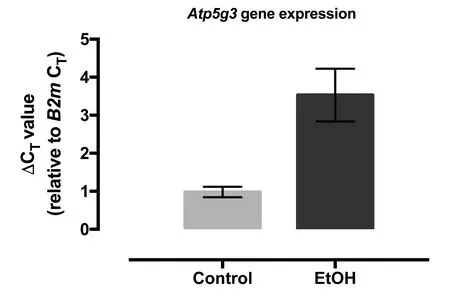
Figure 2 Fold change for Atp5g3 gene expression in cerebellar granular cell cultures.
The present study has several limitations. A single population of neurons was used, thus we cannot infer the impact of different cells' interaction on neuronal viability. The genetic expression analysis was restricted to the Atp5g3 gene and was not done for other subunits of mitochondrial ATP synthase. Likewise, this study did not measure the cellular level of ATP synthase. Future research may focus on examining different aspects of energy metabolism, thus providing more insight into the mechanisms responsible for the energetic failure associated with cell injury after exposure to ethanol and oxygen-glucose deprivation.
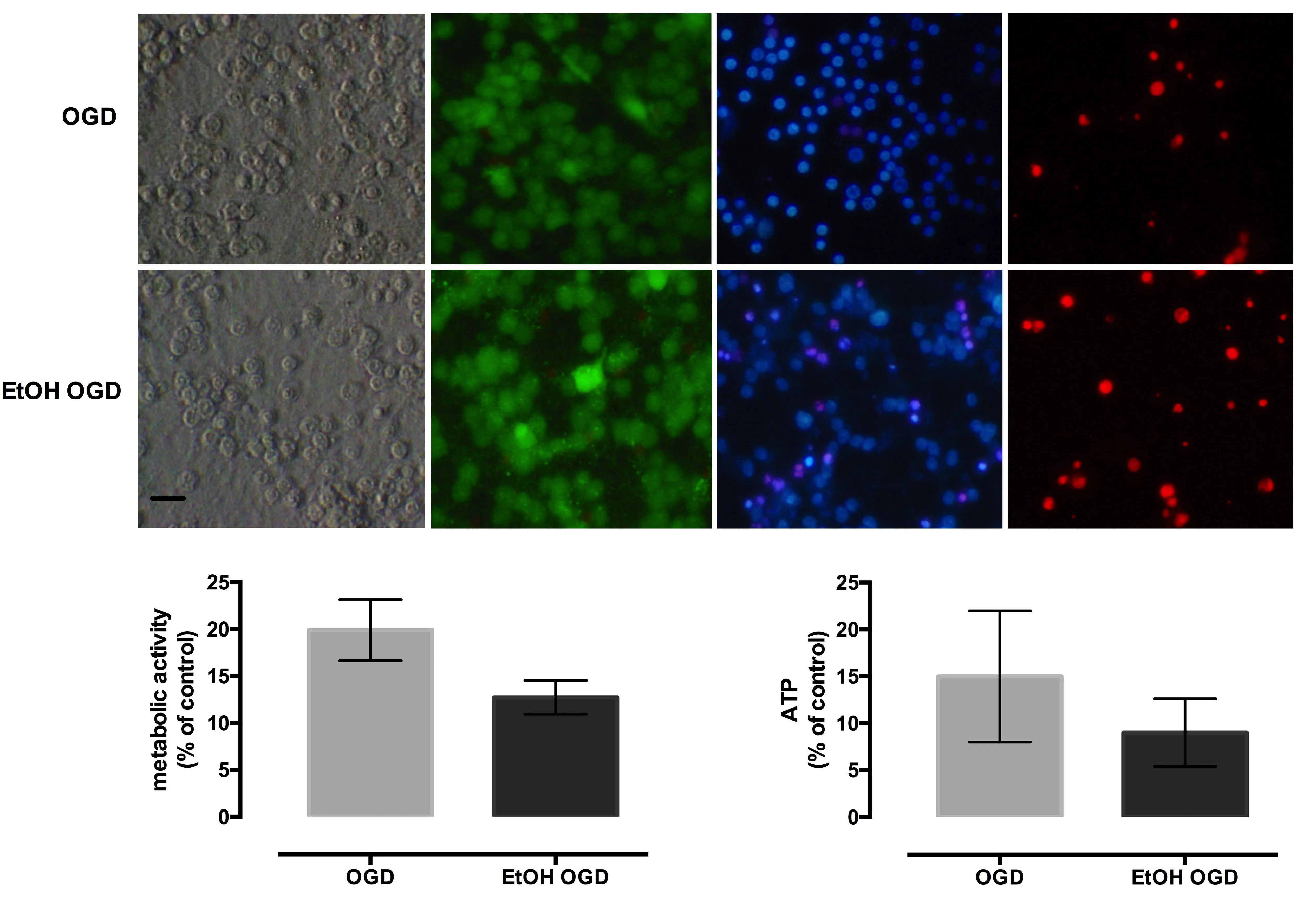
Figure 3 Oxygen-glucose deprivation (OGD)aggravates the ethanol-induced metabolic impairment of cerebellar granule neurons.
In conclusion, our results suggest that chronic ethanol exposure increases vulnerability to oxygen-glucose deprivation and is associated with a marked reduction in cellular ATP,accompanied by a down-regulation in Atp5g3 gene expression. These molecular findings contribute to further understanding the mechanisms of neuronal damage after ethanol exposure. Such an in vitro ethanol exposure model could prove valuable for high-throughput screening toxicology designed to test potential neuroprotective strategies.
Acknowledgments: We are especially thankful to Torsten Schöneberg (Rudolf-Schönheimer-Institut for Biochemistry, Molecular Biochemistry) for helpful comments and suggestions that improved the overall work. We acknowledge support from the German Research Foundation (DFG) and Universität Leipzig within the program of Open Access Publishing.
Author contributions: Study design, experimental procedures, data analysis, and manuscript writing: AS, DLD, and AMZ, manuscript writing: LZ. All authors approved the final manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:The project was supported by a grant of the Romanian National Authority for Scientific Research, project PN-II-PT-PCCA-2011-3, No 80/2012.The funder had no involvement in the study design; data collection, analysis, and interpretation; paper writing; or decision to submit the paper for publication.
Institutional review board statement:All animal procedures were carried out with the approval of the local ethics committee for animal research from Carol Davila University (approval from April 2015), in accordance with the European Communities Council Directive 86/609/EEC on the protection of animals used for scientific purposes.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Ryan Hirschi, University of Utah School of Medicine, USA.
Additional file: Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- The role of Epstein-Barr virus in multiple sclerosis:from molecular pathophysiology to in vivo imaging
- The metabolome identity: basis for discovery of biomarkers in neurodegeneration
- Neuroinflammation as a target for glaucoma therapy
- Basics on the use of acid-sensing ion channels'inhibitors as therapeutics
- Role of axon resealing in retrograde neuronal death and regeneration after spinal cord injury
- Rehabilitation following spinal cord injury: how animal models can help our understanding of exerciseinduced neuroplasticity
