Immune enhancement effect of an herb complex extract through the activation of natural killer cells and the regulation of cytokine levels in a cyclophosphamide-induced immunosuppression rat model
2019-01-05SungMinWooWooRinChoiDoolyJangChunSikYiHaeLimKimKyungHyeonKimJongTaeKimWonHeeChoiSeungHeeJangMinJeungKimJiHyangWeeYeonKiKimBaoLeSeungHwanYangJooWonSuh
Sung Min Woo, Woo Rin Choi, Dooly Jang, Chun Sik Yi, Hae Lim Kim, Kyung Hyeon Kim, Jong Tae Kim, Won Hee Choi, Seung Hee Jang, Min Jeung Kim, Ji Hyang Wee, Yeon Ki Kim, Bao Le, Seung Hwan Yang✉, Joo Won Suh,✉
1Interdisciplinary Program of Biomodulation, Myongji University, Yongin, Gyeonggi 17058, Republic of Korea
2Center for Nutraceutical and Pharmaceutical Materials, Myongji University, Yongin, Gyeonggi 17058, Republic of Korea
3Teazen Co., B-606 Acrotower, 230 Sinim-daero, Dongan-gu, Anyang-si, Gyeonggi-do 14067, Republic of Korea
4Department of Food Science & Biotechnology, Shin Ansan University, Ansan, Gyeonggi 15435, Republic of Korea
5Division of Bioscience and Bioinformatics, Myongji University, Yongin, Gyeonggi 17058, Republic of Korea
6Department of Biotechnology, Chonnam National University, Yeosu, Chonnam 59626, Republic of Korea
Keywords:Immune system enhancement Cyclophosphamide Cornus officinalis Sieb Et Zucc Eriobotrya japonica Lindley Olive leaves
ABSTRACT Objective: To investigate the effects of a herb complex extract (HCE) prepared from Cornus officinalis Sieb. Et Zucc., Eriobotrya japonica Lindley, and olive leaves on immune response of mouse spleen NK cells in vitro and in vivo analysis. Methods: The activity of natural killer(NK) cells was measured in splenocytes and YAC-1 cells. Mice were immunosuppressed using cyclophosphamide (5 mg/kg body weight). Three different doses of HCE (200, 400, and 800 mg/kg body weight) and red ginseng extract (800 mg/kg body weight) which was used as standard immunomodulatory herb were administered orally for 4 weeks. The body weight,dietary, water intake, organs (liver, thymus, and spleen) weight, completed blood count, and cytokines (tumor necrosis factor alpha, interferon gamma, and interleukin-2) production was measured. Results: At the maximum concentration of HCE, the activity of NK cells was increased by 48.5%. HCE increased liver, spleen, and thymus weights without altering numbers of white blood cells, lymphocytes, and neutrophils in a cyclophosphamide-induced immunosuppression rat model. However, HCE recovered the inhibited cytokine expression;HCE (800 mg/kg) increased cytokines levels. The results indicate the immune enhancement potential of this HCE. Conclusion: The HCE enhances immunity by increasing NK cell activity, regulating cytokine levels, and maintaining spleen weight. Therefore, it may be used as a potential immunity enhancer.
1. Introduction
Immune system enhancement is an area of increasing interest.Natural products are being used as alternative therapy for health care and medicinal purposes. Over the past decade, studies have recognized several important aspects of immune systems to protect the body against infection[1]. Emergence of HIV/AIDS, severe acute respiratory syndrome, Ebola hemorrhagic fever, and Zika virus-related encephalopathy has drawn attention to research on immunity-related infections[2]. The immune system is responsible for the identification and elimination of detrimental stimuli and tumor cells[1]. Suppression of the immune system may lead to the pathogenesis of many infections or cancers. A number of literature evidences indicate that herbal medicines were derived from traditional plants which enhance immune response of patients with breast, lung, gynecolical cancers when the host defense mechanism is burdened under chemotherapy[3-5].
Natural killer (NK) cells are lymphocytes that are critical to innate and adaptive immunity[6]. NK cells mainly induce cytotoxicity and promote cytokine production[7]. Cytokines are immune signalling molecules that stimulate the maturation of dendritic cells, active monocytes, and cytotoxic T cells, or induce activation of B cells to enhance the immune response[8]. Activated NK cells produce various pro-inflammatory cytokines such as interferon gamma (IFN-γ),tumor necrosis factor alpha (TNF-α), and interleukin (IL)-2 that contribute to the elimination of abnormal cells[9,10]. IFN-γregulates major histocompatibility complex-1 expression to further activate CD8+T cells to recognize tumor cells[11]. TNF-α acts as a tumorpromoting factor and enhances the production of IL-1β and IL-6 and stimulates nuclear factor-kappa B and signal transduction and activation of transcription inflammatory cascades[12]. IL-2 increases T cell proliferation and TGF-β production by NK cells[13,7]. Risky behaviors stimuli such as alcohol and tobacco abuse, antibiotics,chemotherapy, birth control pills, and other drug therapies can suppress immunity, particularly NK cell activity, which may further result in cancers or infections[14].
Herbs have long been used to prevent and treat many diseases.The beneficial effects of the different herbs are due to their active compounds composition and content[15]. Therefore, several formulas comprising a mixture of different herb types exert various pharmacological effects with less side effects[16]. Cornus officinalis(C. officinali) Sieb. Et Zucc. (Cornaceae) is a deciduous tree native to eastern Asia and first recorded in Shen Nong’s Materia Medica(Shen-Nong-Ben-Cao-Jing) about 2 000 years ago for the treatment of kidney diseases and diabetes[17]. Long-term use of C. officinalis may regulate the immunity and provide renal and neural protection.It contains abundant triterpenoids, iridoids, flavonoids, tannins,organic acids, and polysaccharides[18]. Loquat Eriobotrya japonica Lindl. (E. japonica) is a subtropical evergreen tree and is extensively used in traditional formulations[19]. E. japonica has antitussive, antiinflammatory, and anticancer properties. According to previous studies,the leaf extract of Loquat increased serum IFN-γand TNF-α levels in healthy mice[20,21]. Olive tree [(Olea europaea L., O. europaea)] is widely used in folk medicine in Europe[22]. Different forms of olive are appreciated globally by consumers who are health conscious.Its leaf extract mainly contains phenolics and two secoiridoid compounds: oleacein and oleuropein[23]. It is beneficial in the treatment of AIDS, chronic diseases, and melanomas by enhancing immunity[24,25]. Therefore, it is hypothesized that the herb formulae by combining these herb extract may exert more effective for treatment.
For more than 50 years, cyclophosphamide (CTX) has been widely used to treat various forms of cancers, including lymphoma, breast cancer, and leukemia[26]. However, CTX therapy is often restricted due to its toxicity and adverse effects. Therefore, CTX-induced immunosuppression mouse models have been used to determine the immunomodulatory effects of various herbs[27,28]. We evaluated the immune enhancement effect of an herb complex extract(HCE) comprising C. officinalis, E. japonica, and O. europaea in a cyclophosphamide-induced immunosuppression rat model.
2. Materials and methods
2.1. HCE preparation
The leaf extract of C. officinalis, E. japonica, and O. europaea was supplied by Teazen Co. (Anyang, Gyeonggi-do, Korea). Briefly, the leaves of herbs were dried at 60 ℃ and powdered in a blender. The dried powders were then extracted twice using 70% ethanol at 70 ℃for 3 h. After being filtered, each extract was evaporated in a rotary evaporator and freeze-dried. For the mixture of HCE extracts, each extract was mixed together in a 1:1:1 ratio.
2.2. Cell culture
The spleens of Sprague-Dawley rats were harvested, and a single cell suspension of splenocytes was prepared using tweezers and a mesh. The suspension was washed three times by centrifugation(×1 000 ×g, 5 min, 4 ℃) with RPMI-1640 medium. The suspension was treated with a red blood cell lysis buffer (Sigma-Aldrich, St.Louis, MO, USA) for 3 min to remove red blood cells. YAC-1 T cell lymphoma cells obtained from the Korea Cell Line Bank(KCLB40071) were cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 ℃ and 5% CO2.
2.3. Cell proliferation assay
WST-1 assay kit (ITS Bio, Seoul, Korea) was used to investigate the effects of HCE (1–1 000 μg/mL) on cell proliferation. Splenocytes(1 × 105cells/90 μL/well) and YAC-1 cells (1 × 105cells/90 μL/well) were cultured for 24 h and treated with HCE at different concentrations. The cells were incubated at 37 ℃ and 5% CO2for 24 h and 48 h, and cell proliferation was evaluated by measuring the absorbance at 405 nm with a Multi Detection Reader (Infinite 200;TECAN Group Ltd., Männedorf, Switzerland) after adding 10 μL of WST-1 solution (per 100 μL cell medium) and incubation for 1 h. Cell proliferation rate (%) was calculated as (Absorbance of the sample treatment group/Absorbance of the control group) × 100%.
2.4. NK cell activity assay
The splenocytes were seeded at a density of 5 × 105cells/mL in a 96-well plate, after which HCE (0, 1, 5, 10, 30, 50, 100, 200 μg/mL)were added to the wells. YAC-1 cells (1 × 104cells/mL) were added to the effector cells and target cells at a ratio of 1:50, and then cultured in a 5% CO2incubator at 37 ℃ for 24 h. After incubation, lactate dehydrogenase was measured using a CytoTox detection kit (Takara Bio, Shiga, Japan). Formazan formed by the oxidation of nicotinamide adenine dinucleotide (NAD) in the reaction solution was measured at 490 nm and compared with that in the control group.
2.5. Experimental animals
Specific pathogen-free, 5-week-old Wistar rats were purchased from Sam Taco Bio Korea (Osan, Korea). During the acclimation period, the rats were allowed unrestricted access to a general solid diet (Samtako, Gyunggi, Korea) and filtered water. The housing environment was maintained at (23 ± 1) ℃, (50 ± 5)% relative humidity, a noise level < 60 dB, and a 12-h photoperiod (08:00 to 20:00) with the illumination at an intensity of 150–300 Lux. This experiment was carried out in accordance with the experimental animal guidelines of Wonkwang University (Approval number:AKU17-66).
2.6. CTX-induced immunosuppression
CTX was purchased from Sigma-Aldrich and used at 5 mg/kg in the present study. CTX and HCE were coadministered. CTX was orally administered (5 mg/kg) after dissolving in distilled water.
2.7. Experimental design
During the acclimation period, experimental animals were separated into groups based on body weight using the randomized block design method as described previously with some modifications[29,30]. The experimental design involved normal, control (CTX 5 mg/kg body weight), red ginseng extract 800 mg/kg body weight (RGE) groups as reference group. The HCE groups were administered HCE at 200 mg/kg body weight (HCE 200), 400 mg/kg body weight (HCE 400)or 800 mg/kg body weight (HCE 800) which was selected from preliminary experiment for mice survival. Mice were divided into six groups with 10 rats per group. Individual rats were identified using ear punches.
2.8. Biomarker analysis
Body weights were measured weekly. Food and water intake were also measured weekly by measuring the amount of food and water remaining on the next day. After being anesthetized with diethyl ether, blood was collected from the abdominal vein for analysis, and liver, thymus, and spleen tissues were isolated and weighed. Spleen tissue was fixed in formalin for histological analysis.
2.9. Complete blood cell (CBC) count
Blood drawn from the abdominal vein was collected in EDTA-coated tubes (DB Caribe, Ltd., USA) and in conical tubes.Hematologic testing was performed using a blood analyzer (Hemavet 950 Fs; Drew Scientific, Dallas, TX, USA) after rotating the EDTA tubes containing the blood samples in a roll mixer for about 30 min.White blood cells, lymphocytes, and neutrophils were quantified.Blood collected in the conical tube was used for cytokine analysis.After coagulation at room temperature for 30 min, serum was collected by centrifugation at 1 200 × g for 10 min. Separated serum was analyzed by an ELISA kit (Biolegend, San Diego, CA, USA) to measure TNF-α, IFN-γ, and IL-2.
2.10. Histological analysis
The isolated spleen tissue was fixed in 10% formalin solution,embedded in paraffin, and sliced into 4-μm-thick sections. The sections were treated with xylene to remove the paraffin, dehydrated using a graded ethanol series, and then stained with hematoxylin for 4 min and eosin for 2 min. The stained tissue sections were observed and photographed using a BX50 F4 optical microscope (Olympus,Tokyo, Japan).
2.11. Statistical analysis
The data are represented as Mean ± SD of triplicate measurements.Statistical analysis was performed by ANOVA followed by Dunnett’s post hoc test using the SPSS statistical program (version 12.0, SPSS Inc., Chicago, IL, USA). Significance was at P<0.05.
3. Results
3.1. Cell proliferation
Splenocytes and YAC-1 were treated with 1–1 000 μg/mL HCE.Cell viability was measured at for 24 h and 48 h. Splenocyte viability decreased at doses ≥ 200 μg/mL at 24 and 48 h (Table 1). YAC-1 viability decreased at doses ≥ 500 μg/mL at 24 and 48 h (Table 1).The maximum HCE concentration for NK cells used in subsequent experiments was 200 μg/mL.
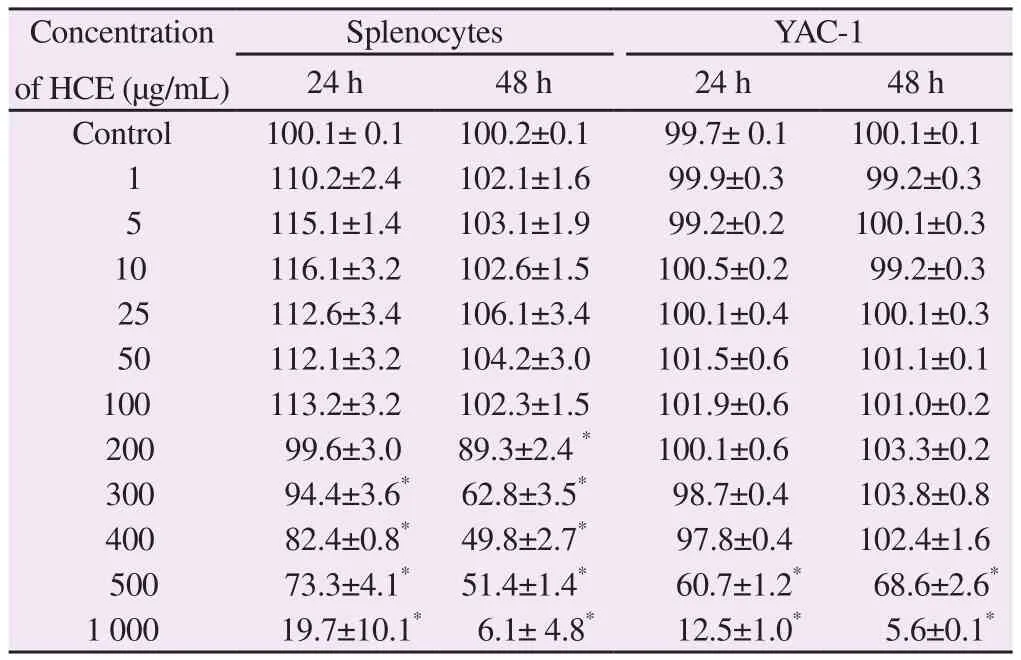
Table 1 Cell viability test for NK cell activity assay of herb complex extracts (HCE)(%).
3.2. NK cell activity
The activity of NK cell treated with HCE began to increase at 10 μg/mL in HCE treatments. At the maximum concentration of 200 μg/mL,the activity increased by 48.5% compared to control.
3.3. Variation in body weight and food and water intake
After oral administration experiment, no mice died after dosing.Compared with the normal group treated with distilled water only,the control group treated with CTX alone displayed decreased body weight gain. At the end of the experiment (4 weeks), the weights were (240.2 ± 5.3) g in the normal group and (238.8 ± 3.0) g in the CTX group. In the HCE 200, 400, and 800 groups, they were (237.2± 3.6) g, (236.3 ± 3.8) g, and (233.0 ± 5.7) g, respectively. Body weight in the RGE 800 group was (246.9 ± 3.6) g. No significant difference was found between the experimental groups. HCE treatment did not significantly affect food and water intake in the CTX-immunosuppressed rats.
3.4. Liver, spleen, and thymus weights
Liver weight was significantly lower in the control group(6.75±0.04) g than in the normal group (8.01±0.09) g. No significant difference in liver weight was observed between the HCE 200 and HCE 400 groups. In the HCE 800 group, the liver weight was (7.25± 0.06) g, which was not significant different from the RGE 800 group (7.40±0.05) g (Table 2).
Spleen weight was (0.55±0.01) g and (0.37±0.01) g in the normal and control groups, respectively, indicating that CTX decreased spleen weight. The spleen weights in the HCE treatments differed slightly from that in the CTX group (Table 2).
The thymus tissue weight was (0.31±0.01) g in the CTX group and (0.54±0.01) g in the normal group. The weights in the HCE 400 group [(0.37±0.01) g] and HCE 800 group [(0.36±0.01) g] were similar to that in the RGE 800 group (0.36±0.01) g; these weights were significantly higher than that in the CTX group (Table 2).

Table 2 Immune-related organ weight (g).
3.5. CBC analysis
White blood cell count was significantly lower in the CTX group than in the normal group. However, no significant difference was found between the experimental groups except for HCE 200 group(Table 3). Lymphocyte count was significantly lower in the control group and HCE groups than in the normal group. No significant difference was found between the experimental groups (Table 3).Neutrophil count in the normal group was higher than that in the CTX group, indicating that CTX significantly reduced neutrophil count. No significant difference was found between the experimental groups (Table 3).
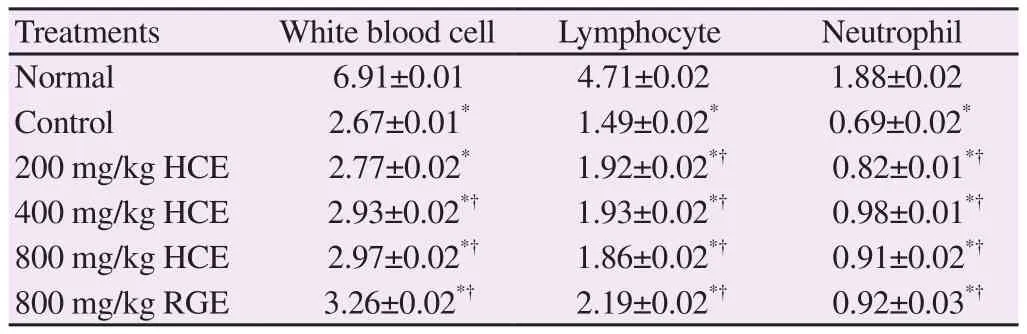
Table 3 CBC analysis (×109 L).
3.6. Serum cytokine analysis
Serum TNF-αcontent in the normal group was significantly higher than that in the CTX group. In the HCE 200 and HCE 400 groups, there were not significantly different from that in the CTX group. However, it was significantly higher in the HCE 800 group(9.45±0.63) pg/mL and the RGE 800 group (10.28±0.26) pg/mL than in the CTX group (Table 4). The same pattern appears in IL-2 cytokine levels. No significant change in IFN-γproduction was observed in HCE treated groups as compared with control group.
3.7. Spleen histological analysis
Optical microscopy was used to investigate the effect of HCE on spleen histology. In the normal group, white pulp was uniformly distributed around the central vein. In addition, the lymph node was surrounded by the marginal zone, and the boundary with the red pulp was clearly distinguished (Figure 1A). However, with CTX treatment, the boundary of the edge zone was unclear, and a remarkable decline in white pulp was observed, and the red pulp was condensed with an irregular arrangement (Figure 1B). HCE 200 did not significantly improve spleen histology. The marginal zone was localized (Figure 1C). In the HCE 400 group, the edge zone around the red pulp was clearly distinguished, unlike that in the control group, where breakdown of white pulp was not observed (Figure 1D). In particular, in the HCE 800 group, breakdown of white pulp by CTX treatment and the condensation of red pulp greatly improved. Overall, the marginal zone of the tissue was clearly observed (Figure 1E). In the RGE 800 group, compared to that in the control group, breakdown of white pulp was not observed, and the condensation of red pulp was greatly alleviated (Figure 1F), similar to that in the HCE 800 group.

Table 4 Cytokine analysis (pg/mL).
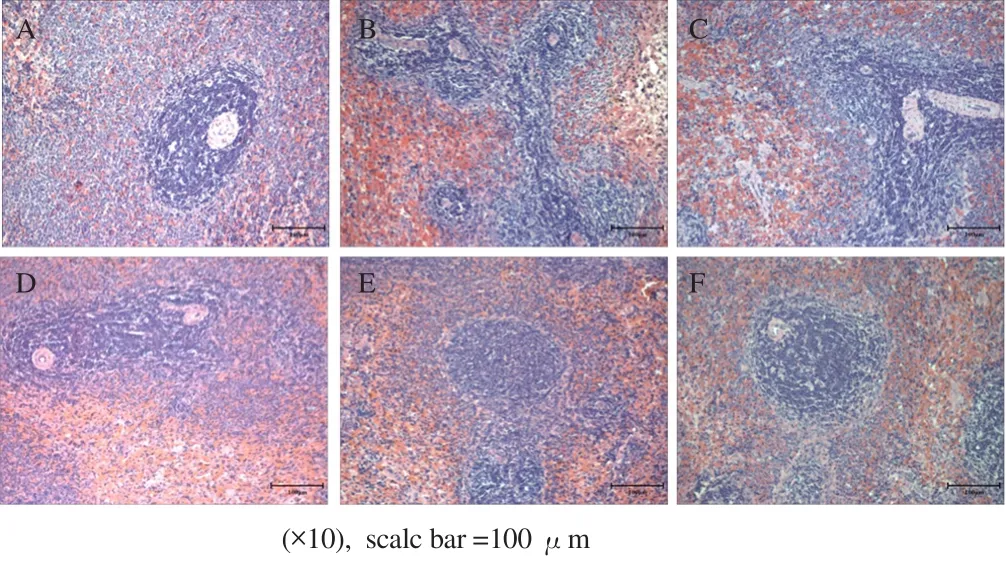
Figure 1. Spleen tissue H & E staining.
4. Discussion
This study was conducted to investigate the immunomodulatory effect of HCE comprising C. officinalis S. et Z., E. japonica Lindley,and olive leaves. RGE, which have found application in antioxidant and anti-inflammatory properties[31,32].
First, we studied the effect of HCE on NK cell activity. NK cell activity was measured in splenocytes and YAC-1 cells. HCE increased the activity of NK cells by about 38% at the maximum treatment concentration of 100 μg/mL. In many previous studies, the increasing of NK cell activity may enhances natural immunity[10,33].The NK cell could be responsible for changing organs and activating of various cytokines[28,34].
With oral administration treatment, we investigated the immunityimproving effect of HCE in immunosuppressed rats. Administration of CTX reduced the weights of the liver, spleen, and thymus,indicating its direct toxicity[35]. However, HCE attenuated this decrease. The same results also noticed in previous studies that C. militaris extract increased spleen weight[36]. These findings constitute indirect evidence that HCE modulated body immune responses.
Generally, the mice enhanced immunity have been reported to increase CBC levels[34]. Cyclophosphamide is an immunosuppressant cause suppression of CBC[37]. In this study,the proliferation of leukocytes, lymphocytes, and neutrophils was enhanced significantly higher than those of in the CTX-treat group.Same results have been found in some herbal medicines[28,37].Moreover, although HCE 800 showed negative effects on splenocytes and YAC-1, the oral administration of rat with HCE 800 treatment indicated that there was no influence on mouse survival rate.
Cytokines are involved in immune regulation. In this study,administration of CTX decreased cytokine levels. However,TNF-α and IFN-γ levels were recovered by HCE treatment.IL-2 also increased. Especially, HCE at 800 mg/kg showed similar effects to RGE at 800 mg/kg. TNF-α is involved in the regulation of macrophages and T cells[38]. IFN-γ modulates IL-2 activity, which affects NK and T cells[39]. CTX is known to cause immunosuppression by damaging DNA of T and B cells[40]. In this study, the administration of HCE attenuated the decrease in cytokine levels. Therefore, the suppressed immune response improved through the activation of NK and T cells.
Improvement in spleen histology is associated with immunity enhancement[41]. The atrophic changes in CTX group was confirmed by histomorphometrical analysis in this study. We found that spleen histology in the HCE 800 group was similar to that in the normal group. These results are direct evidence that HCE exhibits potent immunomodulatory effects through activated NK cells and promoting cytokine on CTX-induced mice.
In conclusion, the in vitro and in vivo results show that HCE enhances immunity by increasing NK cell activity, regulating cytokine levels, and maintaining spleen weight. Therefore, it may be used as a potential immunity enhancer.
Conflict of interest statement
We declare that we have no conflict of interest.
Foundation project
This work was carried out with the support of ‘‘Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01321501)’’ Rural Development Administration, Republic of Korea.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Salacca zalacca: A short review of the palm botany, pharmacological uses and phytochemistry
- Phytocompounds of Anonna muricata leaves extract and cytotoxic effects on breast cancer cells
- Screening of antiproliferative activity mediated through apoptosis pathway in human non-small lung cancer A-549 cells by active compounds present in medicinal plants
- Ultrasound-assisted extraction of antioxidant polyphenolic compounds from Nephelium lappaceum L. (Mexican variety) husk
- High resolution melting real-time PCR detect and identify filarial parasites in domestic cats
- Can miRNA712_3p be a promising biomarker for early diagnosis of toxoplasmosis?
