Extractive Distillation of Methyl Acetate-Methanol Azeotrope Using [DMIM]DMP as Solvent
2019-01-18LiJingWangKeliangLianMingleiLiZhiDuTingzhao
Li Jing; Wang Keliang; Lian Minglei; Li Zhi; Du Tingzhao
(1. College Of Chemistry and Materials Engineering, Liupanshui Normal University, Liupanshui 553004; 2. North China Company, China Petroleum Engineering Co., Ltd., Renqiu 062552)
Abstract: In order to separate methyl acetate and methanol azeotrope, the ionic liquid (IL) 1,3-dimethylimidazolium dimethylphosphate ([DMIM]DMP) was used as the solvent. The Aspen Plus software was used to design and optimize the extractive distillation process. Under the optimized conditions, the mass fractions of methyl acetate and methanol were both above 99.5%. Compared with the dimethyl sulfoxide (DMSO) process, the [DMIM]DMP process has the advantages of saving energy and lower equipment investment cost. The result shows that using [DMIM]DMP as the solvent to separate a methyl acetate and methanol azeotrope has better prospect in industrial application.
Key words: extractive distillation; 1,3-dimethylimidazolium dimethylphosphate; azeotrope; methyl acetate; methanol
1 Introduction
Methyl acetate and methanol are widely used as organic chemical raw materials and solvents. The boiling point of methyl acetate and methanol is 330.15 K and 337.75 K, respectively. The mixture of methyl acetate and methanol, which consists of 66% of methyl acetate and 34% of methanol by molar fraction[1-4], is a typical azeotrope with an azeotropic temperature of 326.69 K. In the production of polyvinyl alcohol (PVA), a large amount of methyl acetate and methanol remains in the waste liquid[5-6]. The traditional distillation technology cannot separate the azeotrope effectively. Currently some special distillation methods, such as the pressureswing distillation, the reactive distillation, the azeotropic distillation, the salt distillation, and the extractive distillation are applied[7-11].
Zhang, et al.[12]used the pressure-swing distillation to separate methyl acetate and methanol. The high pressure and low pressure were set at 1.1 MPa and 0.1 MPa, respectively. The mole fraction of the product obtained thereby was more than 99.5%. Recently, the ionic liquids (ILs) have been proposed to be used as solvents for extractive distillation[13]. ILs are excellent organic solvents featuring a wide range of liquid-phase temperature, high thermal stability, almost zero saturated vapor pressure, low viscosity, and intensified process ability[14-18]. Taking methanoldimethyl carbonate (DMC) azeotrope as an example, Li, et al.[19]studied the interaction mechanism of ILs as solvents through the DFT calculations and found that the interaction between the ILs and methanol was much stronger than that between the methanol and DMC, i.e. ILs could effectively break the initial stable structure formed by the azeotrope. Therefore, ILs are very suitable for being used as solvents in extractive distillation[20].
Recently, many scholars have conducted relevant studies on the solvents for separating methyl acetate and methanol azeotrope. Zhang, et al.[21]measured the isobaric vapor liquid equilibrium for a ternary system composed of methanol, methyl acetate and dimethyl sulfoxide (DMSO) at 101.3 kPa and found that DMSO could effectively remove the azeotropic point of methyl acetate and methanol azeotrope. Cao, et al.[22]studied three kinds of ILs as entrainers to separate methyl acetate and methanol in extractive distillation, in which the ILs covered 1,3-dimethylimidazolium dimethylphosphate ([DMIM]DMP), 1-ethyl-3-methylimidazolium diethylphosphate ([EMIM]DEP), and 1-butyl-3-methylimidazolium dibutylphosphate ([BMIM]DBP). The separation effects of three kinds of ILs were compared. The ability of [DMIM]DMP to break the azeotropic point was stronger than that of [EMIM]DEP and [BMIM]DBP. The test results have shown that the ILs can be used as solvents for separating the azeotrope composed of methyl acetate and methanol.
Therefore, in this work, extractive distillation of methyl acetate and methanol azeotrope using [DMIM]DMP as the solvent was simulated by means of the Aspen Plus software. The feasibility of the IL used in the distillation process of methyl acetate and methanol azeotrope was further investigated. The optimum operating parameters were determined to provide a theoretical basis for the process design. In addition, the separation effect and energy consumption of the process were compared with those of the extractive distillation process using DMSO as the solvent.
2 Property Method and Process Simulation
2.1 Property method
Thermodynamic property data of methyl acetate and methanol are available in the database of Aspen Plus software. However, some data concerning the basic properties of the IL should be added into the Aspen Plus software. Critical properties and constant molar heat capacity of the ILs were calculated by the group contribution method[23-26].
The molar heat capacity at constant pressure of [DMIM]DMP is expressed as:
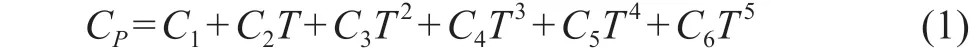
whereCPrepresents the molar heat capacity at constant pressure, andTis the temperature.
Critical properties of [DMIM]DMP and the parameters of heat capacity are listed in Table 1 and Table 2, respectively.

Table 1 Critical properties of [DMIM]DMP
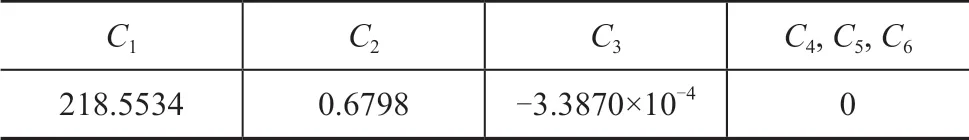
Table 2 Heat capacity parameters of [DMIM]DMP
The vapor-liquid equilibrium data of the methyl acetatemethanol-[DMIM]DMP ternary system were measured by Cao, et al[21]. The eNRTL equation was used to correlate the experimental data, and the results were in good agreement with the experimental data. Therefore, the eNRTL equation was chosen as the thermodynamic model in this work, and the binary parameters which should be added to the Aspen Plus software were calculated, with the results listed in Table 3.
Then they carried Kay and Gerda first to the Finland woman, where they warmed themselves thoroughly14 in the hot room, and she gave them directions about their journey home
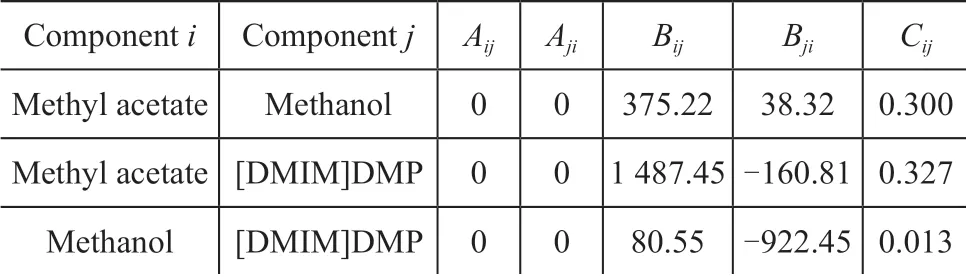
Table 3 Binary parameters in the eNRTL model parameters
The residue curve maps of the two ternary systems are displayed in Figure 1. The methyl acetate and methanol azeotrope is the unstable node, and the solvent [DMIM]DMP or DMSO is the stable node. The methyl acetate and methanol are both saddle points of the residue curves, while no rectification boundary exists. These results show that using the [DMIM][DMP] or DMSO as solvent are both feasible for separating the methyl acetate and methanol azeotrope.
The relative volatility (α12) can be conveniently used to describe the separation effect of extractive solvents on binary azeotropic behavior. The relative volatility of methyl acetate (1) to methanol (2) can be calculated by

whereyiandxiare the mole fractions in vapor phase and liquid phase of the componenti, respectively. Thus, the higher the value ofα12is, the more effective the solvent would be.
The expression relating tox’1(mole fraction not containing the solvent) -α12with different solvents is shown in Figure 2. The addition of the solvent ([DMIM]DMP or DMSO) can obviously increase the relative volatility of methyl acetate to methanol. The effect of [DMIM]DMP is much better than that of DMSO.
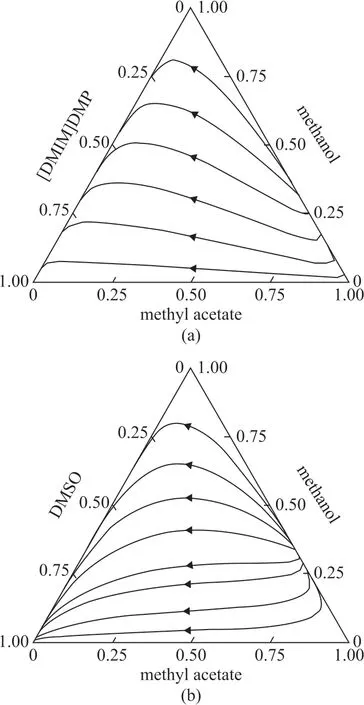
Figure 1 Residue curves of the ternary system with different solvents: (a) [DMIM]DMP and (b) DMSO
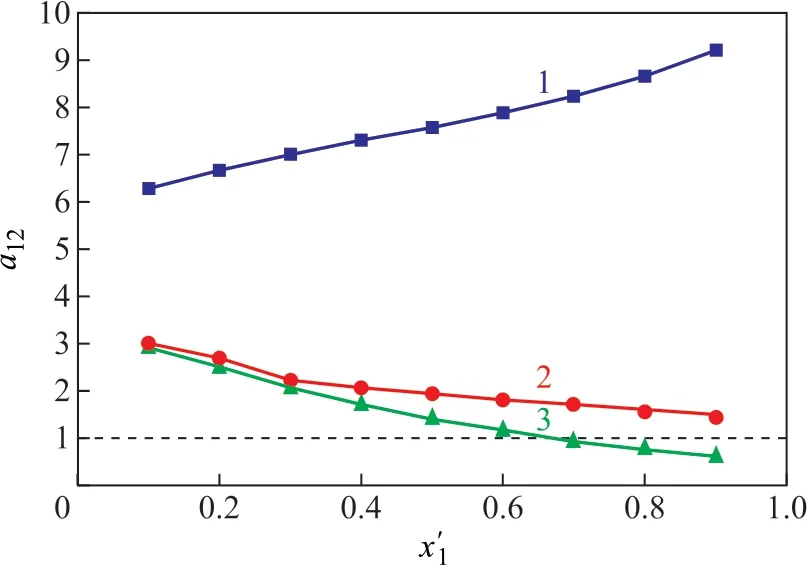
Figure 2 The relative volatility α12 of methyl acetate (1) to methanol (2) in the ternary systems with different solvents: x[DMIM]DMP=0.5 (line 1), xDMSO=0.5 (line 2), and no solvent (line 3)
2.2 Process simulation
The process of separating methyl acetate and methanol azeotrope using [DMIM]DMP as solvent is presented in Figure 3a. A mixture of methyl acetate and methanol is fed into the middle part of the extractive distillation column (C1). The solvent [DMIM]DMP is fed into the top part of C1. After extractive distillation, the high purity product of methyl acetate can be obtained at the top of C1. Meanwhile a mixture of [DMIM]DMP and methanol is obtained at the bottom of C1, which is transported to the solvent recovery column (C2). C2 is operated at vacuum to separate methanol at the top, and the solvent [DMIM]DMP is obtained at the bottom. After cooling, the solvent [DMIM]DMP is recycled to C1 by a pump. The process of separating methyl acetate and methanol azeotrope using dimethyl sulfoxide(DMSO)as the solvent is shown in Figure 3b. The difference between this process and [DMIM]DMP process is that C2 is operated at atmospheric pressure.
3 Results and discussion
3.1 Process optimization
The initial simulation conditions included a mixture feed f low rate of 1 000 kg/h, while the mixture was composed of 81.8% of methyl acetate and 18.2% of methanol. The feed temperature was 313.15K. The theoretical plate number of C1 was 40, while the mixture and the solvent were introduced at the 20th stage and 4th stage, respectively. The whole column operated at atmospheric pressure with a reflux ratio of 2, and the overhead distillate rate was 818 kg/h. The separation process required that the purity of the final products of methyl acetate and methanol should be greater than 99.5%. The Radfrac module was used as the distillation column, and the influence of process parameters on separation effect and energy consumption was investigated.
Figure 4 shows the influence of solvent/feed mass ratio. With the increase in the solvent ratio, the mass fraction of methyl acetate (wD) at f irst increased sharply. When the solvent ratio was greater than 0.8, the trend of change inwDtended to be smooth. However, the trend of change in the condenser duty was just the opposite. The reboiler duty was minimal when the solvent ratio ranged from 0.7 to 0.9. Therefore, the optimal solvent ratio was set at 0.8.
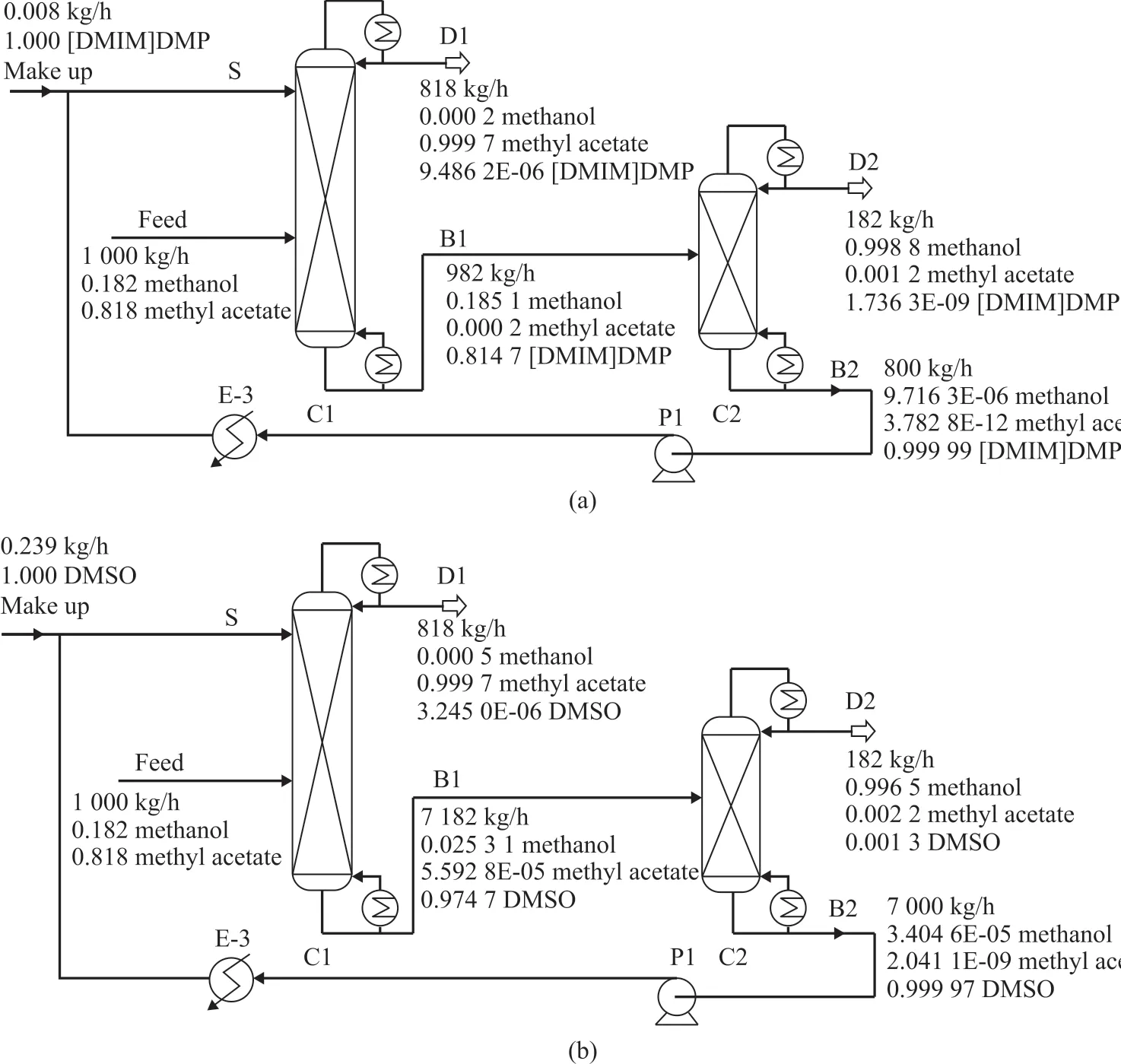
Figure 3 Extractive distillation: (a) using [DMIM]DMP as solvent, and (b) using DMSO as solvent
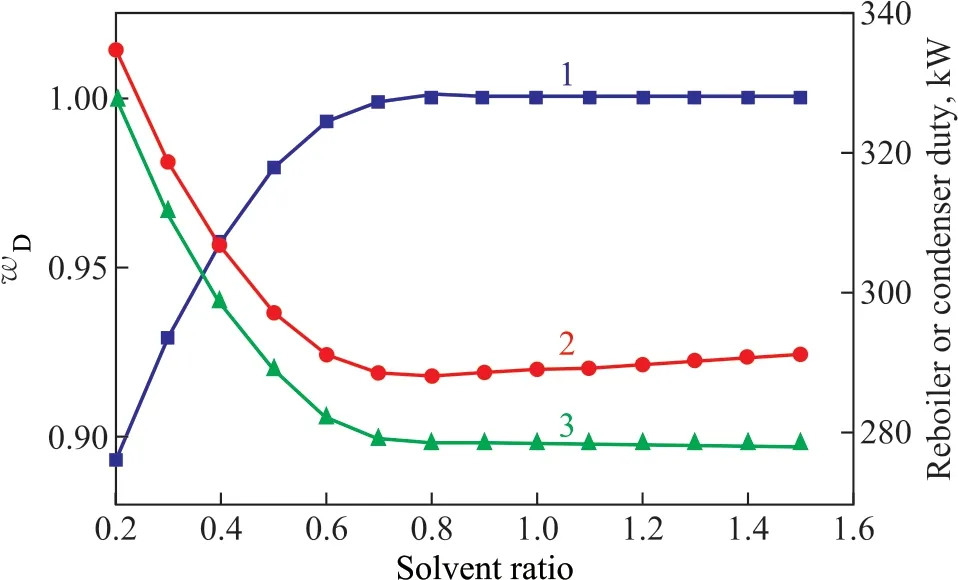
Figure 4 Influence of the solvent/feed mass ratio on: mass fraction of methyl acetate (line 1); reboiler duty (line 2); and condenser duty (line 3)
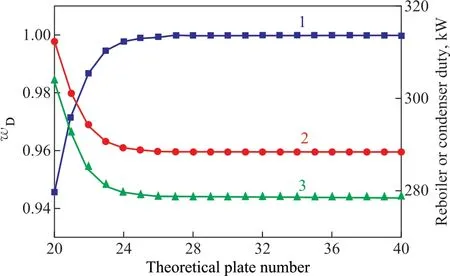
Figure 5 Influence of the number of theoretical plates on: mass fraction of methyl acetate (line 1); reboiler duty (line 2); and condenser duty (line 3)
Figure 6 shows the influence of the mixture feed stage. As the feed position moved down,wDpresented a trend of gradual increase and then sharp decrease, which reached a maximum value of 99.95% at the 19th stage. The condenser duty and reboiler duty showed an opposite trend, which decreased firstly and then increased sharply. The condenser duty and reboiler duty both reached a minimum at the 19th stage. Therefore, the 19th stage was selected as the feed position of raw mixture.
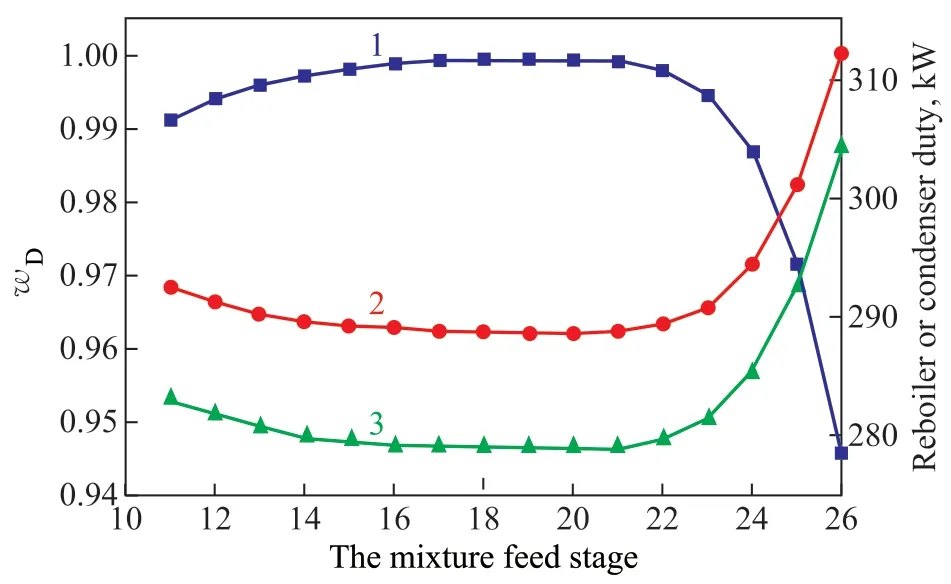
Figure 6 Influence of the mixture feed stage on: mass fraction of methyl acetate (line 1); reboiler duty (line 2); and condenser duty (line 3)
The influence of the solvent feed stage is shown in Figure 7. When the solvent was fed successively from the f irst to the 6th stage,wDf irst increased sharply and then tended to be smooth and slightly decreased. However,wDreached its maximum value at the 2nd stage. There were no changes in condenser duty and reboiler duty after the 2nd stage. Therefore, the 2nd stage was selected as the solvent feed position.
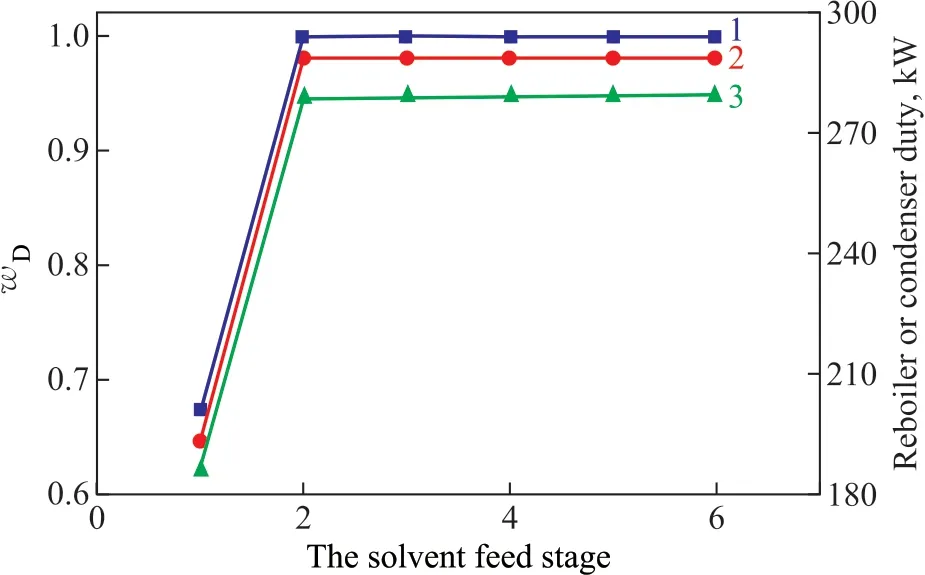
Figure 7 Influence of the solvent feed stage on: mass fraction of methyl acetate (line 1); reboiler duty (line 2); and condenser duty (line 3)
As shown in Figure 8, with the increase of ref lux ratio,wDincreased rapidly till the ref lux ratio was greater than 1.1. The ref lux ratio could affect the separation effect ifit was too large or too small. When the ref lux ratio was too large, it would dilute the solvent, thus reducing the separation effect. When the reflux ratio was too small, the solvent at the top of column would reduce the mass fraction of methyl acetate in the distillate. The reboiler duty and condenser duty showed a linearly increasing trend with the increase of ref lux ratio. Therefore, upon considering the purity of the product and energy consumption, the ref lux ratio was set at 1.1, which could ensure the mass fraction of methyl acetate to reach 99.97% at the top of the column.
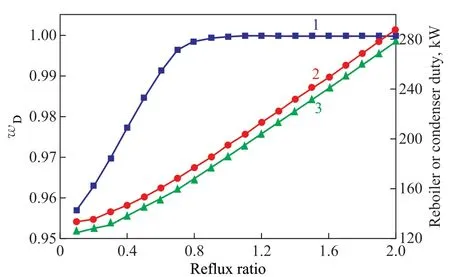
Figure 8 The influence of ref lux ratio on: mass fraction of methyl acetate (line 1); reboiler duty (line 2); and condenser duty (line 3)
The mixture of [DMIM]DMP and methanol from the bottom of C1 column flows into the solvent recovery column C2. Since the boiling point of IL is very high at atmospheric pressure, the solvent recovery column is operated under reduced pressure. The influence of operating pressure on top temperature and bottom temperature of the solvent recovery column is shown in Figure 9.
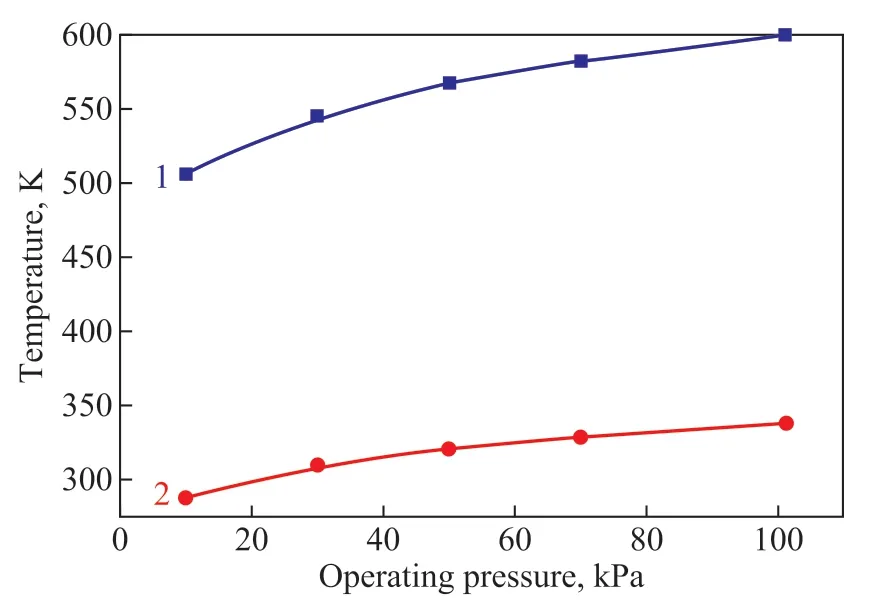
Figure 9 Influence of the operating pressure on: bottom temperature of C2 (line 1); and top temperature of C2 (line 2)
It can be seen that with the decrease of operating pressure, the bottom temperature and top temperature in the column also decreased. If the operating pressure was 101.325 kPa, the bottom temperature of C2 was 599.4 K. It would be difficult to obtain the heating carrier. Therefore, the operating pressure was determined to be 10 kPa and the bottom temperature and top temperature should be 505.81 K and 288.55 K, respectively. The high pressure steam could be used as the heating carrier at the bottom of C2[27]. Similar to the extractive distillation column, the sensitivity analysis tool was also used to determine the optimum process parameters of C2 column as listed in Table 4. Finally, the mass fraction of methanol product was 99.88%, which could meet the separation requirements.
In the course of extractive distillation, a small amount of [DMIM]DMP would be lost. Therefore, in order to meet the separation requirements, it was necessary to add fresh solvent, which should be mixed with the solvent recycled to C1 column. The fresh solvent [DMIM]DMP must be supplemented at a f low rate of 0.008 kg/h by the calculator function in the Aspen Plus software.
3.2 Process comparison
The DMSO process is shown in Figure 3b. The difference between this process and [DMIM]DMP process indicated that C2 was operated at atmospheric pressure. The DMSO process was also optimized to obtain the process parameters by using the sensitivity analysis tool similar to that adopted to the [DMIM]DMP process. The fresh solvent DMSO must be supplemented at a f low rate of 0.239 kg/h. The optimization results and the energy consumption of DMSO process and [DMIM]DMP process are listed in Table 4. It can be seen that the mass f low of solvent used in the [DMIM]DMP process was only 11% of that used in the DMSO process and the amount of makeup solvent needed by the [DMIM]DMP process was much less than that used in the DMSO process. The total energy consumption of the [DMIM]DMP process was only 37% of that in the DMSO process. The [DMIM]DMP process required fewer theoretical plates and smaller reflux ratio. Compared with the DMSO process, the [DMIM]DMP process showed lower energy consumption and less equipment investment cost.
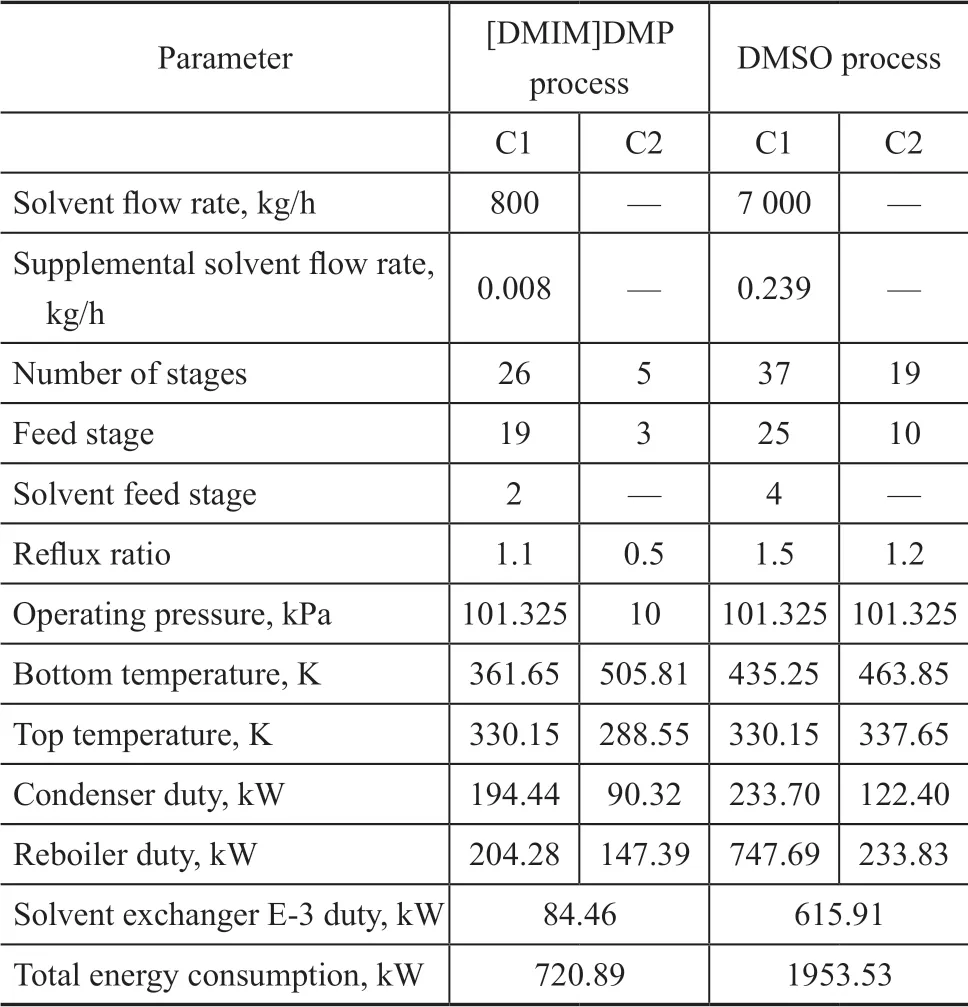
Table 4 Simulation results and energy consumption of two processes
4 Conclusions
In this work, [DMIM]DMP was used as the solvent to separate a methyl acetate and methanol azeotrope. The relevant basic properties of IL were calculated and added into the Aspen Plus software. The feasibility of using [DMIM]DMP or DMSO as solvents was analyzed by the residue curve maps and relative volatility data. The influences of the solvent ratio, theoretical plate number, feed position, and other factors on the separation effect were studied. Under the optimized conditions, the mass fractions of the final products methyl acetate and methanol were both above 99.5%.
Compared with the DMSO process, the amount of solvent needed by the [DMIM]DMP process was much less than that of the DMSO process. The [DMIM]DMP process required fewer theoretical plates, and smaller ref lux ratio. The total energy consumption of the [DMIM]DMP process was only 37% of that needed by the DMSO process. Therefore, the [DMIM]DMP process demonstrated the advantages of saving energy and less investment in equipment cost.
This paper not only shows the fact that [DMIM]DMP can be used to separate methyl acetate and methanol azeotrope effectively but also provides references for the separation of other alcohol-ester azeotropes using ILs as the solvent. It is believed that with the development of synthesis and purification methods of ILs, the price of ILs will gradually decrease, and the strategy of process intensification proposed in this work shall be more easily carried out in industrial application.
Acknowledgement:This work is financially supported by the Guizhou Province Science and Technology United Fund (Qiankehe J zi LKLS[2013]28, LKLS[2013]27), the Guizhou Province Education Department (GZSJG10977201604), the Excellent Engineers Education Training Plan (LPSSY zyjypyjh201702), the Guizhou Province Education Department (Qianjiaohe KY zi [2017]258), Guizhou Solid Waste Recycling Laboratory of Coal Utilization ([2011]278), and the Guizhou Ordinary College Innovation Team of Coal Solid Waste Recycling Technology ([2014]46).
杂志排行
中国炼油与石油化工的其它文章
- Environmentally-Friendly Catalytic Oxidation of Cyclohexanone with 30% H2O2 Solution: A Comparison Study between Hollow Titanium Silicate and Dealuminated HBEA Zeolites
- A Study on the Coking Sites of γ-Alumina Surface Using 1-Methylnaphthalene as the Model Reactant
- Selective Hydrogenation of Butyne-1,4-diol to Butane-1,4-diol over Ni/Al2O3-SiO2 Catalysts
- China Will Increase Ethylene Production Capacity Totaling Around 15 Mt/a by 2025
- Boosted Biodegradability and Tribological Properties of Mineral Base Oil by Methyl Diethanolamine Fatty Acid Esters
- Catalytic Hydrogenation Performance of Methyl Isobutyl Ketone over Ni/γ-Al2O3 Catalysts
