Screening of antiproliferative activity mediated through apoptosis pathway in human non-small lung cancer A-549 cells by active compounds present in medicinal plants
2019-01-05NutanBadgujarKinnariMistryDharamshibhaiRankChaitanyaJoshi
Nutan V. Badgujar, Kinnari N. Mistry✉, Dharamshibhai N. Rank, Chaitanya G. Joshi
1Ashok and Rita Patel Institute of Integrated Study and Research in Biotechnology and Allied Sciences (ARIBAS), Affiliated to Sardar Patel University, Vallabh Vidyanagar, Gujarat 388120, India
2Department of Animal Breeding and Genetics, College of Veterinary Science and Animal Husbandry, Anand Agricultural University, Anand, Gujarat, India
3Department of Animal Biotechnology, College of Veterinary Science and Animal Husbandry, Anand Agricultural University, Anand, Gujarat, India
Keywords:Apoptosis DNA fragmentation assay ROS MTT assay Real time assay DAPI staining
ABSTRACT Objective: To explore the antiproliferative activity and apoptosis in cells caused by active compounds present in plants using different techniques. Methods: We investigated the antiproliferative effects of methanolic extracts from different parts of seven plants on A-549(lung cancer) cells and primary cell culture (chick embryo fibroblast cells, as normal cells)using MTT assay and the potent plant was fractioned further. All these fractions were screened again for anti-proliferative activity. DNA fragmentation and DAPI staining were used to study apoptosis. Quantitative real-time was used to investigate the expression of apoptoticrelated genes. LC-MS and 1H-NMR techniques were used to identify the active compounds present. EnzCheck caspase-3 assay kit was used to measure caspase-3 activity. Results:Methanolic extract of Vitex negundo (V. negundo) was selected as a potent fraction. Among all fractions screened, ethylacetate fraction of V. negundo was selected as the most potent antiproliferative fraction and phytochemical analysis of the extract revealed the presence of secondary metabolites. Ethaylacetate fraction of V. negundo was found to cause characteristic apoptotic morphological changes and generation of ROS in A-549 cells. Ethaylacetate fraction of V. negundo also induced apoptosis in A-549 which was supported by DNA fragmentation and DAPI staining. To investigate the molecular mechanism behind the cytotoxic effect of ethaylacetate fraction of V. negundo, quantitative real-time PCR was used to measure expression levels of p53, bax, bcl2, casp-3 and casp-9. Using LC-MS and 1H-NMR techniques,cytotoxic compounds (luteolin and p-hydroxy benzoic acid) were identified which increased casp-3 activity in a dose and time-dependent manner in A-549 treated cells. Conclusions: It is concluded from the present study that V. negundo is capable of triggering growth-inhibitive and apoptosis effects in A-549 cells, signifying that V. negundo may possesses anti-lung cancer activity.
1. Introduction
Cancer is one of the most serious diseases in developing world.In 2017, it caused as many as 600 920 deaths in United States according to report[1]. Lung cancer is the leading cause of cancer related deaths all over the world[2]. In 2017, 222 500 new lung cancer cases and 155 870 cancer deaths were reported in United States[1]. The two main types of lung cancer are small-cell lung carcinoma and non-small-cell lung carcinoma. Non small-cell lung cancer accounts for more than 85% of all lung cancer cases, with approximately 15% to 20% of patients presenting in early stage.In the first stage of non-small cell lung cancer, lobectomy with systematic lymphnode evaluation followed by chemotherapy, is regular treatment[3]. Post-resection survival chance is only 75%-85% of all lung cancers[4]. India recorded 556 400 cancer deaths in 2010 according to the report[5]. Drug-resistance is a major problem in these patients. So, there is a crucial need to explore new anticancer agents with higher activity and fewer chances of getting resistance against lung cancer. Medicinal plants are the reservoir of secondary metabolites. A number of plant-derived secondary metabolites have been developed into more effective and less toxic medicines[6]. Plants, vegetables and herbs used in folk and traditional medicine have been accepted currently as one of the main sources of cancer prevention drug discovery and development.Secondary metabolites possess anticancer effect and therefore they have been used in cancer treatment. Chemoprevention is one of the prominent approaches to treat cancer. Synthetic or natural agents(alone or in combination) are used to block the development of cancer in human. Drugs used in the cancer treatment have shown side effects such as cardiotoxicity, nephrotoxicity etc. From the natural sources, varied number of chemical structures have been isolated, viz. podophyllotoxin, camptothecin, vinblastine, vincristine,taxol, combretastatins, etc[7-9]. Additional changes in these leads have resulted in therapeutically useful drugs including topotecan,irinotecan, taxotere, etoposide, teniposide, etc[7-11]. Apoptosis is a well regulated and organized death process. It occurs under a variety of physiological and pathological conditions that control the development and homeostasis of multicellular organisms.The apoptotic features comprise cellular morphological change,membrane blebbing, chromatin condensation, cleavage of DNA and caspase family activation[12,13]. Activation of caspase is considered to be a key trademark of apoptosis. Hence, the current study focused on screening anticancer abilities of selected plants [Vitex negundo(V. negundo), Lantana camara (L. camara), Bauhinia variegata (B.variegata), Bauhinia racemosa (B. racemosa), Bauhinia purpurea (B.purpurea), Argyreia nervosa (A. nervosa) and Butea monosperma (B.monosperma)] against A- 549 cells (lung cancer cells) and normal cells. Based on the screening results, role of V. negundo, in apoptosis induction and its molecular mechanism behind apoptosis, was investigated through different methods.
2. Materials and methods
2.1. Chemicals
All chemicals of analytical grade were purchased from Hi-Media and Merck, India. Standard drugs were purchased from Sigma-Aldrich Chemicals. For in vitro experiments, media like DMEM(D5523) and fetal bovine serum (FBS-26140079) for growing cells were purchased from Gibco (USA). Trypsin (25200056) and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTTM5655) were purchased from Sigma Aldrich Company, USA.
2.2. Plant materials
The fresh sample of selected medicinal plants were collected from Pune, in August 2013 under the guidance of a botanist and all materials were authenticated by Dr. Subhash Sadhu Deokule, Head of Botany Department, Savitribai Phule Pune University.
2.3. Cell line
In our study, A-549 cell line was procured from NCCS Pune, India.The cells were cultivated in Dulbecco’s modified eagle medium(DMEM) having 1% (v/v) penicillin streptomycin solution (gibco-15070063) and 10% (v/v) FBS obtained from Gibco (USA). These cells were kept in CO2incubator.
2.4. Preparation of methanolic extracts of selected plants
The selected plants (V. negundo, L. camara, B. variegata, B.racemosa, B. purpurea, A. nervosa and B. monosperma) were washed,shade dried and crushed to get fine powder using a grinder mixer. A total of 10 g of plant powder was dissolved in 200 mL of methanol solvent overnight at room temperature; the next day, the supernatants were filtered by Whatman filter to prepare methanolic extract. For methanolic extract, the filtrate obtained was further concentrated in rotary evaporator (at 40 ℃-50 ℃) under reduced pressure and termed as methanolic extract. The extraction process was repeated three times at different time periods. But no major variation in the percentage yield and content of phytoconstituents were observed.Percentage yield was measured for extraction method. For future assays, plant extracts were kept at –20 ℃ in airtight containers.A total of 10 mg/mL stock solutions were prepared in dimethyl sulfoxide (DMSO) and dilutions were prepared using DMEM medium. Test samples were filtered using 0.22 μm syringe filters(Axiva, Scichem biotech).
2.5. Evaluation of extracts cytotoxicity in normal cells
MTT test was used to evaluate the toxicity of the plant extracts (V.negundo, L. camara, B. variegata, B. racemosa, B. purpurea, A. nervosa and B. monosperma) on primary cell culture [chick embryo fibroblast cells (CFC)]. A total of 1×106cells were seeded in 96 well plates to test toxicity of methanolic extracts toward CFC. After 24 h of incubation,cells were adhere to the plate, and then medium was replaced by medium having different concentration of plant extracts. To measure the viability of cell, MTT assay was used. Purple formazan crystal was formed after reaction was measured at wavelength of 570 nm.Results were calculated in percentage of cellular viability of the test samples.
2.6. Cytotoxicity assessment in cancerous cells
Cytotoxicity assay against human cancer cell line was performed by the MTT method[14]. Methanolic extracts of selected plants (V.negundo, L. camara, B. variegata, B. racemosa, B. purpurea, A. nervosa and B. monosperma) were evaluated for their cytotoxicity against human lung carcinoma cells (A-549). The cells were cultivated in DMEM medium having 10% FBS. In 96-well plates, 1×104cells/well were seeded and treated with varying concentrations (25-500 μg/mL)of test samples for 24 h. Briefly, the cells were added in plates for 24 h and then co-incubated with samples at 37 ℃ for 24 h. Then 20 μL of MTT (5 mg/mL, Sigma) reagent was added. After incubating for 4 h, the medium was discarded and the violet formazan crystals in viable cells were dissolved in DMSO. Absorbance at 570 nm was measured using ELISA plate reader (Promega, USA). The inhibitory rate of cell proliferation was determined using following formulae[15]:

Where O.D.= Optical density
2.7. Partial purification of V. negundo
Based on the results, methanolic extract of V. negundo was selected and bioassay guided liquid-liquid separation was done using separating funnel. Dried powder of V. negundo leaves (1.5 kg) were grounded and extracted with methanol at room temperature. The solvent was evaporated under vaccum to get methanolic crude extract(yield 6.40%). V. negundo’s methanol extract was then suspended in water and fractioned sequentially with n-hexane, chloroform,ethylacetate, butanol and water. Each fraction was evaporated in vacuum to yield the residue of hexane extract (hexane extract:4.50%; chloroform extract: 5.25%; ethylacetate extract:4.90%;butanol extract: 8.40%; water extract: 12.60%). For cytotoxic study against cancerous cells and normal cell, different fractions of V.negundo were suspended in DMSO and dilutions were prepared using DMEM medium. Then, test solutions were filtered through a 0.22 μm membrane filter and used as stock solution for future studies.
2.8. Phytochemical analysis
Methanolic and ethylacetate extract of V. negundo was studied for presence of phytochemical constituents using procedures described by Talla et al.[16] with slight modification.
2.9. Total phenolic and flavonoid determination
Total phenolic content in methanol and ethylacetate extract of V.negundo was determined by Folin-Ciocalteu method[17]. Phenolic content was measured in μg of GAE/mg of dry extracts. Flavonoid content was calculated as described previously[18]. Total flavonoid content was measured in μg of QE/mg of extract.
2.10. Methodology for reactive oxygen species (ROS)determination
ROS generation in A-549 cells was measured by using the cell permeable fluorescent dye 2’,7’-dichlorofluoresceindiacetate(DCFH-DA). Upon entering the cell, the diacetate bond of the fluoroprobe is cleaved by intracellular esterases leaving DCFH which is oxidized to DCF by the oxidants and its fluorescence was taken as an indicator of ROS production in the cell. For microscopy,cells were plated onto the 6 well plates at desired density (seeding density 1××105). After 6 h incubation with V. negundo’s ethaylacetate extract cells were stained with DCFH-DA (50 μM). Cells were set aside in dark for 30 min and observed under fluorescent microscopy.
2.11. Measurement of intracellular ROS levels
Intracellular ROS production was quantified using the fluorescent probes 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA,Himedia). After treatment with ethaylacetate fraction of V. negundo,the cells were harvested, suspended in phosphate-buffered saline(PBS) and then incubated with 10 μM H2DCFDA for 20 min in the dark. The fluorescence intensity was measured by a flow cytometer(Promega) at 488 nm excitation wavelength and 530 nm emission wavelength[19]. ROS production was expressed as % increase in fluorescence relative to control cells.
2.12. DAPI staining
A-549 cells were grown on coverslips for 24 h and then treated with ethaylacetate fraction of V. negundo for different time. Then medium was removed and cells were washed with PBS and fixed with acetone at −20 ℃ for 30 min. DAPI solution (1 μg/mL) was used to stain fixed cells. The cells were again washed twice with PBS and analyzed under fluorescence microscope (Nikon).
2.13. DNA fragmentation assay
In apoptosis, activation of nuclear endonuclease occurs and cleaves deoxyribonucleic acid into small fragments which can be observed as a ladder in agarose gel electrophoresis. A-549 cells were seeded in 6 wells plates and kept the plate in CO2incubator. Cells were treated with IC50value ethaylacetate fraction of V. negundo for 24 and 48 h.After incubation period, the cells were centrifuged for 1 500 rpm for 10 min at 14 ℃. The pellet was resuspended in a lysis buffer for 10 s. (1% NP-40, 50 mM Tris-HCL, 10 mM NaCl, 20 mM EDTA,pH 7.5). Supernatant was collected and extraction was repeated with lysis buffer. The supernatant was brought to 1% SDS and treated for 2 h with RNAase-A (5 μg/mL) at 56 ℃ followed by digestion with proteinase K (2.5 μg/mL) for at least 2 h at 37 ℃. After addition of half volume of 10 M ammonium acetate, the DNA was precipitated with 2.5 volume of ethanol. DNA samples were electrophoretically separated on 1.8% agarose gel containing ethidium bromide (0.4 μg/mL). Untreated cells were used as control. Campothecin was used as positive control.
2.14. Gene expression studies
Gene expression studies were carried out using real-time q-PCR analysis. Briefly, 90%-95% confluent cells were added in 6 well plates and incubated for 24 h. After 24 h, cells were treated with ethaylacetate fraction of V. negundo at its IC50value and incubated further for 4, 16 and 24 h. Total RNA was isolated from ethaylacetate fraction of V. negundo treated and untreated A-549 cells by using the Trizol reagent (Invitrogen, USA). cDNA was prepared using revert aid first strand cDNA synthesis kit(Fermentas, EU). After reverse transcription, cDNA was used for quantitative PCR using gene specific primers for p53, bax,bcl2, casp-3 and casp-9. cDNA amplification was carried out by following PCR conditions: 95 ℃ (10 min), 95 ℃ for 10 s (40 cycles) and 60 ℃ (1 min); holding at 4 ℃ for each of p53, bax, bcl2,casp-3, -9 and gapdh (internal control) gene. Primer sequence for 1) p53 gene (forward primer-TGCTCAAGACTGGCGCTAAA;reverse primer-CAATCCAGGGAAGCGTGTCA); 2) bax gene(forward primer-CAGAGGATGATTGCCGCCG; reverse primer-AAAAGGGCGACAACCCGGCC); 3) bcl2 gene (forward primer-TTTGTGGAACTGTACGGCCC; reverse primer-GTTGACTTCACTTGTGGCCC); 4) casp-3 gene (forward primer-TGTGAGGCGGTTGTAGAAGA; reverse primer-GCACACCCACCGAAAACCAG); 5) casp-9 gene (forward primer- CAGGCCCCATATGATCGAGG; reverse primer-TCGACAACTTTGCTGCTTGC); 6) gapdh gene (forward primer-TGGTATCGTGGAAGGACTCA; reverse primer-ATGCCAGTGAGCTTCCCGTT). To normalize the expression of the apoptotic genes, as an internal reference gapdh gene was used.
2.15. Extraction and isolation
Ethaylacetate fraction of V. negundo which showed potent antiproliferative activity against (non-small cell lung cancer) A-549 cells was subjected to further purification using column chromatography.Column chromatography was passed out on silica gel G (60-120 mesh) above which residue (2 g) was adsorbed on silica gel (4 g)and packed on a silica gel column (25 g) which was pre-equilibrated with n-hexane. Elution was carried out using different solvents like:n-hexane, n-hexane and ethylacetate mixture in different ratios, ethyl acetate, a mixture of ethylacetate and methanol in different ratios and methanol. The fractions with similar Rfvalues in the same solvent systems were pulled together. These were subjected to thin layer chromatography (TLC) in different solvent systems. Preparative TLC was used and two single spots obtained were scraped which was recrystallized using methanol. Structure was confirmed using LC-MS and1H-NMR.
2.16. Caspase-3 activity assay
Cell apoptosis was studied by measuring caspase-3 activity. Briefly,1×106A-549 cells per well in 12-well plates were incubated with IC50value of luteolin and p-hydroxybenzoic acid (PHBA). After 24 h, the cells were collected and processed according to EnzCheck caspase-3 assay kit #1 instructions. EnzChek caspase-3 assay kit#1 was purchased from Invitrogen. Fluorescence microplate reader was used to measure fluorescence using excitation at 342 nm and emission detection at 441 nm.
2.17. Statistical analysis
Data were expressed as the mean±SD of three independent experiments. The statistical differences were calculated in the Student’s t test and P<0.05 was considered statistically significant.GraphPad PRISM software version 5.0 (GraphPad Software, USA)was used for calculation and in all comparisons.
3. Results
3.1. Cytotoxicity assessment of selected plants
The crude methanolic extracts of selected plants were screened for cytotoxicity against A-549 and CFC. As shown in Table 1 and 2, all methanolic extracts except A. nervosa root showed considerable concentration-dependent inhibition of the A-549 cancer cells and CFC cells. But according to the results, V. negundo, L. camara, B. racemosa, B. monosperma were more cytotoxic on A-549 cells than CFC normal cells.
For every extract we calculated IC50value and selective index(SI, SI=IC50for normal cells/IC50for cancer cells)[20]. Among the above mentioned methanol extracts that have selective cytotoxicity against cancer cells compared to normal cells, V. negundo’s methanol extract showed IC50values of (240.12±1.33) μg/mL and(443.25±2.08) μg/mL against A-549 cells and CFC, respectively, the highest SI was obtained in V. negundo (1.85) too (Table 3).
Hence, V. negundo’s methanol extract was selected for partial purification and their fractions were again screened for its cytotoxic activity on A-549 and CFC, respectively.
3.2. Cytotoxicity assessment of different fraction
Among all fractions, ethylacetate fraction at all tested concentrations were more toxic towards A-549 cells but less toxic towards CFC normal cells (Table 4). IC50and SI values of partial purified fractions against A-549 cells (hexane extract, chloroform extract, ethaylacetate extract, butanol extract and water extract) and CFC cells were shown in Table 5. The highest SI was obtained in ethylacetate fraction(2.60)(Table 5). Hence ethaylacetate fraction of V. negundo was chosen for more biological study.All values are expressed as mean±SD (n=3; in triplicates). HE- Hexane extract, CE- Chloroform extract, EAE- Ethylacetate extract, BE- Butanol extract, WEWater extract.

Table 1 Cytotoxic effect of methanolic extracts of different plants against A-549 cell line using MTT assay (% inhibition).

Table 2 Cytotoxic effect of methanolic extracts of different plants against CFC using MTT assay (% inhibition).
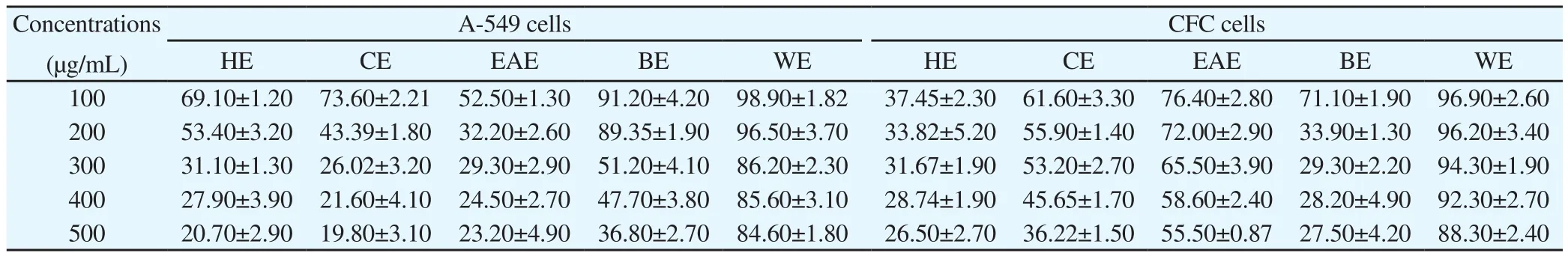
Table 4 Cytotoxic effect of partially purified fractions of V. negundo against A-549 (lung cancer cells) and CFC using MTT assay (% inhibition).

Table 3 IC50 value against A-549 and CFC cells for all nine plant extracts used in the study.
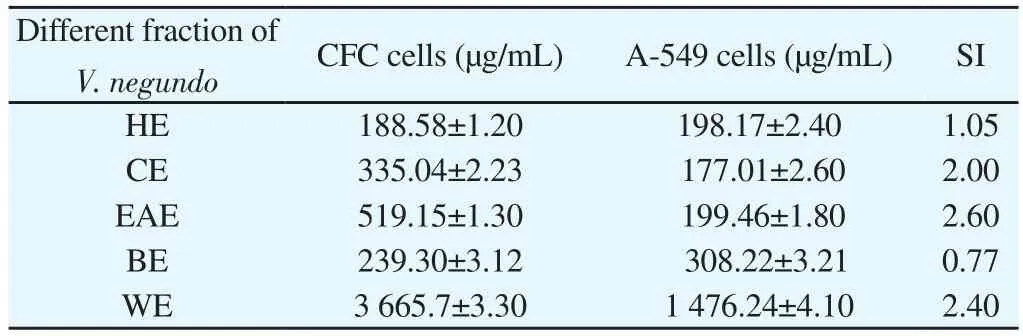
Table 5 IC50 value for different fraction of V. negundo against A-549 and CFC cells.
3.3. Phytochemical analysis
V. negundo’s methanol extract and V. negundo’s ethaylacetate extract showed the presence of steroid, alkaloids, flavanoids, tannins,saponin and terpanoids. Anthraquinone was absent in methanolic and ethylacetate extract of V. negundo.
3.4. Total phenolic and flavanoid content
The total phenolic content in methanolic and ethyl acetate fraction of V. negundo was (175.50±2.20) mg GAE/g dw and (205.00±1.50)mg GAE/g dw. On the other hand, the total flavanoid content in methanolic and ethylacetate fraction of V. negundo was (26.80±3.80)mg QE/g dw and (38.80±2.20) mg QE/g dw.
3.5. Oxidative stress by DCFH-DA
A-549 cells were treated with ethylacetate fraction of V. negundo for 6 h and then treated with DCFH-DA and were observed under fluorescent microscopy at a magnification of 20× (Figure 1A and B).High intensity of fluorescence was observed in the cells treated with ethylacetate fraction of V. negundo compared to the control cells indicating oxidative stress was induced. It provided a qualitative index of the overall oxidation status of a cell (Figure 1).

Figure 1. Cells observed under fluorescent microscopy at a magnification of 20.
3.6. Measurement of ROS level
ROS production was measured in the lung cancer cells (A-549)treated with ethylacetate fraction of V. negundo (0, 100, 300, 400,500 μg/mL) using DCFH-DA. A-549 cells treated with DMSO and H2O2were served as vehicle control and positive control. There was no significant difference observed in ROS generation between untreated cells [(213.00±1.40)%] and DMSO [(215.00±2.40)%]treated A-549 cells, whereas, highly significant increase in ROS production was observed in cells treated with H2O2[(313.3±2.89)%].At lower concentration (100 μg/mL), the ROS production was lower[(209.00±2.50)%], at intermediate concentration (300 μg/mL), the ROS production was [(279.0±1.70)%] but as concentration increased to 500 μg/mL, significant increase in ROS [(318.00±1.34)%] was observed comparing with untreated cells. A constant enhancement in ROS level has been concerned in the pathogenesis of cancer,atherosclerosis, diabetes mellitus, neurodegenerative diseases and rheumatoid arthritis.
3.7. DAPI staining
To detect nuclear condensation and apoptotic bodies, A-549 cells were stained with DAPI. In Figure 2, untreated cells were observed having round intact nuclei. In comparison, cells treated with IC50value of ethaylacetate fraction of V. negundo for 24 h and 48 h showed condensed nuclei and formation of apoptotic body indicating chromosome condensation was increased by ethaylacetate fraction of V. negundo (Figure 2).
3.8. Apoptosis induction
Apoptosis is a type of programmed cell death recognized by condensation of cytoplasm and blebbing of plasma membrane,leading to breakdown of nuclear DNA into multiples of ~200 bp fragments (Figure 3). Many phytochemical compounds can inhibit the growth of tumor cells, but not all of them can trigger apoptosis.Apoptosis was confirmed by the detection of internucleosomal DNA cleavage on agarose gel electrophoresis. Cells treated with ethaylacetate fraction of V. negundo at their IC50value for 24 and 48 h showed fragmented DNA in agarose gel electrophoresis which confirmed antiproliferative effect of ethaylacetate fraction of V.negundo.

Figure 2. Cells visualized under a fluorescence microscope (20×magnification) after staining with DAPI.
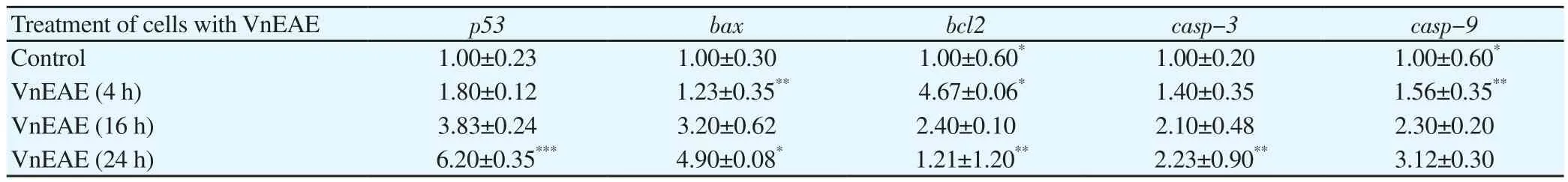
Table 6 Fold change in expression of apoptosis-related genes against controls over 4, 16 and 24 h.

Figure 3. Agarose gel electrophoresis (1.8%) of the chromosomal DNA extracted from A-549 cells.
3.9. Real time qRT-PCR result
To explain the method of apoptosis induced by ethaylacetate fraction of V. negundo, mRNA expression of p53, bax, bcl2, casp-3 and casp-9 was studied using qRT-PCR. In our study, gapdh was chosen as the endogenous control gene. Therefore, gapdh was kept as a reference gene; changes in the expression levels of p53, bax,bcl2, casp-3 and casp-9 after treatment with ethaylacetate fraction of V. negundo (IC50value) were compared and studied. Compared with the control group gene, the mRNA levels of p53, bax, casp-3 and casp-9 genes increased with time for cells treated with IC50value of ethaylacetate fraction of V. negundo (Table 6). bcl2 gene expression level decreased with increasing time. These results indicated that the increased expression of casp-3 and casp-9 may contribute to apoptosis induced by ethaylacetate fraction of V. negundo.
3.10. Isolation of compound
3.10.1. Compound-1
Molecular weight of 3’,4’,5, 7-tetrahydroxy flavone or luteolin is 286.24. As shown in Figure 4, retention time (RT) was obtained around 1.543. Molecular ion peak is obtained on 285.28 which is(n-1)-1of luteolin (Figure 5).1H NMR spectrum was recorded in DMSO at 400 MHZ.1H NMR spectrum of compound: 0.895-0.920(1H, d). 2.032 (1H, s), 4.092-4.145 (1H, m), 6.229 (1H, d), 6.458-6.464 (1H, d), 6.563 (1H, s), 6.911-6.932(1H-t), 7.396-7.421 (2H, t)(Figure 6).
3.10.2. Compound-2
Molecular weight of PHBA is 138.12. As shown in Figure 7, RT was obtained around 1.390. Molecular ion peak was obtained on 137.12 which is (n-1)-1of PHBA (Figure 8).1H NMR spectrum was recorded in DMSO at 400 MHz.1H NMR spectrum of compound:12.429 (1H, s), 10.220 (1H, s), 7.773-7.802 (2H, d-d), 6.804-6.827(2H,d-d) (Figure 9).

Figure 4. HPLC analysis of luteolin.

Figure 5. LC-MS analysis of luteolin.

Figure 6. 1H NMR spectrum of luteolin compound in DMSO at 400 MHz.

Figure 7. HPLC analysis of PHBA.

Figure 8. LC-MS analysis of p-hydroxyl benzoic acid.
3.10.3. Cytotoxicity study of compound 1 & 2
Luteolin and PHBA were studied for their cytotoxicity against lung cancer cells A-549. In Table 7, both compounds inhibit the cancer cell in concentration dependent manner. IC50value for luteolin and PHBA were (43.65±2.30) μM and (53.82±1.40) μM,respectively.
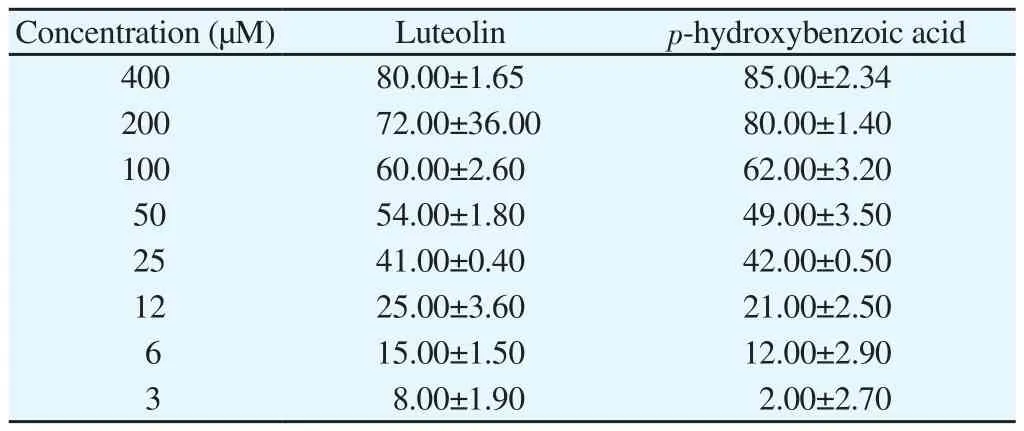
Table 7 Cytotoxic study of compound 1 and 2 (luteolin and p-hydroxybenzoic acid)against lung cancer cells (A-549) assessed using MTT assay (% inhibition).

Figure 9. 1H NMR spectrum of p-hydroxyl benzoic acid in DMSO at 400 MHz.
3.11. Casp-3 activity assay
Casp-3 plays a significant role in proteolytic cleavage of proteins which cause their structural loss, actions and eventually death of cells. The casp-3 activity in the luteolin and PHBA treated cells increased with time (in 12 h and 24 h) in treated cells over that of untreated control cells. Cells exhibited considerable raise of casp-3 activity in a time dependent manner (Table 8). This clearly shows that the growth-inhibitory activity of the luteolin and PHBA could be attributed to apoptosis induction through casp-3 activation. The activation of casp-3 activity suggests that its cytotoxicity was caused by the induction of apoptosis.
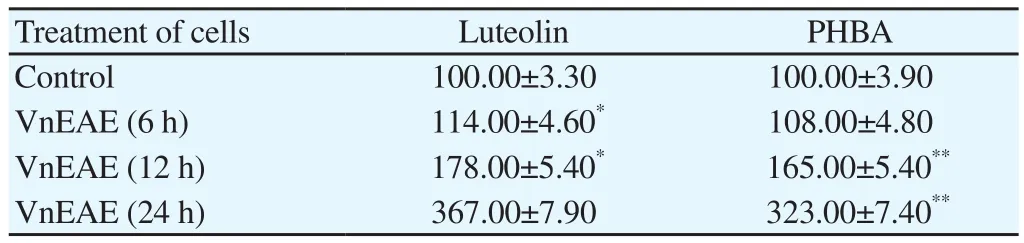
Table 8 Detection of casp-3 activity in A-549 (lung cancer) cells using the EnzChek Casp-3 assay Kit #1 with Z-DEVD-AMC substrate (%).
4. Discussion
From the earlier studies, it was reported that new effective cancer chemopreventive and chemotherapeutic agents are derived from natural origin. Conventional medicine suggests an attractive idea for searching raw material for drug discovery[21]. This is the inspiration of the present study which is to explore medicinal plants in different parts of India and to identify plants which may possess apoptosis and cytotoxic activity.
In current study, anti-proliferative activities of methanolic extracts of selected plants were tested against lung cancer cells (A-549)and primary culture of CFC was used as normal cell. Based on SI value, we selected V. negundo for further extraction process. In the present study, we have also employed bioassay guided fractionation to isolate the most potent cytotoxic fraction from the leaves of V.negundo. Partial purification from leaves of V. negundo was done in the different solvent system and each fraction was checked for its cytotoxic activity against cancerous and normal cells. We calculated the IC50value for all selected fractions against A-549 and CFC. For every extract we calculated SI value and highest SI was obtained in ethaylacetate extract fraction and it was selected for further analysis.
The current results revealed that ethaylacetate fraction of V.negundo had an antiproliferative effect on A-549 cells in a time and concentration dependent manner. Without causing unnecessary damages to normal cells, a good anti-tumor agent should destroy tumor cells, sense least side effects and this perfect situation is achieved by causing apoptosis. Active compound should provide a valuable suggestion for their potential applications in cancer treatment by knowing their modes of action[22]. To evaluate the antiproliferative and apoptotic effects of V. negundo extract on cancer cells several methods were used.
Phytochemical analysis of V. negundo’s methanol extract and V. negundo’s ethaylacetate extract showed a presence of steroid,alkaloids, flavanoids, tannins, saponin and terpanoids. Presence of these secondary metabolites also attributes to cytotoxic effect.The total phenolic content in V. negundo’s methanol extract and V. negundo’s ethaylacetate extract was (175.50± 2.20) and(205.00±1.50) mg GAE/g dw. On the other hand, the total flavonoid content in V. negundo’s methanol extract and V. negundo’s ethaylacetate extract was (26.80±3.80) and (38.80±2.20) mg QE/g dw. Phenolic extracts of plant materials have been shown to neutralize free radicals in various model systems and play a role in anticancer activity[24]. Flavonoids consist of the most well-known and varied group of polyphenolic plant secondary metabolites. These compounds play an important role in various biological and chemical activities[5].
In current study, the results indicated that ethaylacetate fraction of V. negundo’s ethaylacetate extract treatment induced an increased attack of intracellular ROS after treatment in A-549 cells. Literature reports indicate that DNA is sensitive to ROS-induced oxidative injury and that DNA is the most frequent target of cytoprotection.The chemotherapeutic drugs are proved to produce high ROS in cancer cells and high ROS has been considered to be a reason for DNA damage[25]. In the present study, we showed that ROS levels increased transiently in A-549 cells after ethaylacetate fraction of V. negundo treatment, followed by apoptosis. Results obtained from other groups suggested that the increase in ROS level activates the JNK pathway and finally activates caspases[26]. Increases in ROS levels cause anomalous signaling pathways and expression of the gene, hence cause tumor inhibition by induction of apoptosis in affected cells whereas leaving normal cells intact[27].
Since apoptotic cells showed some typical morphological features,nuclear changes were studied using DAPI staining. After 24 h and 48 h of treatment with ethaylacetate fraction of V. negundo, A-549 cells demonstrated signs of chromatin condensation and nuclear shrinkage. In DAPI staining, morphological examination revealed cell shrinkage in ethaylacetate extract of V. negundo treated A-549 cells. This indicated that A-549 cells underwent apoptosis when treated with ethaylacetate fraction of V. negundo. It was reported that the loss of surface attachment is an early sign of apoptosis once in touch with an apoptotic inducing agent. The method behind many anticancer drugs are based on their capability to induce apoptosis.A-549 cells treated with ethaylacetate fraction of V. negundo showed the presence of fragmented DNA which confirmed antiproliferative activity of ethaylacetate fraction of V. negundo compared to control cells. Programmed cell death is both biochemically and morphologically different from the process of necrosis. Apoptosis is characterized biochemically by fragmentation of the genome and cleavage or degradation of several cellular proteins including the activation of caspases through extrinsic or intrinsic mitochondrial pathways[28].
Result of qRT-PCR technique showed considerable increase of p53,bax, casp-3, casp-9 gene expression, on the other hand, decrease in bcl2 mRNA expression. In the intrinsic pathway of apoptosis, antiapoptotic and pro-apoptotic proteins are key regulators by controlling the main checkpoint and setting the threshold for maintaining death mechanism[29]. The ratio of bax to bcl2 determines the vulnerability of cells to death signals according to previous reports[30]. For that reason, Bcl2 proteins have been used as a major target for the expansion of new anticancer agents[31]. According to earlier reports,changes in bcl2/bax occurs due to decrease in bcl2 expression and increase in bax expression[32,33]. In present study, bcl2 expression was considerably inhibited while p53 and casp-3 and casp-9 expressions were increased depending upon their concentration.Apoptosis can be triggered through two main signaling pathways:extrinsic pathway mediated by the death receptor and intrinsic pathway mediated through the mitochondrion. In mitochondrionmediated apoptosis, family of caspase gene play a essential role[34].Casp-3 is a frequently activated protease in mammalian cell apoptosisviathe activation, proteolysis and hydrolysis of particular substrates such as DNA-dependent protein kinases[35]. Casp-3 is activated by casp-9. Our qRT-PCR study showed that ethaylacetate fraction of V. negundo increased the casp-3 and casp-9 gene expression, suggesting that ethaylacetate fraction of V. negundo’s ethaylacetate extract stimulates apoptosis via a caspase-dependent pathway. Our results also demonstrate that the ethaylacetate fraction of V. negundo’s ethaylacetate extract induces apoptosis by regulating genes involved in apoptosis.
From ethylacetate fraction, luteolin and PHBA were isolated using column chromatography technique. Both compounds showed cytotoxic activity against A-549 cells. From the leaves of V. negundo, a bioactive flavanoid isolated and characterized was luteolin. In conclusion, the series of proceedings is well-matched with the assumption that luteolin activates the mitochondrial pathway of apoptosis, possibly by causing DNA damage. Seidel et al identified three novel 4-hydroxybenzoic acid derivatives as panhistone deacetylase inhibitors that increased protein acetylation levels, arrested cell cycle progression and triggered apoptotic cell death, without affecting the viability of normal cells[36]. In 2005,Pugazhendhi D and his colleagues studied that PHBA can give oestrogenic responses in human breast cancer cells[37].
In recent times, several chemotherapeutic drugs have been shown to trigger apoptotic pathways which lead to drug-induced cell death and the activation of caspases plays an essential task in the biological actions related with apoptosis. In particular, casp-3 has been reported as the executioner upon which many apoptotic pathways converge[38]. Therefore, we studied the activity of casp-3 in the treated cells using a casp-3 specific substrate, Z-DEVD-AMC,which is cleaved to afford a fluorescent product. It was shown that casp-3 activity was elevated with increasing time in the treated cells.Although the active constituents isolated from the V. negundo, its activity has not been examined to a large amount. Bioactivity-guided isolation of bioactive compounds needs to be done.
To conclude, in the present study, it was observed that V. negundo was capable of triggering growth-inhibitive and apoptosis effects in A-549 cells, signifying that V. negundo may possesse anti-lung cancer activity with lower IC50value for lung cancer cells compared to normal cells, which might be a new and striking remedial contender for cancer treatment in clinical practice. The current findings will give precious information for its potential application in lung cancer treatment in the upcoming years. Based on the present study, researchers can translate these compounds to in vivo cancer study by using different animal models. The subject area of this animal model will spread out the doorways for the researchers to find out the exact mechanism of action of the active compound as one of the potential candidates for the cancer treatment.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Salacca zalacca: A short review of the palm botany, pharmacological uses and phytochemistry
- Immune enhancement effect of an herb complex extract through the activation of natural killer cells and the regulation of cytokine levels in a cyclophosphamide-induced immunosuppression rat model
- Phytocompounds of Anonna muricata leaves extract and cytotoxic effects on breast cancer cells
- Ultrasound-assisted extraction of antioxidant polyphenolic compounds from Nephelium lappaceum L. (Mexican variety) husk
- High resolution melting real-time PCR detect and identify filarial parasites in domestic cats
- Can miRNA712_3p be a promising biomarker for early diagnosis of toxoplasmosis?
