Influence of Dechlorination Temperature on Propane Dehydrogenation over Pt-θ-Al2O3
2019-01-18LiuJieLiuChangchengMaAizengDaZhijianZhengHuidong
Liu Jie ; Liu Changcheng; Ma Aizeng; Da Zhijian; Zheng Huidong
(1. College of Chemical Engineering, Fuzhou University, Fuzhou 350116; 2. SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Abstract: Several Pt-θ-Al2O3 catalysts with similar ultra-low Cl contents were used to investigate the influence of dechlorination temperature on propane dehydrogenation reaction. The Pt-θ-Al2O3 catalyst treated at a highest dechlorination temperature showed a lowest propane rate and propylene selectivity. The scanning transmission electron microscopy showed that the dispersions of Pt nanoparticles decreased with an increasing dechlorination temperature. The temperature-programmed reduction analysis showed that higher dechlorination temperature could lead to strong interactions between the metal and support, making it difficult to reduce Pt nanoparticles. The temperature-programmed oxidation analysis implied that more coke was deposited on the metal for catalyst treated at higher dechlorination temperature. The Raman spectra and the H/C ratio showed that more side-reactions, such as cracking and severe deep dehydrogenation reactions, occurred on catalysts treated at higher dechlorination temperatures. Therefore, the lower the dispersion of Pt nanoparticles was, the stronger the metal-support interactions and increased side-reactions would be, resulting in lower catalytic activity for Pt-θ-Al2O3 treated with higher dechlorination temperature.
Key words: dechlorination temperature; metal-support interactions; θ-Al2O3; Pt nanoparticles; propane dehydrogenation reaction
1 Introduction
Propane dehydrogenation (PDH) is becoming a promising approach for producing propylene with the depletion of oil reserves and prevalence of shale gas extraction[1]. Generally, the reaction temperature ranges from 550 ºC to 750 ºC, and such a high temperature can cause severe agglomeration of Pt nanoparticles over the Al2O3support and further lead to catalyst deactivation. Numerous methods have been employed to enhance the anti-sintering of Pt nanoparticles, such as introducing promoters and alternative supports[2-9]. The more stable phases of alumina, such as θ-Al2O3or α-Al2O3, were also employed as supports for PDH reaction thanks to their weaker acidity and higher stability.
In the preparation of supported θ-Al2O3catalysts for dehydrogenation reactions, Prakash, et al.[10]found that the PtSn/θ-Al2O3catalyst prepared in acid solvents (HCl and HNO3) exhibited increased stability and activity. Promoters were commonly used to enhance the catalytic performance of PtSn/θ-Al2O3. Both Nagaraja[11]and Lee, et al.[12]provided evidence of the effect of potassium and proposed that the addition of an appropriate amount of potassium could block the acidic sites and that excess potassium addition could weaken the interactions between Pt, Sn and K. The role of θ-Al2O3support was elucidated by Shi, et al. and our group. Shi, et al.[13]considered that the θ-Al2O3support, due to its large pore volume, large pore diameter, and strong interactions with Sn, could promote the PDH reaction. The reaction conditions and catalyst regeneration process also attracted the interests of other researchers[14-15].
The phase of support, component of Cl, and dispersion of Pt are the main factors that affect the catalytic performance of Pt/Al2O3catalysts in the PDH reaction. In a previous study, we investigated the effect of the Al2O3support and Cl content on the PDH reaction[16]. The dispersion of Pt is sensitive to temperature and can be regulated by changing the dechlorination temperature. The effect of the sizes of Pt nanoparticles can also be clarified by investigating the influence of the dechlorination temperature. In the present study, three catalysts with similar Pt and ultra-low Cl contents were employed to investigate the influence of dechlorination temperature on propane dehydrogenation reaction.
2 Experimental
2.1 Materials
The θ-Al2O3support was obtained via the calcination of γ-Al2O3in air at 1 000 ºC, which was purchased from Sasol. The aqueous solution of HCl was acquired from the Beijing Xingqinghong Fine Chemical Technology Co., Ltd. H2PtCl6and another Cl-containing reagent were of analytical grade purity.
2.2 Catalyst preparation
The catalysts were prepared by the incipient wetness impregnation method. The θ-Al2O3support was impregnated in the mixed aqueous solutions of H2PtCl6, HCl and another Cl-containing dispersant. All samples were air-dried at 120 ºC for 12 h. After calcination of samples in HCl and in an H2O vapour atmosphere at 510 ºC, 650 ºC, and 700 ºC, respectively, the reduction reaction occurred in H2f low; the products were denoted as Pt-510, Pt-650, and Pt-700, respectively. Then, the catalysts were preserved in bottles under nitrogen blanketing.
2.3 Characterizations
The scanning transmission electron microscopy (STEM) was used on a JEOL TEM-ARM 200F transmission electron microscope equipped with a Cs corrector on an illumination system to determine the distribution of Pt nanoparticles.
The temperature-programmed reduction (TPR) analysis was performed on a Micromeritics Autochem II 2920 chemisorption analyser with H2gas used as the reducing agent. The temperature-programmed oxidation (TPO) analysis of the spent catalysts was also performed on the same chemisorption analyzer. The dispersion of Pt was determined by temperature-programmed titration[17-19].
The Raman spectrometric analysis was performed on a JY LabRAM HR Raman spectrometer with a 325 nm laser beam. The H/C atomic ratio of the coke deposits formed on the spent catalysts was determined by an Elementar Vario Micro Cube elemental analyser. The Pt weight loading of the catalysts was analyzed on a Shimadzu UV-2401PC spectrophotometer. The Cl content of the catalysts was determined using a Metrohm 905 Titrando automatic potentiometric titrator.
2.4 Catalytic tests
Assessment of propane dehydrogenation reaction was performed in a fixed-bed stainless steel microreactor lined with quartz, the length and inner diameter of which were 750 cm and 11 mm, respectively. The microreactor was initially heated from 25 ºC to 580 ºC in a N2f low for 3 h and then heated from 580 ºC to 600 °C in a H2f low for 1 h. Propane was introduced into the reactor at this time and then the dehydrogenation reaction was initiated. Typically, the weight hourly space velocity (WHSV) of propane was 3.8 h-1, the molar ratio of H2to C3H8was 0.5, the reaction pressure was 0.22 MPa and the catalyst load was 2 g. The products were analysed by an online GC-14C gas chromatograph.
The propane rate and propylene selectivity were calculated as follows:

whereFC3H8represents the flow rate of propane.Xandmcatare the conversion of propane and catalyst loading, respectively.wPtis the Pt weight loading.[Fi]inand [Fi]outare the inlet and outlet f low rates of the hydrocarbon, respectively.
3 Results and Discussion
3.1 Characterizations of the as-prepared catalysts
The phase of support, content of Cl, and dispersion of Pt are the main factors that affect the catalytic performance of catalysts for the PDH reaction. The physicochemical properties of the as-prepared catalysts are listed in Table 1, showing that the Pt loadings and Cl contents were similar among the Pt-510, Pt-650 and Pt-700 catalysts and that all of their supports were θ-Al2O3. Thus, the effect of the dechlorination temperature on the PDH reaction could be well elucidated through a comparative investigation of these three catalysts. The dispersion of Pt nanoparticles obviously decreased with an increasing dechlorination temperature.
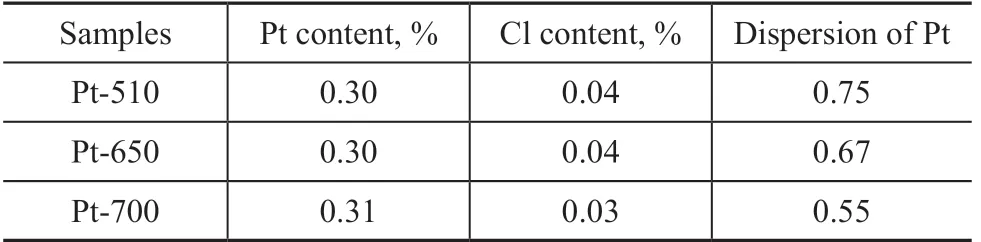
Table 1 Physicochemical properties of the as-prepared catalysts
STEM was employed to directly observe the distribution of the Pt nanoparticles. The Pt nanoparticles were uniformly distributed on the θ-Al2O3support in samples Pt-510, Pt-650, and Pt-700. The sizes of Pt nanoparticles over Pt-510 ranged from 0.6 nm to 0.8 nm and those over Pt-650 and Pt-700 ranged from 0.9—1.0 nm and 1.1—1.2 nm, respectively. The size of the Pt nanoparticles increased with an increasing dechlorination temperature, which was consistent with the dispersion results, as shown in Figure 1. This effect might be caused by the fact that the agglomeration of Pt nanoparticles could reduce the surface energy, making the Pt nanoparticles more stable in the higher temperature dechlorination process.

Figure 1 Scanning transmission electron microscopy images of the as-prepared catalysts, with the insets showing the catalyst particle size distributions
Besides, the dechlorination temperature exerted little influence on the acid properties of Pt-510, Pt-650, and Pt-700 catalysts, and the types of OHs and interactions between the Pt species and OHs were also similar among the three catalysts (Figure 2).
Generally, there were two reduction peaks for the TPR profiles. The peak located at the lower temperature region indicated a weaker interaction between the metal and the support; by contrast, the higher reduction peak indicated a stronger metal-support interaction[20]. However, there were three reduction peaks at approximately 200 ºC, 360 ºC, and 600 ºC for all three catalysts Pt-510, Pt-650 and Pt-700, as shown in Figure 2. Lee and colleagues considered that the reduction peaks at 548 ºC were attributed to the reduction of Pt oxychlorides[21]. In the present study, the Cl contents in the three catalysts were ultra-low and the corresponding dispersions of Pt nanoparticles decreased with an increasing dechlorination temperature (Table 1). Therefore, the peaks located at approximately 600 ºC might be ascribed to the reduction of Pt species, which were sintered to interact more strongly with the support θ-Al2O3. Additionally, the intensity of the reduction peaks appearing at approximately 600 ºC increased with an increasing dechlorination temperature, implying that the contents of Pt species interacting strongly with the corresponding supports could increase. Therefore, the higher the dechlorination temperature, the more difficult it would be to reduce the Pt species on the support.
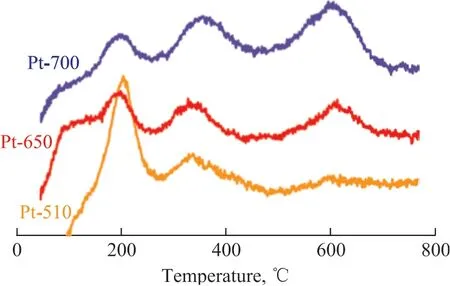
Figure 2 Temperature-programmed reduction profiles using H2 as a reducing agent for the as-prepared catalysts
3.2 Catalytic performance and characterization of spent catalysts
Figure 3 shows the catalytic activity of the as-prepared catalysts in the PDH reaction. The effect of the reactor on the reaction can be ignored as evidenced by the blank test. The results indicate that Pt-510 demonstrated the highest conversion rate of propane and propylene selectivity; conversely, Pt-700 exhibited the lowest catalytic performance. The conversion rates of propane on the three catalysts were similar, while the propylene selectivity on Pt-510 was close to that on Pt-650, both of which showed higher selectivity than that of Pt-700.

Figure 3 Reaction rate of propane (a) and selectivity of propylene (b) over the as-prepared catalysts during propane dehydrogenation (PDH) reactions
Figure 4 shows the TPO profiles of the as-prepared catalysts. It can be observed that there were two peaks located at approximately 430 ºC and 500 ºC for Pt-510, Pt-650, and Pt-700, which were consistent with the results obtained by He, et al[22]. Generally, the peak at the lower temperature represented the carbon deposits interacting with the metal, while that at the higher temperature was attributed to coke deposition on the support[23]. Correspondingly, “Area I” in Table 3 denoted the coke deposition on Pt nanoparticles, while “Area II” was attributed to coke deposits on the θ-Al2O3support. As shown in Table 3, there was a positive correlation between the amount of coke deposits on the metal and the dechlorination temperature. The greater the coverage of coke on the metal, the greater the hindrance of the contact between the active sites and the reactant; moreover, the degree of aggregation of Pt nanoparticles was similar among the spent Pt-510, Pt-650, and Pt-700 catalysts, and no severe sintering occurred on the three catalysts after PDH reaction (Figure S3). The dispersion of Pt nanoparticles decreased (Figure 1 and Table 1) and the difficulty of reducing Pt species increased with an increasing dechlorination temperature (Figure 2). Therefore, the excess coke on the metal, the low dispersion of Pt nanoparticles and even the strong interactions between Pt nanoparticles and the θ-Al2O3support could denote the lower activity of catalysts treated at higher dechlorination temperatures.
Figure 5 shows the fitted Raman spectra of the spent catalysts. Generally, the spectra that appeared at approximately 1 393 cm-1and 1 593 cm-1were assigned to the D band and G band, irrespective of the coke deposits[24-25]. All locations of the D and G bands were similar. The ratio of the area for the D band versus that for the G band (ID/IG) was used to measure the degree of graphitization of coke, and a higher value indicated a lower degree of graphitization[26]. Therefore, the degree of graphitization for coke deposition on the spent catalysts decreased with an increasing dechlorination temperature.
As shown in Table 2, the amount of coke was similar among the spent Pt-510, Pt-650 and Pt-700 catalysts, which was consistent with the results of acid properties; with an increasing size of the Pt nanoparticles (Figure 1 and Table 1), the H/C ratios of the preceding two catalysts were similar and both of these values were larger than that of the Pt-700, implying that there was a severe deep dehydrogenation reaction over the Pt-700 catalyst.

Figure 4 Fitted temperature-programmed oxidation curves of the spent catalysts

Table 2 Properties of the spent catalysts

Table 3 Curve fitting results of the temperatureprogrammed oxidation profiles over the spent catalysts

Figure 5 Fitted Raman spectra of the spent catalysts

Table 4 Curve fitting results of the Raman spectra over the spent catalysts
Additionally, theID/IGvalue increased with the increase in dechlorination temperature, suggesting that the lower the dechlorination temperature of the catalyst, the higher the degree of graphitization of the corresponding coke and the fewer the side-reactions, such as the cracking reaction.
Thus, catalysts operating at lower dechlorination temperatures possessed higher propylene selectivity for the PDH reaction.
4 Conclusions
The effect of dechlorination temperature on the hightemperature PDH reaction was investigated by designing and preparing three catalysts with similar chlorine contents and the same support, labelled as Pt-510, Pt-650, and Pt-700. With an increasing dechlorination temperature, there was little change in the acid properties, the type of surface hydroxyl groups, and the interactions between Pt species and OHs of the catalysts. However, the dispersion of the Pt nanoparticles decreased with an increasing dechlorination temperature, leading to more structure-sensitive side-reactions, such as coke deposition. The strong interactions between the metal and the support made it difficult to reduce the Pt nanoparticles, leading to the reduction of active sites. Additionally, the increase in coke deposition on the metal, along with the severe deep dehydrogenation reactions and increased cracking reactions contributed to the lower catalytic performance of Pt-θ-Al2O3operating at higher dechlorination temperature during the hightemperature PDH reaction.
Acknowledgements:The present study was financially supported by grants from the State Key Laboratory of Catalytic Materials and Reaction Engineering (RIPP, SINOPEC), the National Natural Science Foundation of China (Grant No. 21706036), the Natural Science Foundation of Fujian Province (Grant No. 2018J05019), the Fujian Educational Bureau (Grant No. JAT170073), and the Talent Foundation of Fuzhou University (Grant No. XRC-1650). The authors are grateful for the experimental assistance from Wang Chunming, Zhou Yiran, Liu Chen, and Yang Yanpeng.
杂志排行
中国炼油与石油化工的其它文章
- Selective Hydrogenation of Butyne-1,4-diol to Butane-1,4-diol over Ni/Al2O3-SiO2 Catalysts
- Mass Loss Behavior and Volatile Composition during Pyrolysis of a Bituminous Coal
- Catalytic Hydrogenation Performance of Methyl Isobutyl Ketone over Ni/γ-Al2O3 Catalysts
- Environmentally-Friendly Catalytic Oxidation of Cyclohexanone with 30% H2O2 Solution: A Comparison Study between Hollow Titanium Silicate and Dealuminated HBEA Zeolites
- Boosted Biodegradability and Tribological Properties of Mineral Base Oil by Methyl Diethanolamine Fatty Acid Esters
- A Study on the Coking Sites of γ-Alumina Surface Using 1-Methylnaphthalene as the Model Reactant
