Boosted Biodegradability and Tribological Properties of Mineral Base Oil by Methyl Diethanolamine Fatty Acid Esters
2019-01-18DingJianhuaFangJianhuaChenBoshuiZhangNanFanXingyuZhengZhe
Ding Jianhua; Fang Jianhua; Chen Boshui; Zhang Nan; Fan Xingyu; Zheng Zhe
(Department of Oil, Army Logistics University, Chongqing 401311)
Abstract: Methyl diethanolamine fatty acid esters, viz. methyl diethanolamine octanoate and methyl diethanolamine oleate, were prepared. Their impacts on the biodegradability and tribological properties of mineral base oil 400SN were evaluated by a tester for fast evaluating the biodegradability of lubricants and by a four-ball tester, respectively. The results showed that methyl diethanolamine octanoate and methyl diethanolamine oleate both could markedly promote the biodegradation of the oil and improved its tribological properties. The improvement of biodegradability was attributed to the enhanced growth and quantity of microbes by methyl diethanolamine fatty acid esters. The worn surfaces were analyzed by a scanning electron microscope (SEM) equipped with an energy dispersive spectrometer (EDS) and an X-ray photoelectron spectroscope (XPS). The results indicated that the enhancement of friction-reducing and anti-wear properties of the mineral oil was attributed to the formation of complicated boundary lubrication f ilms composed of species such as Fe2O3, Fe3O4 and organic nitrogen-containing compounds with a structure of –C-N- or R-NH2.
Key words: biodegradation, wear, friction, methyl diethanolamine fatty acid esters
1 Introduction
With the rapid development of industrialization, the demand and consumption of lubricants is rising continuously, however their pollution to the environment is also increasing. Because mineral base oils possess the advantages of low cost and good comprehensive performance, it is not realistic that they would be completely replaced by biodegradable base oils in a short time[1-4]. However, because of their low-biodegradable nature and inherent eco-toxicity, mineral lubricants may seriously pollute the environment[5-7]. In addition, although biodegradable base oils such as synthetic esters or vegetable oils are prepared and have been applied practically in the formulation of biodegradable lubricants, the thermal oxidation and low temperature fluidity of vegetable oils are bad, while the hydrolytic stability of synthetic esters is poor and its price is relatively high[8-9]. Meanwhile, traditional additives can hardly make lubricants meet simultaneously the requirement for performance and ecological efficiency, and may be harmful to the active microbes or degrading enzyme, resulting in reducing the biodegradation of lubricant and even increasing the toxicity of lubricants[10-11]. Hence, it is significant and indispensable to develop additives meeting the usable requirements and ecological requirements, especially in terms of enhanced biodegradability of mineral lubricants.
Many researches on environmental microbiology have indicated that the addition of organic or inorganic compounds containing N and P in the degradation process can increase the nutrients needed for microbial growth, improve the microbial activity, and promote the biodegradation of petroleum hydrocarbons[12-16]. In this paper, methyl diethanolamine fatty acid esters, namely methyl diethanolamine octanoate and methyl diethanolamine oleate, were prepared, with their impacts on biodegradability and tribological properties of mineral base oil were reported.
2 Experimental
2.1 Materials
The AR grade experimental reagents (methyl diethanolamine, caprylic acid, oleic acid, toluene, and toluenesulfonic acid monohydrate) were purchased from the Chengdu Kelong Chemical Reagent Company. A non-polarized paraffinic lubricating base oil (400SN), the kinematic viscosity of which at 40 °C (was equal to 84.93 mm2/s), was provided by the Shenzhen Lubricating Oil Industry Company.
2.2 Preparation of additives
Figure 1 shows the synthetic pathways and molecular structures of methyl diethanolamine octanoate (denoted as MDEAC) and methyl diethanolamine oleate (denoted as MDEAO). The synthetic process is demonstrated as follows:
30 mL of toluene serving as the solvent and water entraining agent, 0.05 mol of methyl diethanolamine, 0.1 mol of oleic acid and catalyst (p-toluenesulfonic acid) were added into a 500-mL three-necked flask equipped with a thermometer, a reflux condenser, a dean-stark trap for water separation, and a stirrer. The reaction was carried out under ref luxing and stirring at 160 °C until no obvious water production could be observed. After being cooled down, the mixture was distilled to remove toluene under reduced pressure. MDEAO was then obtained.
Following the similar procedure described above, MDEAC was also obtained by substituting oleic acid with caprylic acid.
The products were characterized by Fourier transform infrared spectroscopy (FT-IR). Figure 2 and Figure 3 give the FT-IR spectra of MDEAC and MDEAO, respectively. The significant features of MDEAC covered the bands corresponding to the CH3/CH2stretching vibration at 2 925.9 cm-1and 2 855.2 cm-1, the C=O in ester group at 1 735.6 cm-1, and the C-O-C in ester group at 1 162.3 cm-1. The significant features of MDEAO covered the bands corresponding to the CH3/CH2stretching vibration at 2 925.4 cm-1and 2 852.8 cm-1, the C=O in ester group at 1 737.5 cm-1, and the C-O-C in ester group at 1 166.2 cm-1. The results showed that MDEAC and MDEAO were synthesized.
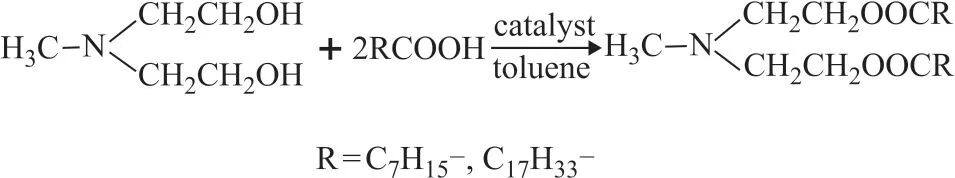
Figure 1 The pathway for synthesis of MDEAC and MDEAO

Figure 2 FT-IR spectra of MDEAC

Figure 3 FT-IR spectra of MDEAO
2.3 Biodegradation test
In order to study the impact of MDEAC and MDEAO on biodegradation of mineral base oil, different contents of additives, viz.: 0, 0.5%, 1.0%, 1.5% and 2.0%, respectively, were incorporated into the base oil 400SN. The biodegradability of the formulated oils was tested by the method introduced in our previous work[17]. In short, test of lubricant biodegradability by means of this method was achieved by the reference substance oleic acid and the parallel biodegradation reactions of the lubricant. The carbon dioxide created by the test sample was measured every two days under formulated conditions, after 10 days of biodegradation, while the accumulated amount of CO2generated from oleic acid (abbreviated asMO) and the tested lubricant (abbreviated asML) was measured, respectively. The biodegradability index (BDI), a comparative parameter of the percentage ratio of the amount of CO2produced by single tested lubricant against that produced by oleic acid, was used to determine the biodegradability of the lubricant. BDI was calculated by the following equation:

The higher the BDI value was, the better the biodegradability of the lubricant would be. The diagrammatic drawing of biodegradation test is presented in Figure 4[18]. The CO2degasser and absorber contained NaOH solution and Ba(OH)2solution, respectively. This test was conducted under dark condition in order to prevent light degradation.

Figure 4 Diagrammatic drawing of biodegradation test
The optical density of microbial culture medium at a wavelength of 600 nm (abbreviated as OD600) was measured by an ultraviolet spectrophotometer every two days. The OD600value indicated the growth situation of microbes. The higher the OD600value was, the more the microbial quantity would be.
2.4 Tribological properties
The tribological properties of MDEAC and MDEAO in the mineral base oil 400SN were studied on a four-ball tribotester following the procedures of GB/T 3142―1982, which was a Chinese standard method for determining the friction and wear properties of lubricants. The four-ball tribotester was composed of a rotating ball that glided on three fixed balls under chosen loads. The steel balls were made of standard GCr15 steel balls with a diameter of 12.7 mm, a hardness of 59―61 HRC, and a surface roughnessof Ra 0.040 μm. The wear scar diameters (WSD), friction coefficient and the maximum nonseizure loads (PB) were measured. The WSD and friction coefficient were obtained under a load of 392N and a rotary speed of 1 500 r/min for 30 min. The duration of testingPBwas 10 seconds under a selected load and a rotary speed of 1 450 r/min.
2.5 Surface analysis
The steel balls were lubricated by a 400SN base oil sample and the formulated oil samples including 2.0% of MDEAC or 2.0% of MDEAO under a load of 392 N and a rotary speed of 1 500 r/min for 30 min, then they were ultrasonically cleansed with petroleum ether for 10 min. The surface morphology and elemental composition of the steel balls were analyzed by a scanning electron microscope (SEM) equipped with an energy dispersive spectrometer (EDS). The chemical features of the typical elements on the worn surfaces were analyzed by an ESCALab250 X-ray photoelectron spectroscope (XPS).
3 Results and Discussion
3.1 Biodegradable properties
Figure 5 suggests that the BDI varies with different mass fraction of MDEAC and MDEAO. It can be found that MDEAC and MDEAO, added into 400SN mineral oil at different concentrations, can improve the BDI. Especially at the content of 1.0% and 0.5%, the BDI increased from 33.6% to 59.2% and 65.7%, respectively, showing that MDEAC and MDEAO could enhance the biodegradation of base oil. Studies have shown that many compounds containing nitrogen and phosphorus elements can effectively promote the biodegradation of hydrocarbons for remediation of petroleum polluted water or soil[19-20]. Enhanced biodegradation of mineral lubricating oil by MDEAC and MDEAO is thus evidentially possible, because they both contain the nitrogen needed by microbial growth.
Figure 6 indicates that the OD600of microbial culture medium with and without additives (MDEAC and MDEAO) varies with the biodegradation time. The results reveal a significantly higher OD600of microbial culture medium containing additives in the exponential period of microbial growth. It testifies that MDEAC and MDEAO can increase the growth rate and quantity of microbes, which contributes to the enhanced degradation of the mineral base oil 400SN.
3.2 Anti-wear properties

Figure 5 Variation of BDI with different content of MDEAC and MDEAO

Figure 6 Variation of OD600 with biodegradation time
Figure 7 gives the variation of WSD with different contents of MDEAC or MDEAO under a rotary speed of 1 500 r/min and a load of 392 N for 30 min. As Figure 7 shows, incorporation of additives into the mineral oil 400SN both can ensure lower WSD value than that achieved by the neat mineral oil. The WSDs decrease dramatically from 0.64 mm to 0.46 mm and 0.49 mm respectively, when the concentration of additives increases to 1.0%, indicating that MDEAC and MDEAO can boost the anti-wear performance of the base oil. Moreover, MDEAC provides lower WSD than MDEAO does. It can be seen that the content of N element in MDEAC molecule is relatively higher.
Figure 8 displays the impact of different loads (namely, 196, 294, 392 and 490 N, respectively) on WSD of steel ball lubricated by base oil and by the oil containing 1.0% of MDEAC or 1.0% of MDEAO under a rotary speed of 1 500 r/min for 30 min. Figure 8 shows that MDEAC and MDEAO both exhibit excellent antiwear performance by providing lower WSD than those achieved by the neat mineral oil at the applied loads ranging from 196 N to 490 N. In addition, WSDs increase slowly with an increasing load. The reason may be that, with the increase of loads, the molecular chains of MDEAC and MDEAO are decomposed sharply to become shortened. Then the strength of protective f ilm of friction surface is weakened, resulting in the increase of WSDs[21].

Figure 7 Variation of WSD with different content of MDEAC and MDEAO

Figure 8 Variation of WSD with different loads
3.3 The maximum non-seizure load (PB value)
Shown in Figure 9 is the variation ofPBvalues with different contents of MDEAC or MDEAO. Figure 9 shows that thePBvalues of test oils all increased with an increasing concentration of additives. Compared with the base oil, thePBvalues are improved from 495 N to 618 N and 696 N, respectively, at a fatty acid ester additive content of 1.0%. Figure 9 also shows that MDEAO provided higherPBvalue than MDEAC. The reason maybe that MDEAO has longer fatty acidic chain length than MDEAC, providing a thicker triboadsorption film on the surfaces of steel balls to better keep the surfaces from direct contact under boundary lubrication conditions[22].

Figure 9 Variation of PB values with different mass fraction of MDEAC or MDEAO
3.4 Friction-reducing properties
Figure 10 shows that the friction coefficient of base oil and the oil containing 1.0% of MDEAC or 1.0% of MDEAO varies with test duration under a rotary speed of 1 500 r/min and a load of 392 N. Figure 10 displays that the base oil with additives can provide lower friction coefficient than the neat base oil in the whole test durations, suggesting that MDEAC and MDEAO both can effectively enhance the friction-reducing properties of the base oil 400 SN.
3.5 Mechanism analysis
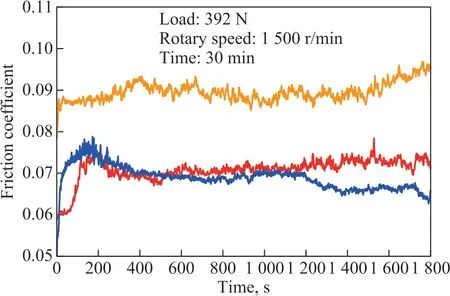
Figure 10 Variation of friction coefficient with test duration
Shown in Figure 11 is the SEM morphology of the worn surfaces of steel balls lubricated by the base oil 400 SN and the oil containing 2.0% of MDEAC or 2.0% of MDEAO under a load of 392 N and a rotary speed of 1 500 r/min for 30 min. Figure 11 suggests that the worn surface obtained from the oil containing additives is smoother than that achieved by the neat base oil. This is in agreement with the better friction-reducing and antiwear ability of additives as shown above.
Figure 12, Table 1, Figure 13 and Table 2 give the EDS analytical results of the worn surfaces of steel balls lubricated by the oil containing 2.0% of MDEAC or 2.0% of MDEAO under a load of 392 N and a rotary speed of 1 500 r/min for 30 min. Figure 12, Table 1, Figure 13, and Table 2 show that nitrogen element is deposited on the worn surface, indicating that nitrogen element has been involved in the tribochemical reaction.
Figure 14 and Figure 15 display the XPS spectra of worn surfaces of steel balls lubricated by the oil containing 2.0% of MDEAC or 2.0% of MDEAO under a rotary speed of 1 500 r/min and a load of 392 N for 30 min. The XPS spectrum of C1s suggests a peak in a binding energy range of 284.5―288.4 eV, which is attributed to organic species of C-C or O=C-O- bonds and indicates that additives are absorbed on the friction surface of steel balls. The peak of O1s at a binding energy of 531.3 eV and 529.9 eV may be ascribed to C=O or C-O and iron oxide, respectively. The peak of Fe2p at a binding energy of 710.6 eV shows that the iron is oxidized into Fe2O3or Fe3O4. The peak of N1s around a binding energy of 399.5 eV may be attributed to –C-N- or R-NH2. The enhancement of friction-reducing and anti-wear ability is caused by the formation of a complex boundary lubrication film resulted from the adsorption and complicated tribochemical reactions of additives on the worn surface.

Figure 11 SEM images of the worn surfaces

Figure 12 The EDS images of the worn surfaces lubricated by MDEAC-doped oil

Table 1 Weight percentage and atomic percentage of elements on the worn surface lubricated by oil containing 2% of MDEAC
4 Conclusions

Figure 13 The EDS images of the worn surfaces lubricated by MDEAO-doped oil
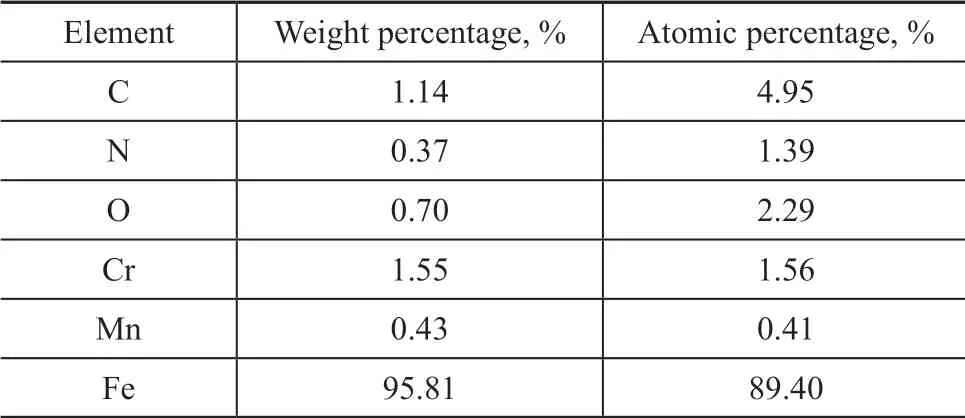
Table 2 Weight percentage and atomic percentage of elements on the worn surface lubricated by oil containing 2% MDEAO
Methyl diethanolamine fatty acid esters (methyl diethanolamine octanoate and methyl diethanolamine oleate) have been prepared, with their chemical structures characterized by FT-IR spectroscopy. Upon being added into the mineral base oil 400SN, MDEAC and MDEAO both can accelerate the biodegradation of the oil effectively. The BDI increases from 33.6% to 59.2% and 65.7%, respectively, which is ascribed to the enhanced growth and increased quantity of microbes. They also can improve the anti-wear ability and friction-reducing ability of the oil. The WSDs decrease dramatically from 0.64 mm to 0.49 mm and 0.46 mm, respectively, which is attributed to the formation of the complicated boundary lubrication film composed of the adsorbed films and tribochemical species such as Fe2O3and Fe3O4, and organic nitrogencontaining compounds.

Figure 14 XPS spectra of typical elements on worn surfaces lubricated by MDEAC-doped oil

Figure 15 XPS spectra of typical elements on worn surfaces lubricated by MDEAO-doped oil
Acknowledgment:The authors are grateful to the financial supports from National Defense Science Technology Foundation (Project No.3604003), National Natural Science Foundation of China (Project No.51375491), Natural Science Foundation of Chongqing (Project No. CSTC 2014JCYJAA50021) and Natural Science Foundation of Chongqing (Project No. cstc2017jcyjAX0058).
杂志排行
中国炼油与石油化工的其它文章
- Environmentally-Friendly Catalytic Oxidation of Cyclohexanone with 30% H2O2 Solution: A Comparison Study between Hollow Titanium Silicate and Dealuminated HBEA Zeolites
- A Study on the Coking Sites of γ-Alumina Surface Using 1-Methylnaphthalene as the Model Reactant
- Selective Hydrogenation of Butyne-1,4-diol to Butane-1,4-diol over Ni/Al2O3-SiO2 Catalysts
- China Will Increase Ethylene Production Capacity Totaling Around 15 Mt/a by 2025
- Extractive Distillation of Methyl Acetate-Methanol Azeotrope Using [DMIM]DMP as Solvent
- Catalytic Hydrogenation Performance of Methyl Isobutyl Ketone over Ni/γ-Al2O3 Catalysts
