Neuroprotective effect of Notch pathway inhibitor DAPT against focal cerebral ischemia/reperfusion 3 hours before model establishment
2019-02-13JunJieWangJunDeZhuXianHuZhangTingTingLongGuoGeYanYu
Jun-Jie Wang, Jun-De Zhu, Xian-Hu Zhang, Ting-Ting Long, Guo Ge, Yan Yu
Department of Anatomy, School of Basic Medicine, Guizhou Medical University, Guian New District, Guizhou Province, China
Abstract As an inhibitor of the Notch signaling pathway, N-[N-(3,5-difluorohenacetyl)-l-alanyl]-S-phenylglycine tert-butyl ester (DAPT) may protect brain tissue from serious ischemic injury. This study aimed to explore neuroprotection by DAPT after cerebral ischemia/reperfusion (I/R)injury. DAPT was intraperitoneally injected 3 hours before the establishment of a focal cerebral I/R model in the right middle cerebral artery of obstructed mice. Longa scores were used to assess neurological changes of mice. Nissl staining and TdT-mediated dUTP-biotin nick-end labeling staining were used to examine neuronal damage and cell apoptosis in the right prefrontal cortex, while immunofluorescence staining was used to detect glial fibrillary acidic protein- and Notch1-positive cells. Protein expression levels of Hes1 and Hes5 were detected by western blot assay in the right prefrontal cortex. Our results demonstrated that DAPT significantly improved neurobehavioral scores and relieved neuronal morphological damage. DAPT decreased the number of glial fibrillary acidic protein- and Notch1-positive cells in the right prefrontal cortex,while also reducing the number of apoptotic cells and decreasing interleukin-6 and tumor necrosis factor-α contents, and simultaneously downregulating Hes1 and Hes5 protein expression. These findings verify that DAPT alleviates pathological lesions and strengthens the anti-inflammatory response after cerebral I/R injury. Thus, DAPT might be developed as an effective drug for the prevention of cerebral I/R injury.
Key Words: nerve regeneration; DAPT; cerebral ischemia; glial fibrillary acidic protein; Notch1; Hes1; Hes5; neural regeneration
Introduction
Stroke is a major cause of death, adult chronic disability,and dementia. Ischemic stroke accounts for approximately 80% of stroke cases, which may be caused by hypertension,dyslipidemia, lifestyle, and/or genetic factors (Zhao et al.,2017). Many clinical methods, such as anticoagulation and thrombolysis treatment, have been used to treat cerebrovascular diseases; however, vascular recanalization could aggravate brain tissue damage and cause delayed nerve cell death,thus eliciting cerebral ischemia/reperfusion (I/R) injury(Certo et al., 2015; Sadana et al., 2015; Hartig et al., 2017).Despite widespread research, there is no effective treatment for cerebral I/R injury so far. Therefore, understanding its molecular mechanisms may provide novel insights into the development of effective treatments for cerebral I/R injury.
The Notch pathway, an evolutionarily conserved signaling system, has been recognized as an important adaptive signaling pathway that participates in various biological processes,such as neuronal proliferation, differentiation and apoptosis(Nemir and Pedrazzini, 2008; Wickremasinghe et al., 2011).Previous studies have shown that the classical biological effects of Notch signaling are mediated through interactions between Notch receptors and their ligands to release the Notch intracellular domain, which then forms a transcription-activating complex to regulate Notch-dependent gene expression,such as Hes1 and Hes5 (Mahler et al., 2010; Osathanon et al.,2013; Zhang et al., 2015). N-[N-(3,5-difluorohenacetyl)-l-alanyl]-S-phenylglycine tert-butyl ester (DAPT), a common γ-secretase inhibitor, can effectively inhibit release of the Notch intracellular domain from the Notch receptor during the enzymatic digestion process (Lee et al., 2017).
Inflammatory responses and apoptosis are crucial mechanisms involved in cerebral I/R injury (Broughton et al., 2009;Siniscalchi et al., 2014). Recently, several studies found a significant reduction of blood flow in the ischemic core, whereby programmed cell apoptosis and inflammatory responses occurred in the ischemic penumbra (Xu and Zhang, 2011;Ghosh et al., 2012; Kalogeris et al., 2012; Hu et al., 2017).Moreover, DAPT could protect against renal I/R injury by suppressing inflammation and apoptosis (Huang et al., 2011).However, no direct evidence has indicated involvement of the Notch pathway in the protective effects of glial fibrillary acidic protein (GFAP) and/or Hes1 and Hes5 protein expression on cerebral I/R injury. Therefore, this study aimed to investigate the role of DAPT and its putative mechanism in mice with cerebral I/R injury to provide a new strategy for the prevention and treatment of cerebrovascular disease.
Materials and Methods
Animals
In total, 204 healthy male Kunming mice, aged 3-4 months and weighing 30-35 g, were purchased from the Experimental Animal Center of Guizhou Medical University, China[License No. SYXK(Qian) 2012-0004]. All mice were housed at 50-70% humidity and 22-24°C under a 12-hour light/dark cycle. All experiments were conducted in accordance with the guidelines of the Ministry of Health of China for Animal Care and Use. The study protocol was approved by the Experimental Animal Research Committee of Guizhou Medical University, China. Food and water were freely available during all phases of the experiment. Mice were randomly divided into a sham group (n = 68), I/R group (I/R only) (n = 68) and DAPT group (I/R + DAPT treatment) (n = 68). Each group was divided into four subgroups: 1, 3, 7 and 14 days.
Mouse models of focal cerebral ischemia/reperfusion injury
Mice were acclimated to the facility for one week prior to surgery. I/R mouse models were established as previously described by Longa et al. (1989). In brief, mice were intraperitoneally anesthetized with 5 mL/kg of 10% (w/v) chloral hydrate and secured on the operating table. Through a midline cervical incision, the bilateral common carotid arteries were exposed and gently separated from the carotid sheath and vagus nerve. The right common carotid artery, internal carotid artery, and external carotid artery were exposed and isolated. A microvascular clamp (Roboz, Berlin, Germany)was used to temporarily clip the internal carotid artery. A small cut was made at the common carotid artery from the bifurcation 3-4 mm. A 2.0-cm nylon suture (diameter 0.16 ±0.02 mm) with its tip rounded by heating into the internal carotid artery was made until it closed the origin of the middle cerebral artery. Sham group mice underwent identical surgery, but did not have the suture inserted. The body temperature of mice was maintained at 37°C through all surgical and postoperative procedures until mice regained consciousness.After recovery from anesthesia, all mice were kept in the animal quarters with free access to food and water.
Drug intervention
DAPT solution (20 mg/mL; MCE Co., Monmouth Junction,NJ) was prepared by dissolving DAPT powder in dimethyl sulfoxide. In the DAPT group, DAPT solution (5 mL/kg)was injected intraperitoneally 3 hours before middle cerebral artery occlusion. Mice in the other two groups were injected with 5 mL/kg dimethyl sulfoxide solution (Wang et al., 2015).
Neurobehavioral assessment
According to the Longa scoring method (1989), neurobehavioral deficits were evaluated by an observer blinded to the identity of groups at 2 hours before sampling and rated on a scale of 0-4. Score 0: No obvious behavioral deficit. Score 1:Left forelimb showed a mild obvious neurobehavioral deficit and failed to straighten. Score 2: Mice showed a trend of circling to the left. Score 3: Mice spontaneously walked in a leftcircle. Score 4: Mice could not walk spontaneously and lost consciousness. Mice scored 1-3 were included in this study,but mice not showing behavioral deficits at the four time points were excluded from the study.
Preparation of paraffiin sections
In each subgroup, mice (n = 5) were anesthetized with 5%(8 mL/kg) chloral hydrate and perfused with physiological saline via an aortic root catheter until the liver appeared to be white, followed by 4% paraformaldehyde solution that had been cooled to 4°C. Brains were removed and post-fixed in 4% paraformaldehyde solution overnight at 4°C. After dehydration and embedding in paraffiin, 7-μm coronal sections of brain were used for immunofluorescence, Nissl, and TdT-mediated dUTP-biotin nick-end labeling (TUNEL) staining.
Nissl and TUNEL staining of the right prefrontal cortex
Paraffin sections were stained with Nissl (Solarbio Biotechnology, Beijing, China) and imaged using a light microscope(Olympus, Tokyo, Japan) at 400× magnification. Apoptotic cells in brain sections were identified by the TUNEL staining kit (KeyGen BioTech, Nanjing, China) as previously described(Wicha et al., 2017). Paraffin sections were deparaffinized,treated with 3% hydrogen peroxide and TdT enzyme, and incubated with digoxigenin-conjugated antibodies. Sections were photographed using a light microscope (Olympus). Nissl- and TUNEL-positive cells were counted using Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA).
Immunofluorescence staining
To detect Notch1- and GFAP-positive cells in the right cerebral cortex of the ischemic hemisphere, brain sections were incubated for 30 minutes in 2.0 M HCl to denature DNA, and the reaction was neutralized in 0.1 M boric acid for 10 minutes.Thereafter, brain sections were rinsed in phosphate-buffered saline (PBS) containing 0.3% Triton for 30 minutes, preincubated in 10% normal goat serum for 2 hours at room temperature, and incubated with polyclonal rabbit anti-Notch1 (1:150;Biosynthesis Biotechnology, Beijing, China) and monoclonal rabbit anti-GFAP (astrocyte marker) antibody (1:200; Boster Biotechnology, Wuhan, China) at 4°C overnight, and then incubated with Cy3-conjugated affiinity-purified goat anti-rabbit IgG (1:100; Sigma, St. Louis, MO, USA) in a humidified chamber for 1 hour at 37°C. Anti-Notch1 and anti-GFAP were used as cell-type specific markers in each brain section. Numbers of Notch1- and GFAP-positive cells were counted by laser-scanning confocal microscopy (Olympus).
Western blot assay
Mice (n = 5) from each subgroup were anesthetized with 5%(8 mL/kg) chloral hydrate, and perfused with physiological saline rapidly via an aortic root catheter. Brains were removed quickly and frozen at -80°C. Brain tissue from the right cerebral cortex was homogenized by adding protein extraction buffer at a 1:5 volume to tissue weight in a glass homogenizer. Sample protein concentrations were determined using a bicinchoninic acid assay kit (Beyotime Biotechnology,Shanghai, China). Hes1 and Hes5 proteins were loaded onto 5% stacking/10% separating sodium dodecyl sulphate-polyacrylamide gels (Solarbio Biotechnology, Beijing, China) for electrophoresis, and then transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). After blocking with 5% skimmed milk powder, membranes were incubated with rabbit polyclonal anti-Hes1, anti-Hes5 (1:200;Biosynthesis Biotechnology), and rabbit polyclonal anti-β-actin (1:4000; ImmunoWay, Plano, TX, USA) overnight at 4°C.After washing with PBS for approximately 15 minutes, membranes were incubated with horseradish peroxidase-conjugated secondary goat-anti-rabbit antibody (1:6000; Proteintech,Chicago, IL, USA) for 2 hours at room temperature. Proteins were visualized using a sensitivity-enhanced chemiluminescence solution (Millipore). Finally, the intensity of blots was quantified using a Multi-Functional Imaging System (Bio-Rad, Hercules, CA, USA). Relative amounts of proteins were calculated as the ratio of Hes1 or Hes5 to β-actin.
Transmission electron microscopy
Transmission electron microscopy was used to evaluate neuronal ultrastructural changes within the right prefrontal cortex in each subgroup (n = 2). Brain tissues from the right prefrontal cortex were cut into 1 mm3sections and immediately fixed in 2.5% glutaraldehyde solution at 4°C overnight.After washing with 0.1 M PBS (pH 7.4), sections were fixed with 1% osmic acid (Pelco, CA, USA) for approximately 1 hour. Following dehydration in ethyl alcohol, brain tissues were embedded in Epon-Araldite resin (EMS, San Francisco, CA, USA), cut into ultrathin sections (approximately 50 nm), examined with transmission electron microscopy (Hitachi-7500, Tokyo, Japan), and imaged.
Enzyme-linked immunosorbent assay
Remnant mice from each group were sacrificed at different time points. The right prefrontal cortex was separated and cleared by cold saline to prepare 10% fresh brain tissue homogenates. According to the manufacturer's protocol (Neo Bioscience, Beijing, China), amounts of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) released in the right prefrontal cortex were measured by enzyme-linked immunosorbent assay.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). All data were analyzed using SPSS 19.0 software (SPSS, Chicago,IL, USA) by an observer blinded to experimental groupings.A one-way analysis of variance followed by Student-Newman-Keuls test was used to assess statistical significance. A value of P < 0.05 was considered statistically significant.
Results
DAPT improves neurobehavioral deficits of cerebral I/R mice
The results of neurobehavioral scores are shown in Figure 1. In the sham group, there was no obvious neurologic dysfunction in mice. Cerebral I/R mice showed more severe neurological function injury compared with the sham group(P < 0.01). After DAPT treatment, neurobehavioral deficits were significantly improved (P < 0.05).
DAPT alleviates pathological injury of neurons
The sham group showed normal neural structures with clear cell outlines, central nuclei, and abundant Nissl bodies in the cytoplasm (Figure 2A and B). In contrast, neural cells of the I/R group showed obvious injury, with shrunken cell bodies,pyknosis, and breaking and dissolution of nuclei. In the 1-day
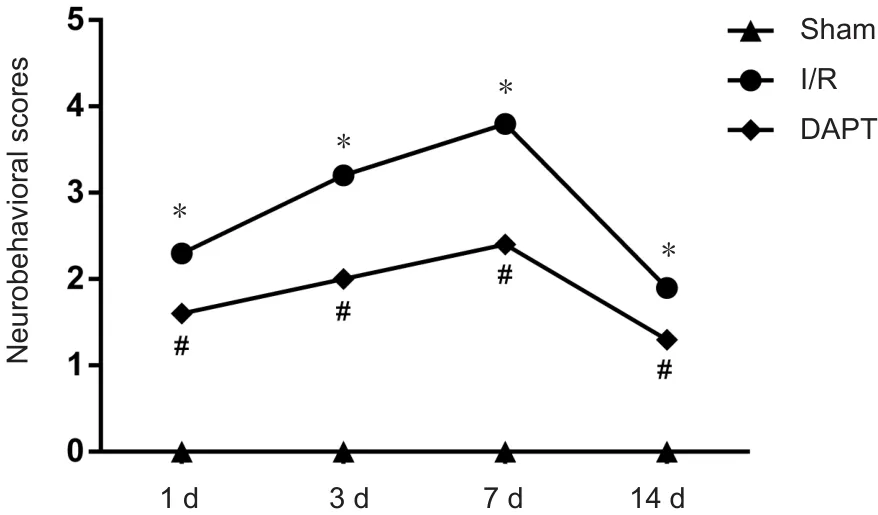
Figure 1 Neurobehavioral scores of cerebral I/R mice following DAPT treatment.
I/R subgroup, neurons presented slight injury. In the 3-day I/R subgroup, neurons were disorganized and nuclear pyknosis occurred. Neurons exhibited the worst damage (Nissl bodies dissolved or disappeared) in the 7-day I/R subgroup.Cellular kernel pyknosis displayed as triangular-shaped cells.The clearance surrounding between neural cells was widened(Figure 2D). Numbers of neurons in the 14-day I/R subgroup increased and the damage was slightly relieved compared with the 7-day I/R subgroup. However, compared with the I/R subgroup, DAPT could alleviate neuronal damage in the DAPT group at different time points (Figure 2I and K).
DAPT decreases cell apoptosis
In the sham group, no obvious TUNEL-positive cells were observed in the right prefrontal cortex of brain tissues (Figure 3). Compared with the sham group, a few TUNEL-positive cells appeared in cerebral I/R mice (P < 0.01; Figure 3J).Quantitative analyses (Figure 3J) demonstrated significantly less TUNEL-positive neurons in the DAPT group than in the I/R group (P < 0.05).
DAPT decreases GFAP-positive cells
In the sham group, a few GFAP-positive cells appeared(Figure 4A). In the 1-day I/R subgroup, GFAP-positive cells began to increase compared with the sham group (Figure 4B). In the 3-day I/R subgroup, GFAP-positive cells exhibited star shapes and were also significantly enhanced compared with the sham group, but GFAP-positive cells were significantly decreased in the DAPT group (P < 0.01)(Figure 4C and J). In addition, among the four subgroups of the I/R group, GFAP-positive cells reached a peak at 7 days, and showed thickened and shortened processes (Figure 4D). Compared with the 14-day I/R subgroup, numbers of GFAP-positive cells decreased and cell staining became shallower in the DAPT group (P < 0.01; Figure 4E and J).These results indicated that cerebral I/R injury increased the expression of GFAP-positive cells and induced activation of gliosis. However, DAPT treatment evidently suppressed the activation of astrocytes with small cell bodies and slender processes at four time points (Figure 4F-J).
DAPT reduces Notch1-positive cells
Notch1-positive signal was concentrated in the cytoplasm,while nuclei were dyed blue by 4′,6-diamidino-2-phenylindole (DAPI). As shown in Figure 5A, a few Notch1-positive cells were observed in the sham group. Compared with the sham group, more Notch1-positive cells appeared in the cerebral cortex of the I/R group at each time point (P < 0.01;Figure 5B-J). After DAPT treatment, Notch1-positive cells were decreased compared with the I/R group at different time points (P < 0.05; Figure 5F-J). Among the four DAPT treatment subgroups, numbers of Notch1-positive cells remained highest at 7 days.
DAPT inhibits expression of Hes1 and Hes5
Hes1 and Hes5 protein expression levels in the right prefrontal cortex were measured by western blot assay, as shown in Figure 6. Compared with the sham group, protein expression of Hes1 and Hes5 was increased in the I/R group(P < 0.01). However, the two proteins were remarkably decreased in the DAPT group compared with the I/R group at different time points (P < 0.05).
DAPT alleviates ultrastructural damage of neurons
In the sham group, neurons exhibited abundant organelles in the cytoplasm (Figure 7A). However, neurons in the prefrontal cortex of the I/R group showed varying degrees of damage at different time points (Figure 7B-E). In the 1-day I/R subgroup, organelles were disordered, intracellular spaces became large, and the mitochondria swelled slightly(Figure 7B). In the 3-day I/R subgroup, nuclei became pyknotic and mitochondria were markedly swelled (Figure 7C).In the 7-day I/R subgroup, neuronal ultrastructural damage was most severe with incomplete nuclear envelopes and dissolved organelles (Figure 7D). In the 14-day I/R subgroup,the cristae of some mitochondria were fractured and the number of organelles was increased compared with the 7-day subgroup (Figure 7E). Results shown in Figure 7F-I revealed that DAPT treatment alleviated neuronal ultrastructural damage in mice after middle cerebral artery occlusion.
DAPT inhibits production of IL-6 and TNF-α
As shown in Figure 8, compared with the sham group, IL-6 and TNF-α contents were significantly increased in each I/R subgroup (P < 0.01). Indeed, contents of IL-6 and TNF-α in the I/R group became gradually elevated within the first 7 days and decreased from 7 to 14 days. However, IL-6 and TNF-α contents were significantly inhibited following DAPT treatment (P < 0.05).
Discussion
Stroke is a common cerebrovascular disorder in the aging population and constitutes the second highest principle cause of mortality (Guan et al., 2017). Ischemic stroke involves a complex pathophysiological process, appearing as calcium overload, inflammatory response, oxidative stress,and brain edema (Lee et al., 2017; Tamaki et al., 2017). Despite use of effective endovascular therapy, clinical outcomes after acute cerebral ischemic stroke remain poor (Goyal et al., 2016). As the result of severe neurological complications caused by stroke, neuroprotection in this disorder has recently become an increasingly hot topic (Zhu et al., 2012).Studies have found that the infarct area is formed by longterm ischemia, which is first focused within the ischemic center and then involves surrounding tissue, leading to an ischemic penumbra (Huang et al., 2015; Zhu et al., 2017).Restoring the perfusion of ischemic penumbra is a common clinical treatment to maintain the structural and functional integrity of neurons by inhibiting the inflammatory response(Heiss et al., 1997; Song and Yu, 2014; Liu et al., 2017).
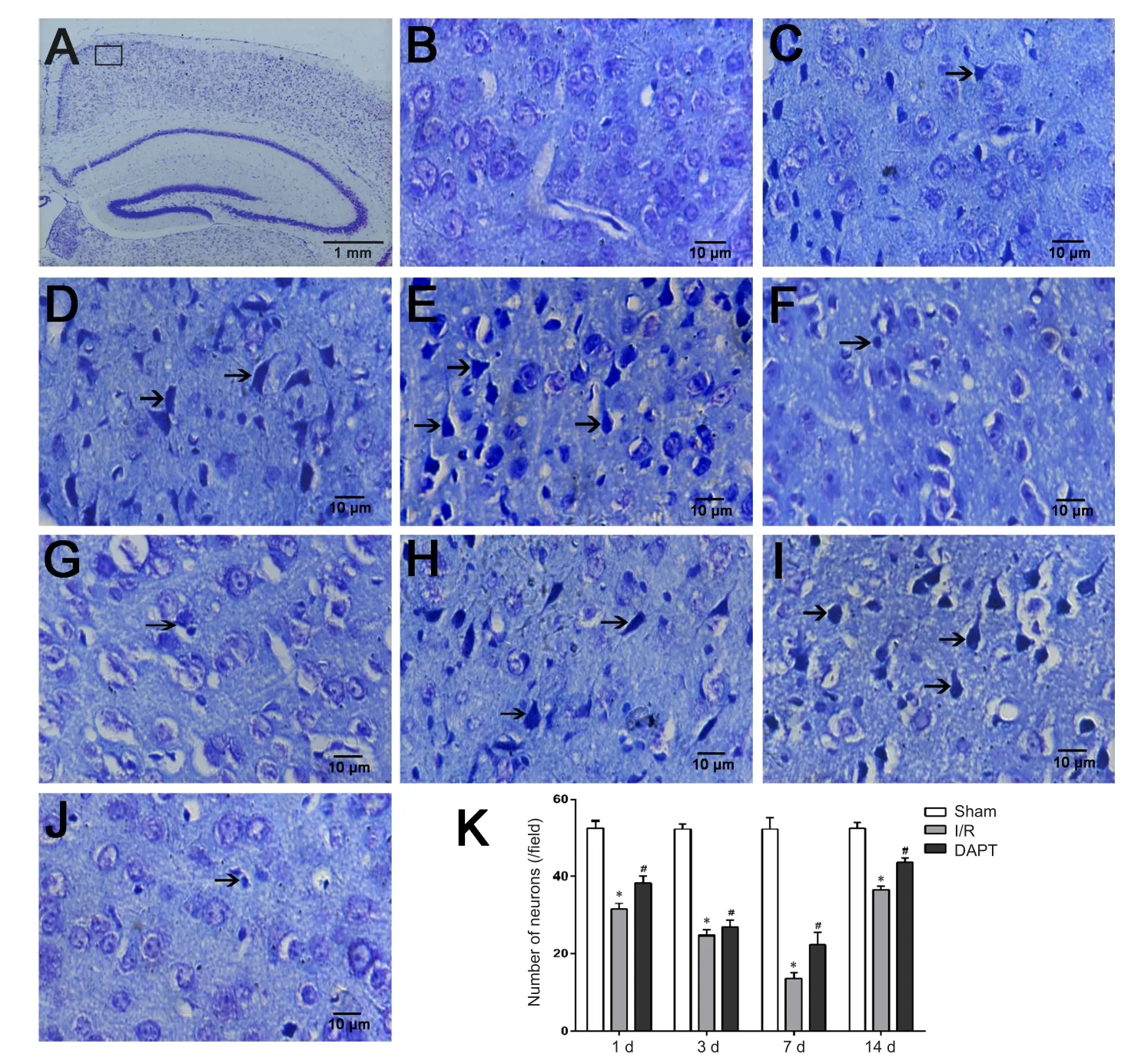
Figure 2 Neurons of the right prefrontal cortex in cerebral I/R mice following DAPT treatment as assessed by optical microscopy.
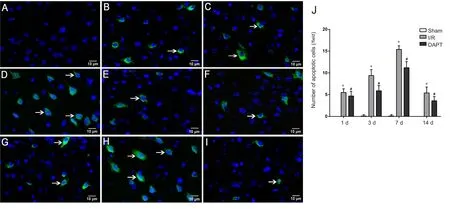
Figure 3 Cell apoptosis in the right prefrontal cortex area in cerebral I/R mice following DAPT treatment, as assessed by fluorescence microscopy.
Many studies have shown that middle cerebral artery infarction has become the main cause of ischemia in the clinic(Wijdicks et al., 2014; Chen et al., 2018). The focal cerebral ischemia model of middle cerebral artery occlusion could simulate pathological processes of ischemic cerebrovascular disease and cause mice to show symptoms similar to humans.Previous studies demonstrated a strong association between the hippocampus and prefrontal cortex and age-related cognitive decline (Paulesu et al., 1993; Levy and Goldman-Rakic,1999). Ischemic stroke is a serious disease and threat to the cognitive abilities of people. Thus, in our present study, the right prefrontal cortex was chosen to investigate the function of neural cells.
Nissl staining is a commonly used histological method to access morphological changes of cells (Shen et al., 2014). From the results of our light microscopy and transmission electron microscopy, neural structure was normal in the sham group,with abundant organelles exhibiting clear structures. In the I/R group, the distribution and arrangement of neural cells was irregular and mitochondria presented swelling, vacuolization,and even dissolution. Apoptosis is one of the major pathogenic mechanisms underlying cerebral I/R injury (Broughton et al., 2009). Blocking neuronal apoptosis could prevent the loss of neuronal cells, minimize brain injury induced by I/R damage, and slow the onset of cerebral ischemia and necrosis(Xie et al., 2011). In the present study, I/R surgery induced increases of TUNEL-positive cells in the right prefrontal cortex.Consistent with previous studies, cerebral I/R injury could cause severe brain damage, increased apoptosis, and destroy normal structure (Deng et al., 2015; Tang et al., 2017).
Proinflammatory cytokines, such as TNF-α, IL-6 and IL-1α, have emerged as significant contributors to myocardial and cerebrovascular dysfunction (Sun et al., 2012; Kraft et al., 2017). Among several inflammatory factors, TNF-α appears to be the major pro-inflammatory cytokine with multiple mechanisms that promote inflammatory reactions (Di Paola et al., 2013). IL-6, which is produced by monocytes,macrophages and endothelial cells, is a multi-functional single chain glycoprotein cytokine with broad biological activity (Aloisi et al., 1992; Choi et al., 2017). It exhibits a rapid increase during the early stage of cerebral I/R injury,along with TNF-α and IL-1β (Frangogiannis, 2007; Wong and Crack, 2008). In this study, IL-6 and TNF-α expression in the I/R group increased significantly, in line with the results of previous studies (Baskaya et al., 2000; Shichita et al.,2012; Sanderson et al., 2013). Furthermore, when cerebral I/R injury occurred, release of IL-1β, TNF-α, IL-6 and other inflammatory factors increased the inflammation reaction,leading to irreversible neuronal damage and even apoptosis or necrosis (Siniscalchi et al., 2014).
The Notch pathway is known to regulate homeostasis and development in diverse organs, such as lung (Xu et al., 2012),liver (Kobayashi et al., 2002; Zhang et al., 2017), kidney (Barak et al., 2012) and heart (Yu et al., 2015; Luxan et al., 2016).Moreover, the Notch pathway plays a vital role in immune homeostasis, which exploits the ability to mediate intercellular communication (Nistri et al., 2017). In mammalian cells,four distinct Notch receptors (Notch1-4) and five Notch ligands have been identified (Guruharsha et al., 2012; Zhang et al., 2015; Lu et al., 2018). DAPT, a γ-secretase inhibitor, is regarded as a Notch signaling pathway inhibitor (Cao et al.,2015). In recent years, DAPT has been widely used in the study of Alzheimer's disease, atherosclerosis, and neuroblastoma (Espinoza and Miele, 2013; Qin et al., 2016). DAPT could inhibit IL-6 secretion and restrain the activation of inflammatory responses during rheumatoid arthritis (Jiao et al., 2012). Similarly, Arumugam et al. (2006) reported that DAPT could reduce neuronal damage in the focal ischemic stroke model by restraining activation of microglial cells.
Hes1 and Hes5 are important downstream target genes in the Notch signaling pathway. Indeed, expression of Hes1 and Hes5 correlates with expression of Notch1, an important component of Notch signaling. Expression levels of Notch1, Hes1, and Hes5 respond well to regulation of the Notch signaling pathway (Yoon and Gaiano, 2005; Du et al., 2013). In our study, we demonstrated that DAPT was an effective inhibitor that could markedly depress expression of Notch1, Hes1, and Hes5. By Nissl staining and transmission electron microscopy, DAPT treatment could increase the number of intact neurons, and extenuate cellular edema and mitochondrial swelling. In the DAPT group, numbers of apoptotic cells and contents of IL-6 and TNF-α were obviously reduced. Accordingly, DAPT may have a positive effect on protecting brain tissue by inhibiting the inflammatory response and decreasing apoptosis.
Astrocytes are the most abundant glial cells in the brain and GFAP is considered one of the major marker proteins in mature astrocytes (Gomes et al., 1999; Middeldorp and Hol, 2011). In addition, astrocytes play a vital role in providing general structural, metabolic, and trophic support to neurons in brain tissue (Kamphuis et al., 2012). It was found that when stroke occurred, astrocytes proliferated and became activated; that is, the cell soma enlarged and processes became shortened and thickened (Hol and Pekny, 2015;Minhas et al., 2017). Astrocytes could promote the recovery of nerve function by isolating the infarct, reconstructing the blood-brain barrier, and repairing damaged neurons(Kimelberg and Nedergaard, 2010; Sofroniew and Vinters,2010). In addition, GFAP expression was suppressed by DAPT treatment in neonatal rats following hypoxic-ischemic brain injury (Xu and Zhang, 2011). Hence, increasing evidence suggests an important role of astrocytes in the nervous system. Our results showed that compared with the I/R group, expression of GFAP decreased after DAPT treatment.DAPT could inhibit gliosis and further improve cerebral ischemia.
In conclusion, DAPT ameliorates neuronal structural damage of the right prefrontal cortex and improves neural function. DAPT may activate mechanisms of anti-inflammation and anti-apoptosis by inhibiting the expression of GFAP. Our findings offer further theoretical evidence for DAPT as a potential therapeutic drug for cerebral I/R injury. However, the effects of DAPT on other mechanisms of cerebral I/R injury, such as reactive oxygen species and leukocyte infiltration, still require further investigation in our future studies.
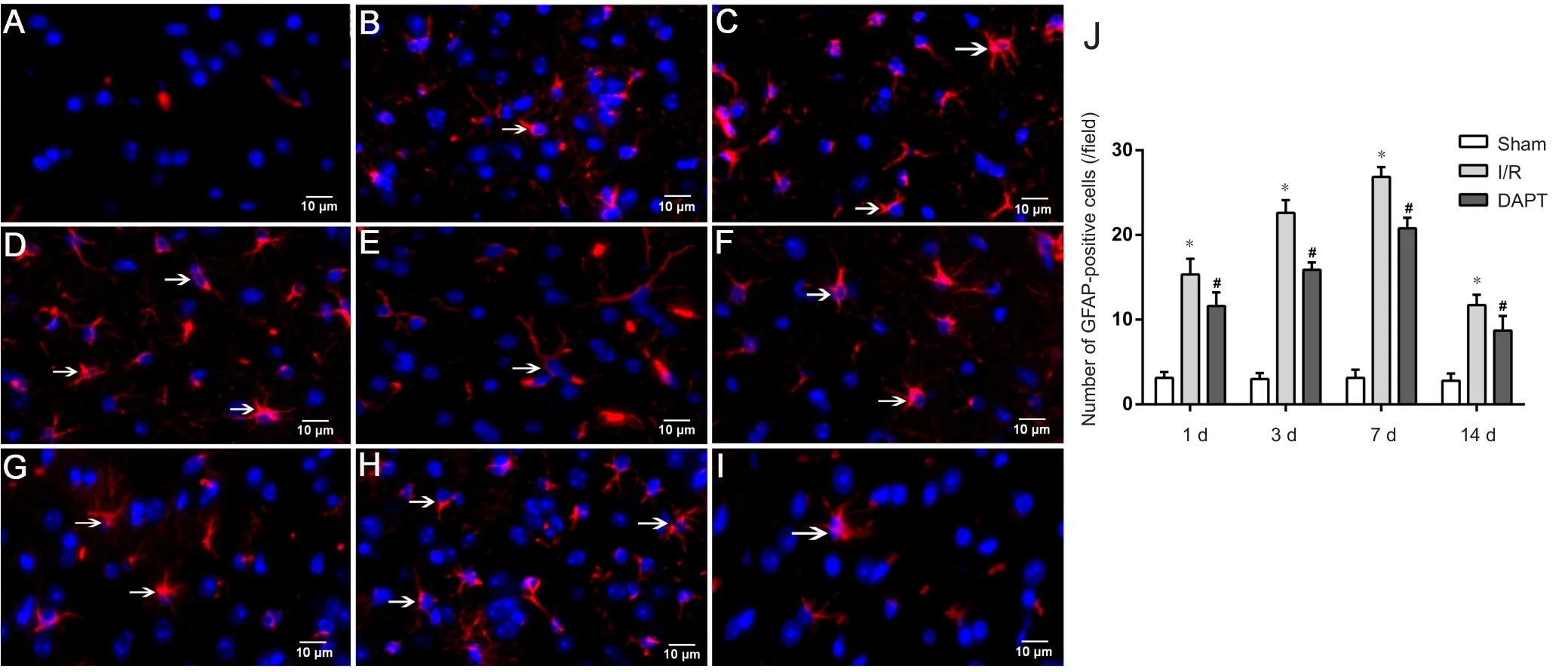
Figure 4 Immunofluorescence microscopy of GFAP in the right prefrontal cortex of cerebral I/R mice following DAPT treatment.
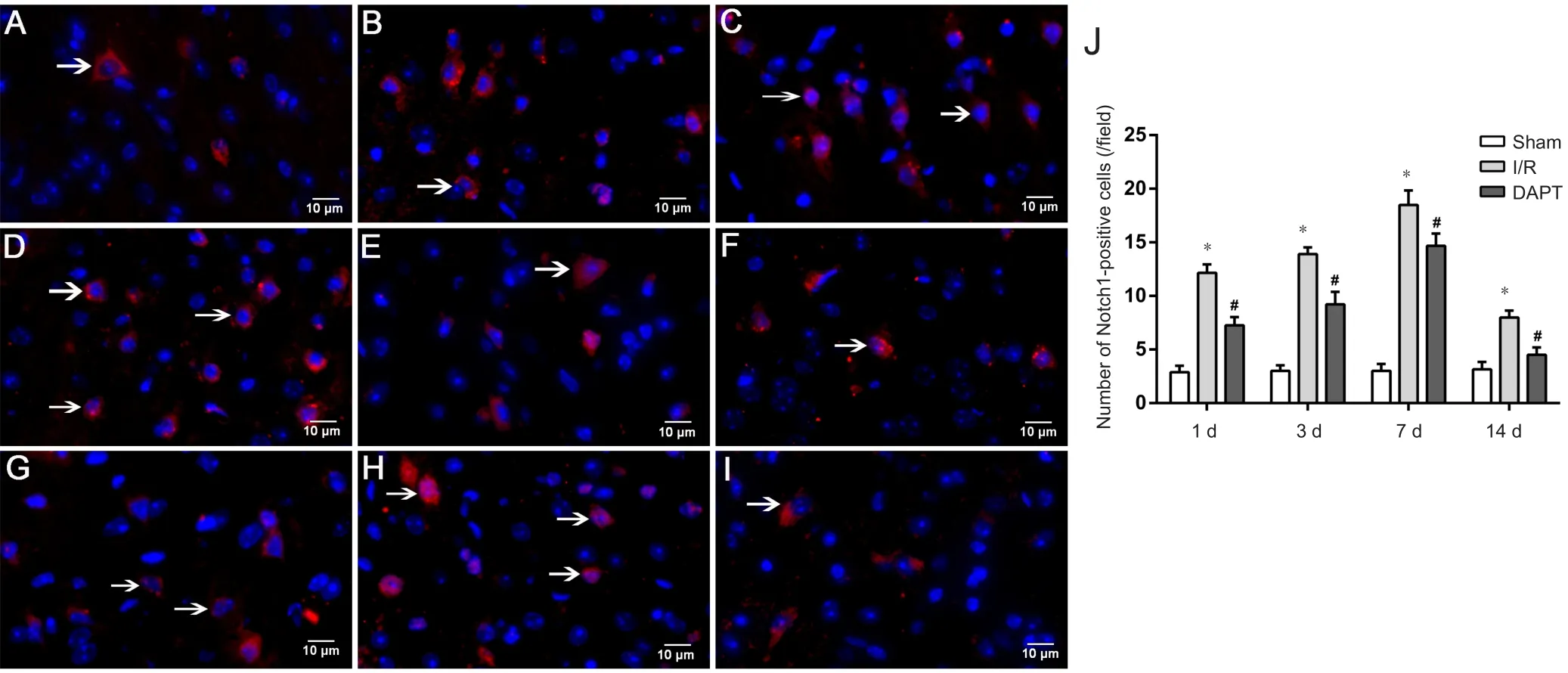
Figure 5 Immunofluorescence microscopy of Notch1 in the prefrontal cortex area of cerebral I/R mice following DAPT treatment.

Figure 6 Western blot assay for Hes1 (A) and Hes5 (B) protein expression in the right prefrontal cortex of cerebral I/R mice following DAPT treatment.

Figure 7 Ultrastructure of neurons of the right prefrontal cortex in cerebral I/R mice following DAPT treatment under a transmission electron microscope.

Figure 8 Contents of TNF-α (A) and IL-6 (B) in the prefrontal cortex in cerebral I/R mice following DAPT treatment (enzyme linked immunosorbent assay).
Author contributions:Concept and design of the study: JJW and JDZ;experiment implementation: JDZ, JJW, XHZ, YY, and TTL; data analysis: GG; provided reagents/materials/analysis tools: JJW and XHZ; paper writting: JJW. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that no conflict of interest exists.
Financial support:This study was supported by the National Natural Science Foundation of China, No. 81660243 (to JDZ); a grant from the Social Development Science and Technology Plan Project of Science and Technology Department of Guizhou Province of China, No. SY [2015] 3041(to JDZ); a grant from the Science and Technology Department of Guizhou Province of China, No. LG [2012] 028 (to JDZ). The funders did not participate in data collection and analysis, article writing or submission.
Institutional review board statement:The study protocol was approved by the Experimental Animal Research Committee of Guizhou Medical University of China. The experimental procedure followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals(NIH Publications No. 8023, revised 1985).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer: Maxime Gauberti, GIP Cyceron, Bd Henri Becquerel, France.
Additional file: Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- The role of Epstein-Barr virus in multiple sclerosis:from molecular pathophysiology to in vivo imaging
- The metabolome identity: basis for discovery of biomarkers in neurodegeneration
- Neuroinflammation as a target for glaucoma therapy
- Basics on the use of acid-sensing ion channels'inhibitors as therapeutics
- Role of axon resealing in retrograde neuronal death and regeneration after spinal cord injury
- Rehabilitation following spinal cord injury: how animal models can help our understanding of exerciseinduced neuroplasticity
