Antibiotic resistance of Neisseria species in Iran: A systematic review and meta-analysis
2019-01-30FarzadKhademiAmirhosseinSahebkar
Farzad Khademi, Amirhossein Sahebkar
1Department of Microbiology, School of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran
2Biotechnology Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, Mashhad, Iran
3Neurogenic Inflammation Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
4School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran
Keywords:Antibiotic Neisseria Resistance Meta-analysis Iran
ABSTRACT Objective: To estimate the prevalence of antibiotic resistance of Neisseria species in Iran.Methods: A systematic and electronic search using relevant keywords in major national and international databases was performed until 6th July, 2018 in order to find studies reporting the prevalence of antibiotic resistance of Neisseria species in Iran. Results: A total of nine studies were found to be eligible based on prede fined inclusion and exclusion criteria. Our analysis indicated that the prevalence of Neisseria gonorrhoeae resistance to different antibiotics was as follows: 66.9% to penicillin, 59.1% to cipro floxacin, 11.1% to ceftriaxone, 21.6% to spectinomycin, 13.8% to ce fixime, 82.4% to co-trimoxazole, 52.7% to tetracycline, 29.9% to gentamicin, 87.5% to ampicillin, 11.1% to azithromycin, 2.2% to chloramphenicol, 50.1% to cefepime and 50.0% to vancomycin. Antimicrobial resistance rates of Neisseria meningitidis was as follows: 30.0% to penicillin, 33.3% to amoxicillin, 33.3% to cephalexin, 55.6% to ampicillin and 0.0% to ciprofloxacin, ceftriaxone, cefotaxime, amikacin, co-trimoxazole,gentamicin, kanamycin, chloramphenicol and ceftizoxime. Conclusion: Neisseria gonorrhoeae and Neisseria meningitidis isolates of Iran show resistance to different types of antibiotics.Therefore, care should be exercised for the use of penicillin, cipro floxacin, co-trimoxazole,tetracycline, gentamicin, ampicillin, cefepime and vancomycin for gonococcal infections,and also with respect to the use of penicillin, amoxicillin, ampicillin and cephalexin for meningococcal infections in Iran.
1. Introduction
The genusNeisseriacomprises aerobic, Gram-negative diplococci and obligate human pathogens that share common properties such as being oxidase-positive and catalase-positive[1-3].Neisseria gonorrhoeae(N. gonorrhoeae) andNeisseria meningitidis(N.meningitidis) are two clinically importantNeisseriaspecies in the familyNeisseriaceae[1-3].N. gonorrhoeaeinfects only humans and causes a spectrum of diseases including urogenital infections (urethritis in men and women and cervicitis in women),epididymitis and pelvic inflammatory disease along with serious reproductive health consequences in untreated infections(e.g.infertility in women), conjunctivitis such as ophthalmia neonatorum in neonates during passageviathe birth canal and rarely disseminated gonococcal infection[4]. Gonorrhea is a sexually transmitted infection and the most common local infection caused byN. gonorrhoeaethat is acquired through direct humanto-human contact during sexual activities[4]. Despite the presence of effective antimicrobial treatments, gonorrhea still remains as a major public health issue worldwide due to dwindling of treatment options. Based on the World Health Organization (WHO) reports,approximately 106 million people are infected with gonorrhea as a new infection each year[5]. Owing to the lack of any vaccine againstN. gonorrhoeae, antibiotic therapy is the only way to manage gonococcal infections[6]. However, bacteria have developed resistance to different classes of antimicrobial agents used to treat the disease including penicillins, early-generation cephalosporins,macrolides, tetracyclines, fluoroquinolones and sulphonamides,resulting in increased rates of treatment failure[4,7,8]. Recently,WHO announced a list of bacteria divided into three categories,critical, high and medium, based on urgently need to develop new antibiotics.N. gonorrhoeaeis placed in the high priority categories with increasingly resistance to cephalosporin and fluoroquinolone antibiotics[9]. Hence, it is necessary to update the healthcare systems with accurate information on the antibiotic resistance pro file ofN.gonorrhoeaeisolates to prevent spreading of treatment failure. Similar to gonococcus that infect human mucosal surfaces of the genitourinary tract, meningococcal strains interact with mucosal surfaces of the nasopharynx and cause life-threatening systemic infections such as bacteremia, fulminant septicemia and meningitis[10].Meningococcus acquired by respiratory droplets or throat secretions from carriers and along withHaemophilus influenzatype b (Hib)andStreptococcus pneumonia(pneumococcus) are three important causative agents of bacterial meningitis characterized by stiff neck,sensitivity to light, high fever, headaches, confusion and vomiting as the most common symptoms[11]. The pathogen only infects humans and is responsible for 75 000 deaths within 24 to 48 hours in the world, but bacterial meningitis is curable and the mortality rate can decrease from 100% in untreated patients to less than 10% in those receiving effective antibiotic treatment[2,11,12]. Therefore, this fatal meningococcal disease is listed as a medical emergency and should be treated with effective antibiotics including penicillin, ampicillin,chloramphenicol and ceftriaxone[13]. However, evidences indicate increased prevalence of antibiotic-resistant meningococcal strains that threatens successful antibiotic therapy in a large number of countries[14].
To our knowledge, there has been no previous study to present pooled estimates of antibiotic resistance ofNeisseriaspecies in Iran.The present systematic review and meta-analysis was undertaken to fill this gap.
2. Materials and methods
2.1. Search strategies
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement[15]. Until 6th July, 2018, we searched for all studies addressing the prevalence of antibiotic resistance ofNeisseriaspecies in Iran. The search was performed in major international databases including PubMed, Scopus and Web of Science as well as Iranian databases including Scienti fic Information Database (www.sid.ir) and Magiran (www.magiran.com). Relevant English and Persian medical terms including “drug resistance” OR “antibiotic resistance” AND “Neisseria′′ AND “Iran” were used for searching.After searching reference lists to find any additional study, a complete list of articles were collected in a digital library for further evaluations and selection of eligible articles based on inclusion and exclusion criteria.
2.2. Inclusion and exclusion criteria
The titles, abstracts and full texts of numerous original studies were collected in a digital library and reviewed to determine their eligibility by two independent investigators. Our inclusion criteria were: (1) published cross-sectional studies in English or Persian languages, (2) studies which focused on antibiotic resistance ofNeisseriaspecies and (3) studies in an Iranian population.Additionally, duplicate reports and non-original articles were excluded.
2.3. Data extraction
Author′s name, year of the study, location of the study,Neisseriaspecies, number of isolated strains, methods used for antimicrobial susceptibility testing and antibiotic resistance rates of bacteria to different antibiotics were extracted from the included articles.
2.4. Meta-analysis
Antibiotic resistance rates ofN. gonorrhoeaeandN. meningitidisreported in different studies were calculated and expressed as percentage and 95% confidence intervals (95%CIs). Fixed- or random-effects models were applied to estimate pooled effect size on the basis of heterogeneity. Heterogeneity in study results and publication bias risk were determined byI2statistic and funnel plots, respectively. All statistical analyses were performed by Comprehensive Meta-Analysis software (Biostat, Englewood, NJ).
3. Results
3.1. Characteristics of included studies
Briefly, a total of 27 records involving 17 studies in English and 10 studies in Persian languages were identified following databases search for antibiotic resistance ofNeisseriaspecies in Iran. Six duplicate studies were excluded and 15 studies did not meet our inclusion criteria and were removed after full text review.
An additional 3 studies were added after checking the reference lists of articles and ultimately 9 studies met the inclusion criteria and were included in the meta-analysis. As presented in Table 1, 3 studies from Tehran, 2 studies from Kashan, 1 study from Zahedan and 1 study from Mashhad evaluated the prevalence of antibiotic resistance ofN. gonorrhoeae. Additionally, 2 studies from Tabriz and Hamadan reported antibiotic resistance pro files ofN. meningitidis.In these studies,Neisseriaspecies were collected from various specimens including endocervix, vagina, urethra, cerebrospinal fluid and blood. On the other hand, standard microbiological methods including samples culturing on chocolate agar, sheep blood agar and modi fied Thayer-Martin agar mediums for 48 h at 35 °℃ under microaerophilic condition along with biochemical tests such as gram staining, morphology, oxidase, catalase and carbohydrate utilization tests were used for bacterial identi fication. Figure 1 shows forest plots of the meta-analysis of the prevalence ofN. gonorrhoeaeresistance to cipro floxacin, ceftriaxone, ce fixime and cefepime in Iran. Additionally, as shown in Figure 2, there is some evidence of the publications bias in the study due to asymmetrical distribution of studies.
3.2. Characteristics of N. gonorrhoeae antibiotic resistance
Disk diffusion was the most frequent technique applied to determine antimicrobial susceptibility pattern ofNeisseriaspecies in Iran. The prevalence ofN. gonorrhoeaeresistance to different antibiotics was as follows: 66.9% (95%CI: 52.4-78.8) to penicillin,21.6% (95%CI: 3.1-70.0) to spectinomycin, 82.4% (95%CI: 46.4-96.2) to co-trimoxazole, 52.7% (95%CI: 9.1-92.5) to tetracycline,29.9% (95%CI: 20.7-41.0) to gentamicin, 87.5% (95%CI: 71.1-95.2) to ampicillin, 11.1% (95%CI: 3.2-31.7) to azithromycin, 2.2%(95%CI: 0.4-10.1) to chloramphenicol, and 50.0% (95%CI: 5.9-94.1) to vancomycin (Table 2).
3.3. Are cephalosporin- and fluoroquinolone-resistant N.gonorrhoeae a public health problem in Iran?
Our results in the current study showed thatN. gonorrhoeaeantibiotic resistance rate to the fluoroquinolone cipro floxacin was high (59.1%; 95%CI: 43.9-72.7). On the other hand, resistance rate to cephalosporins including ceftriaxone (11.1%; 95%CI: 2.9-34.4)and ce fixime (13.8%; 95%CI: 8.5-21.6) were low, with cefepime as the only exception (50.1%; 95%CI: 0.7-99.3). Therefore, the use of cipro floxacin and cefepime for treatingN. gonorrhoeaeinfections is not recommended in Iran.

Table 1 Characteristics of studies included in this meta-analysis.
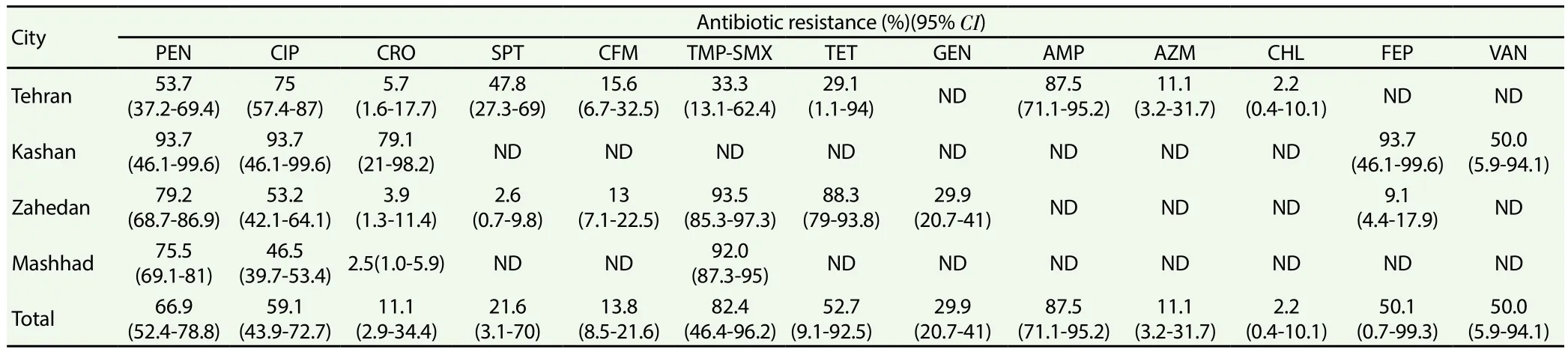
Table 2 Antibiotic susceptibility pro files of N. gonorrhoeae in different cities of Iran.

Table 3 Antibiotic susceptibility pro files of N. meningitidis in different cities of Iran.

Figure 1. Forest plots of the meta-analysis of the prevalence of N.gonorrhoeae resistance to cipro floxacin, ceftriaxone, ce fixime and cefepime in Iran.

Figure 2. Funnel plots of the meta-analysis of the prevalence of N.gonorrhoeae resistance to cipro floxacin and ceftriaxone in Iran.
3.4. Characteristics of N. meningitidis antibiotic resistance
As shown in Table 3, the prevalence ofN. meningitidisresistance to different antibiotics was as follows: 30.0% (95%CI: 10.0-62.4)to penicillin, 33.3% (95%CI: 11.1-66.7) to amoxicillin, 33.3%(95%CI: 11.1-66.7) to cephalexin, 55.6% (95%CI: 25.1-82.3)to ampicillin and 0.0% to ciprofloxacin, ceftriaxone, cefotaxime,amikacin, co-trimoxazole, gentamicin, kanamycin, chloramphenicol and ceftizoxime.
4. Discussion
The emergence of antibiotic-resistantN. gonorrhoeaestrains has led to some problems in the prevention and control of gonococcal infections[6,25]. The spread of resistance to the lastline cephalosporins, penicillins, sulfonamides, tetracyclines,quinolones and macrolides has limited treatment options forN.gonorrhoeaeinfections and can lead to increased morbidity and mortality rates, a conditions resemble the pre-antibiotic era[6,25].Therefore, it is important to obtain accurate information on the nationwide and global antibiotic susceptibility pro file ofNeisseriaspecies to guide the treatment of infected patients. In the present study, antibiotic resistance ofN. gonorrhoeaeandN. meningitidisto β-lactam antibiotics and other antibiotics inhibiting bacterial cell wall synthesis including penicillins, cephalosporins and vancomycin were summerized. According to our results, antibiotic resistance ofN. gonorrhoeaeto penicillins, such as penicillin (66.9%) and ampicillin (87.5%), and vancomycin (50.0%) was high. A review of other studies shows that drug resistance findings varies widely among countries such as Germany (penicillin, 18.8%), Tunisia(penicillin, 55.3%) and Korea (penicillin, 100%)[9,26,27]. Similar resistance rates were observed forN. meningitidisstrains: penicillin(30.0%), ampicillin (55.6%) and amoxicillin (33.3%). The results of other studies are as follows: 30.4% in Tunisia, 0.0% in Scotland, and 40.9% in Belgium[26]. Major mechanisms of antimicrobial resistance ofN. gonorrhoeaeandN. meningitidisto β-lactam antibiotics including (1) TEM-1 type β-lactamase-encoding plasmids and(2) chromosomally mediated resistance by the modification ofponAandpenAgenes that encode penicillin-binding protein 1 and 2 (PBP1 and 2),mtrRgene that encodes efflux pump,porBgene that encodes outer membrane porin andpilQgene that encodes a component of the gonococcal outer membrane. Enzymatic destruction of the antibiotic and the last two resistance genes are most often contributed toN. gonorrhoeae[4,10,26]. Included studies did not investigate the mechanisms of resistance to antibiotics in the Iranian isolates. For gonorrhea treatment, third-generation of cephalosporins and quinolones were proposed[1]. However, WHO reported that resistance to third-generation cephalosporins has emerged in some geographic areas such as the United Kingdom,Norway, Sweden, Australia, Japan and China[28]. In Iran, gonococcal resistance rate to cephalosporins including ceftriaxone (11.1%),cefixime (13.8%) and cefepime (50.1%) were variable. Therefore,as WHO reported, cefepime-resistantN. gonorrhoeaecan be a threat to human health in Iran[9]. Additionally, meningococcal antibiotic resistance to cephalosporins such as ceftriaxone (0.0%), cefotaxime(0.0%) and ceftizoxime (0.0%) were low, with cephalexin (33.3%)as the only exception. The antibiotic of choice for meningococcal infections therapy is penicillin G[2]. However, treatment can be changed to ceftriaxone or cefotaxime if the organism is penicillinresistant[2]. In Iran, according to the present results, meningococcal resistance to penicillin is high. Therefore, the use of ceftriaxone or cefotaxime instead of penicillin is recommended. Ciprofloxacin,a quinolone antibiotic which acts via inhibition of bacterial DNA synthesis by interfering with the DNA gyrase and topoisomerase IV enzymes, along with ceftriaxone are commonly prescribed as chemoprophylaxis drugs against meningococcal infection[26]. In the current study, resistance ofN. meningitidisto cipro floxacin was 0.0% but high rates of resistance were detected inN. gonorrhoeaestrains (59.1%). This result in the current meta-analysis is consistent with WHO′s concerns about the emergence of fluoroquinoloneresistantN. gonorrhoeaein the world [9]. Cipro floxacin resistance ofNeisseriaspecies can be achieved through mutations in genesgyrA,gyrB,parC,parEandmtrR, and there have been reports of emerging ciprofloxacin-resistantN. meningitidisstrains in Australia, France,Spain, Argentina and Hong Kong[26]. Gonococcal and meningococcal resistance to antibiotics inhibiting protein synthesis were evaluated in the current meta-analysis and the results showed a low resistance rate to gentamicin, amikacin, kanamycin and chloramphenicol inN.meningitidis. However,N. gonorrhoeaeresistance to spectinomycin,tetracycline and gentamicin was high. Chloramphenicol resistance is mediated by chloramphenicol acetyltransferase enzyme (encoded bycatgenes) but the global resistance ofNeisseriaspecies to this antibiotic is low[26]. Similar results were found in the present study andN. meningitidis(0.0%) andN. gonorrhoeae(2.2%) showed low resistance rates to chloramphenicol. Spectinomycin and tetracycline,especially in patients allergic to penicillin, have been suggested as alternatives to treat gonorrhea and actviablocking bacterial protein synthesis by binding to the 30S ribosomal subunit[4,6,26].Unlike some countries like South Korea in which susceptibility to spectinomycin is high (100.0%), in Iran 21.6% of strains were resistant to this antibiotic[27]. Based on WHO reports, there are high rates of tetracycline resistance worldwide and in the majority of countries gonorrhea treatment with this drug is not recommended[28].Similar to other countries, tetracycline resistance inN. gonorrhoeaewas high in Iran (52.7%). InN. gonorrhoeae, mechanisms of resistance may be attributed to the mutations ofmtrR,penB,pilQandtetMgenes[4]. However, none of the included studies investigated the mechanisms underlying resistance. Due to the resistance ofN. gonorrhoeaeto first-line antibiotics as well as the extendedspectrum cephalosporins that lead to treatment failure, the Centers for Disease Control and Prevention recommended combination therapy with ceftriaxone and azithromycin or doxycycline, and this therapeutic regimen has been used in many regions such as USA,Asia and Europe[2,27]. In light of the present study, the antimicrobial susceptibility ofN. gonorrhoeaeto azithromycin was low (11.1%)in Iran. Similar results were reported from Germany (11.9%) and Korea (5%)[9,27]. Azithromycin binds to the 50S ribosomal subunit and inhibits bacterial protein synthesis. Mutations in the23S rRNAandmtrRgenes play a major role in the resistance to this macrolide antibiotic[4,29]. In the late 1930s, sulphonamides, which prevent folic acid synthesis in bacteria, were used to treat gonorrhea[1,4].However,N. gonorrhoeaeisolates resistant to these drugs were soon identi fied and the use of these drugs was stopped in many countries such as USA[4]. In this meta-analysis, we observed a high level of co-trimoxazole resistance inN. gonorrhoeaestrains (82.4%) in Iran.However, while sulfonamides have lost their effectiveness against meningococcal infections due to the spread of resistant strains, cotrimoxazole resistance rate was 0% inN. meningitidisisolates of Iran[1].
To conclude, high rates of gonococcal resistance to penicillin,cipro floxacin, co-trimoxazole, tetracycline, gentamicin, ampicillin,cefepime and vancomycin were detected in the current study.Therefore, the use of these antibiotics is not recommended for the treatment of gonococcal infection in Iran. Furthermore, penicillin,amoxicillin, ampicillin and cephalexin are not suitable antibiotics to treat meningococcal infections in Iran. Overall, global and national monitoring of antibiotic resistance profile ofNeisseriaisolates is fundamental to guide proper treatment and prevent spreading of resistant species. Finally, further research is required to evaluate the major mechanisms involved in the acquisition of antibiotic resistance inNeisseriaspecies.
Conflict of interest statement
The authors declare that they have no con flict of interests.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Resistance status of main malaria vector, Anopheles stephensi Liston(Diptera: Culicidae) to insecticides in a malaria Endemic Area, Southern Iran
- Frequency of typhoon occurrence accounts for the Poisson distribution of human leptospirosis cases across the different geographic regions in the Philippines
- Cytotoxic, antioxidant and antimicrobial activities of Nerium oleander collected in Morocco
- Diagnosis of Toxoplasma gondii infection in pregnant women using automated chemiluminescence and quantitative real time PCR
- Surveillance of Chikungunya virus activity in some North-eastern states of India
- Potentiating activity of rhein in targeting of resistance genes in methicillin-resistant Staphylococcus aureus
