Efficacy and safety of Vidangadi Yoga (ayurvedic polyherbal medicine) in type 2 diabetes mellitus:a randomized controlled clinical study
2019-01-02ShaileshVinayakDeshpandeKrutikaSubhashJadhav
Shailesh Vinayak Deshpande , Krutika Subhash Jadhav
1 Parul Institute of Ayurved, Parul University, AP Limda, Tal Waghodia, Vadodara, Gujarat, India
2 PDEA's College of Ayurved and Research Centre, Sector 27, Akurdi, Pradhikaran, Pune, India
Abstract
Key words:type 2 diabetes mellitus; ayurvedic polyherbal medicine; Vidangadi Yoga; herbal treatment; metformin; blood glucose; randomized controlled trial
INTRODUCTION
Background
Diabetes mellitus (DM) is a group of metabolic disorders that are characterized by hyperglycemia caused by reduced insulin secretion, decreased glucose utilization and increased production of glucose.1Metabolic derangement taking place in the body as an outcome of the pathology of DM causes secondary pathological changes in multiple organs and systems.It imposes unbeatable load on systems, leading to increased incidences of end stage renal disease, nontraumatic amputations, adult blindness, and cardiovascular diseases, and finally resulting in death.Prevalence of DM is predicted to double globally from 171 million in 2000 to 366 million in 2030 with a maximum increase in India.2With more than 62 million diabetics currently present in India, it is fast gaining the status of a potential epidemic.It is predicted that by 2030 DM may afflict up to 79.4 million individuals.3This rising prevalence undoubtedly is going to pose a potential burden on the healthcare system.Over the past 30 years, the status of DM has changed as a mild disorder of the elderly to one of the major causes of mortality and morbidity affecting the youth and middle-aged people.Though advances in modern medicine have helped scholars to develop multiple oral hypoglycemic agents (OHA) and insulin therapy, achieving glycemic control becomes the sole purpose in clinical practice, let alone think of reversing the pathology.Control of hyperglycemia also becomes tricky in some individuals due to primary and secondary failures.Along with it, limited role of good glycemic control in preventing long term complications has always kept the window open for better remedy for overall control of the disease.
Researchers have correlated DM with Prameha mentioned in Ayurveda.The disease has been explained in great details in all ancient ayurvedic treatises.In Ayurveda, treatments of DM have been described in details, including multiple mono-herbal, multi-herbal, herbo-mineral combinations and Panchakarma (~bio-purification treatments) treatments.Such mono or multi-herbal medicines have been widely studied, but many potential combinations remain unexplored.Vidangadi decoction (kwatha) is one of such classical Ayurvedic formulations.It is widely used in DM treatment.All the ingredients of the combinations are herbal in origin and are known to have antidiabetic/hypoglycemic effects.There are no controlled studies describing the effects of the combinations.For better palatability and uniformity, Vidangadi decoction was processed into tablets.All standard parameters were followed while preparing decoction and tablets.
According to modern sciences, metformin is selected for treatment of initial-stage DM.4,5Metformin is known to reduce hepatic glucose production and control blood glucose level in DM.6A previous study has shown that metformin's primary effect occurs in the gut.7Metformin has been widely used as a gold standard for the treatment of type 2 DM (T2DM).8Therefore, it was used as a standard control medicine in this study.
Study objectives Primary objective
To investigate the efficacy of Vidangadi Yoga tablets in the management of T2DM compared with metformin tablet based on serum levels of fasting and postprandial blood glucose.
Secondary objectives
a) To investigate the efficacy of Vidangadi Yoga tablets in the management of T2DM compared with metformin tablet based on serum levels of fasting and postprandial blood glucose.
b) To investigate the efficacy of Vidangadi Yoga tablets in the management of T2DM compared with metformin tablet based glycosylated hemoglobin, lipid profile and clinical symptoms.
c) To evaluate the safety of Vidangadi Yoga based on hemogram, renal and hepatic profiles, and the occurrence of adverse events, adverse drug reaction and serious adverse events.
SUBJECTS/METHODS
Study design
This is a prospective, randomized, open-label, active-controlled, two-arm study with a 1:1 allocation ratio (Figure 1).
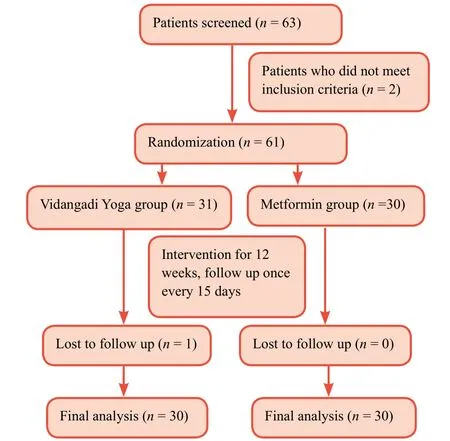
Figure 1:Study flow chart.
Ethical consideration
This study was approved by the institutional ethics committee of PDEA's College of Ayurved and Research Centre, Pune, Maharashtra, India (approval number 6833) on March 22, 2014.Written informed consent of all study subjects was obtained prior to screening.This study was registered with Clinical Trials Registry-India (CTRI) (No.CTRI/2015/04/005719)on April 25, 2015.
Eligibility criteria
All subjects were recruited from PDEA's College of Ayurved and Research Centre and screened for their eligibility to participate in the study at the visit.
Inclusion criteria
Either patients with known T2DM on treatment or patients with newly diagnosed DM were included.In case of undiagnosed cases, diagnosis of DM was confirmed according to American Diabetic Association criteria, 2010 (fasting blood glucose level > 7.0 mM, 2-hour postprandial blood glucose level > 11.1 mM).9The age limits were ≥ 25 years old and ≤60 years old.Patients with fasting blood glucose level up to 13.9 mM, postprandial blood glucose level up to 27.8 mM and showing good to moderate control in glycosylated hemoglobin were included.Ranges of blood glucose level and glycosylated hemoglobin were decided after consulting with consulting physicians of the study center.
Exclusion criteria
Patients with T2DM receiving insulin or those with increased blood glucose level (fasting blood glucose level > 13.9 mM and postprandial blood glucose level > 27.8 mM) and showing normal or poor to very poor control in glycosylated hemoglobin were excluded from this study.Patients having acute or chronic complications of DM and immunocompromised patients were not included.
Study intervention
The medicine studied is a classical ayurvedic preparation named vidangadi kwatha (decoction).10It is mentioned in classical ayurvedic treatises as decoction that is freshly prepared for each use.To make the medicine more palatable and avoid variations in making decoction, it was used in the form of tablets.The tablets were named as Vidangadi Yoga.Barks of all raw drugs were procured from the market and were authenticated from Agharkar Research Institute, Pune,India.According to ayurvedic pharmacopeia of India, raw materials were analyzed in terms of macro and microscopic structure, moisture, ash, extract percentage, and phytochemical properties at in house research laboratory.11Decoction was prepared by the standard procedure mentioned in Ayurveda.Medicines were mixed together and soaked in water overnight.Medicines were mixed with 16 parts of water and boiled till 2 parts remained.The solution was filtered to get decoction.The decoction was further heated to get solidified extract.10% gum acacia was added as a binding agent in the tablets of solidified decoction.Care was taken to make sure each tablet contains 500 mg of the solid extract.Compositions of each tablet are outlined in Table 1.The studied medicine was tested for its average weight, diameter, hardness, friability,and disintegration time.The studied medicine was prepared at in house pharmacy.Patients in the control group received metformin tablet 500 mg (Glyciphage®(500 mg), Franco Indian Pharmaceuticals, Mumbai, Maharashra, India) which was purchased from the market.
Randomization
Sixty-one patients were randomly divided into two groups using a computer generated randomization table to receive Vidangadi Yoga (n= 31) or metformin (n= 30).Allocation concealment was achieved using sealed, opaque envelops for packing study and control medicines.These envelops were sequentially numbered.
Study procedure
This study was conducted at Outpatient Department (OPD)of Ayurvedic Medicine at PDEA's Ayurved Rugnalaya and Sterling Multispecialty Hospital, Pune, India.A special case record form was prepared for each patient to record the medical details from baseline till final follow up.
Baseline data included demographic details, signs and symptoms, medical history, and details of previous physical examination.
Funduscopy was performed on each patient by an in house ophthalmologist to rule out diabetic retinopathy.Each patient was asked to visit again on the next day on empty stomach for at least 12 hours for measuring blood parameters including hemogram, fasting and postpradial blood glucose levels, glycosylated hemoglobin, lipid profile, renal and hepatic profiles,and undergoing routine urine test.If above parameters meet the eligibility inclusion, patients were included and randomized into Vidangadi Yoga group or metformin group.Patients in the Vidangadi Yoga group received Vidangadi Yoga tablet 500 mg thrice daily before food with water and patients in the metformin group received metformin tablet 500 mg after food twice daily.Patients in the Vidangadi Yoga group received 60 tablets of Vidangadi Yoga, and patients in the metformin group received 40 tablets of metformin at each visit.Patients were instructed about use of the study medicine and dosage and asked to visit for subsequent follow ups at the interval of 15 days, till completion of 90 days (3 months).At each follow up, in addition to sign and symptom evaluation and physical examination, fasting blood glucose level and postpradial blood glucose level were also measured.The remaining medicine was checked for compliance.At the last follow up (day 90), blood parameters were measured, including hemogram, glycosylated hemoglobin, lipid profile, renal and hepatic profile, fasting blood glucose level and postpradial blood glucose level, and routine urine test was performed.Details about adverse events or adverse drug reaction, if any, were asked throughout the study period.
Study outcomes
Primary outcome measure was change in blood glucose level measured at baseline and after completion of 90-day study.Secondary outcome measures were changes in glycosylated haemoglobin and lipid profile measured at baseline and at the end of study, changes in symptoms according to five-point scale measured at each follow up (Table 2).Adverse events,serious adverse events, and adverse drug reaction were evaluated at each follow up.Haemogram, hepatic and renal profiles were evaluated at baseline and at the end of study.
Sample size
Sample size was calculated using WINPEPI (version 11.0,2010).It was calculated based on the assumption that a sample size of 55 completers would be needed to assess the study objective at an 85% power and a 5% level of significance.Considering a patient dropout rate of 10%, 61 patients were enrolled to get a minimum of 55 patients completing the study.
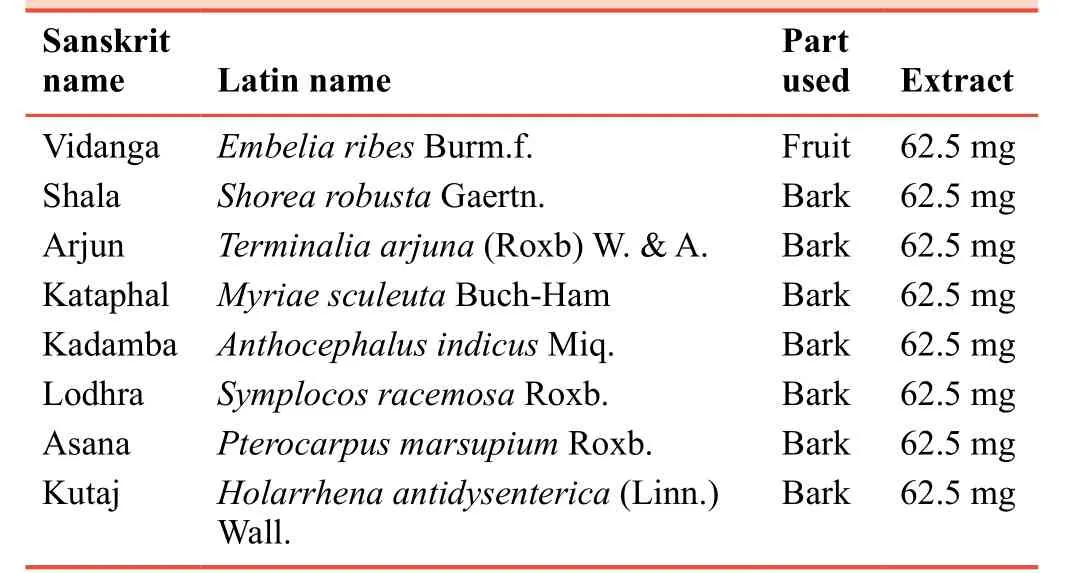
Table 1:Compositions of each 500 mg tablet of Vidangadi Yoga
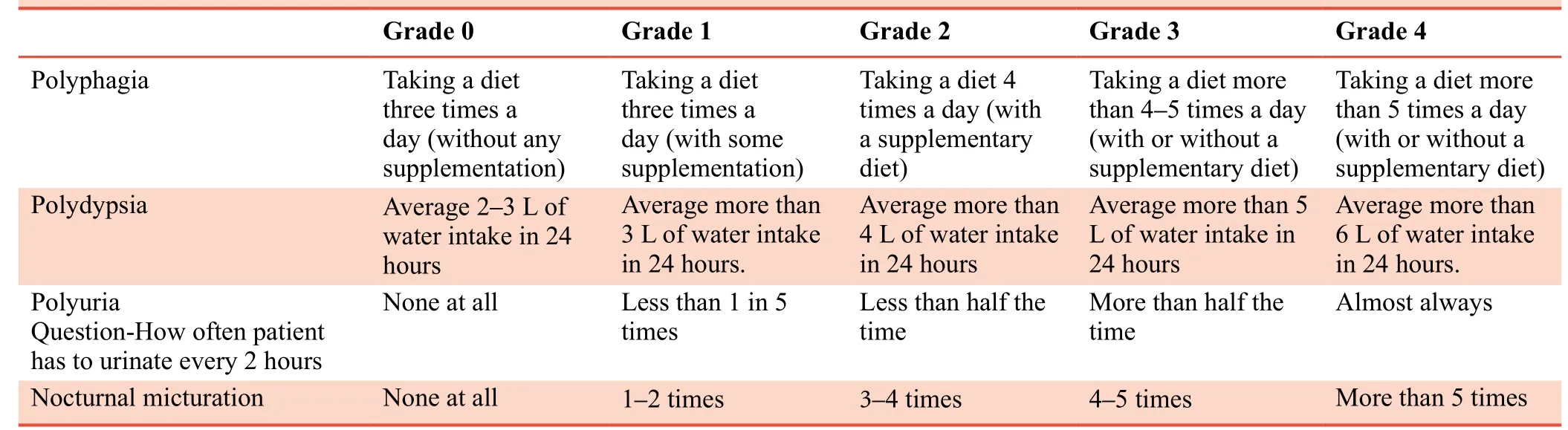
Table 2:Five-point scale for assessment of subjective parameters in patients with type 2 diabetes mellitus
Statistical analysis
Statistical analysis was done by a qualified statistician using SPSS 16.30 software (SPSS, Chicago, IL, USA).All values were expressed as the mean ± SD.Tests used for analysis were Student'st-test and if there were multiple measurements,repeated measures analysis of variance was also used.The statistical values obtained from the study were tested for 95%confidence level.
RESULTS
Baseline of patients
Sixty-three patients were screened for the study over the period of 14 months starting from March 2015.Among these 63 patients, two patients did not meet inclusion criteria and were rejected for this study.Thus, 61 patients completed the study.Study flow chart is outlined in Figure 1.Among them,there were 29 males and 32 females.The mean age of patients included in the Vidangadi Yoga and metformin groups was 49.8± 10.4 years and 48.5 ± 10.6 years respectively.The sample was homogenous, and there were no significant differences in baseline data between Vidangadi Yoga and metformin groups(Table 3).
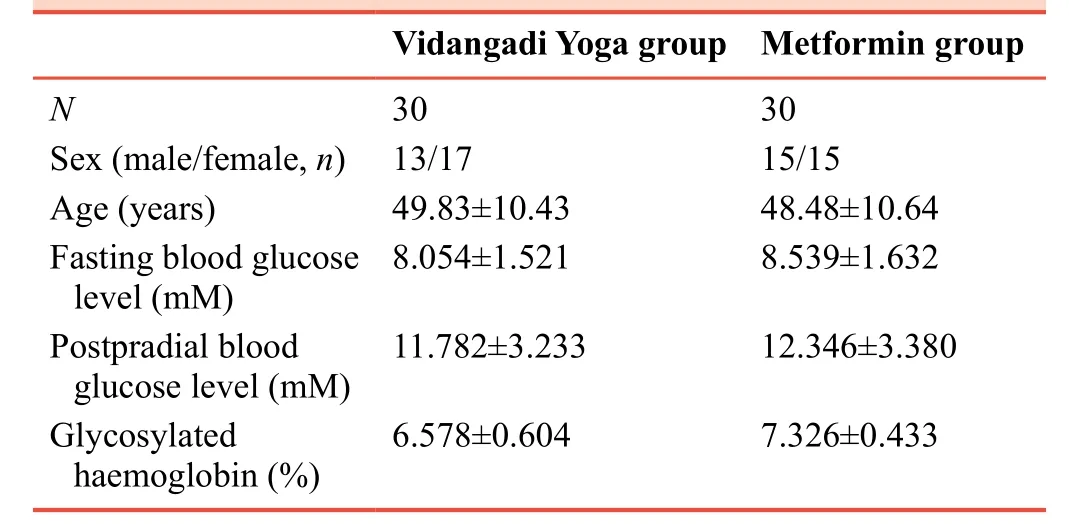
Table 3:Demographic distribution of patients with type 2 diabetes mellitus
Effect of Vidangadi Yoga on fasting and postpradial blood glucose levels in patients with T2DM
There were no significant differences in the mean fasting and postpradial blood glucose levels and glycosylated haemoglobin between Vidangadi Yoga and metformin groups.Fasting and postpradial blood glucose levels in both Vidangadi Yoga and metformin groups showed decreasing treads on day 90 when compared with baseline (P< 0.001).However, there were no significant differences in fasting and postpradial blood glucose levels between both groups.Glycosylated haemoglobin was slightly, but not significantly, reduced in both groups (Table 4).The difference of mean fasting and postpradial blood glucose levels as compared to baseline was 22.99% and 23.97 %respectively in the Vidangadi Yoga group and it was 17.00%and 22.44% respectively in the metformin group (Figure 2).
In the Vidangadi Yoga group (n= 30), fasting blood glucose level was reduced in 23 patients, increased in 2 patients, and was not altered in 5 patients.In the Vidangadi Yoga group(n= 30), postpradial blood glucose level was significantly reduced in 22 patients before and after the study, and it was deranged in 1 patient, and was not greatly altered in 7 patients.As compared to the baseline, mean glycosylated haemoglobin level decreased in 8 patients, increased in 3 patients, and was not greatly altered after the treatment in 19 patients.In the metformin group (n= 31), fasting blood glucose level was reduced in 17 patients, increased in 1 patient, and was not greatly altered in 12 patients.In the metformin group (n= 31),postpradial blood glucose level was reduced in 23 patients,increased in 3 patients, and was not altered in 4 patients.As compared to the baseline, mean glycosylated haemoglobin level was decreased in 8 patients, increased in 12 patients, and was not greatly altered after treatment in 10 patients (Figure 3).
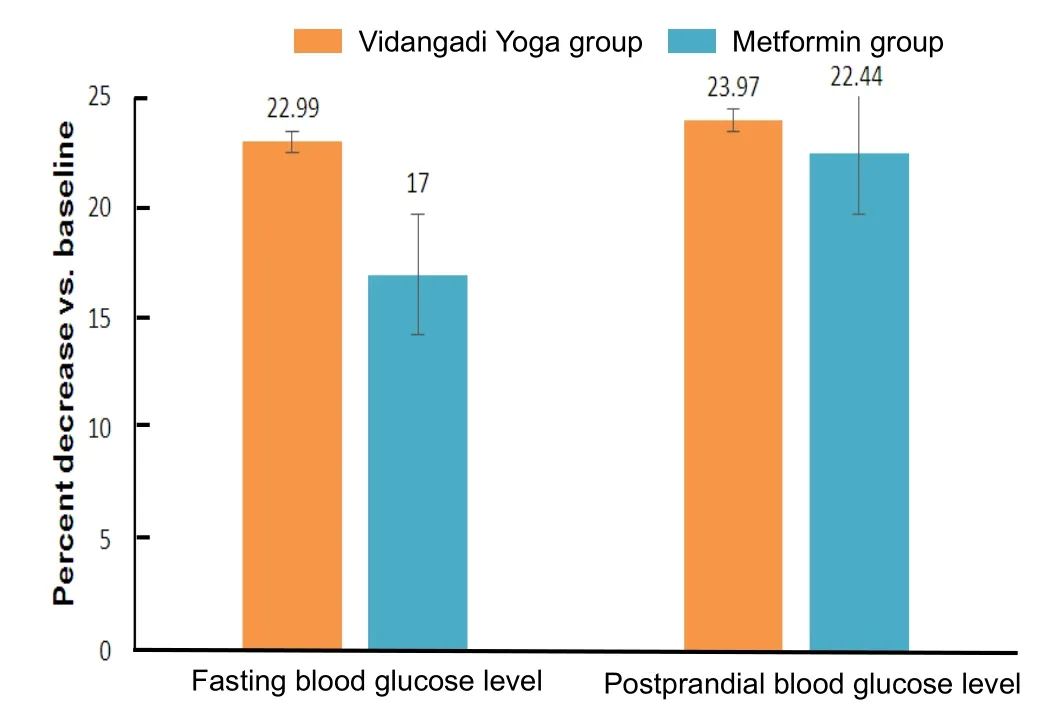
Figure 2:Mean percentage of reduction in fasting and postprandial blood glucose levels in patients with type 2 diabetes mellitus as compared to baseline.
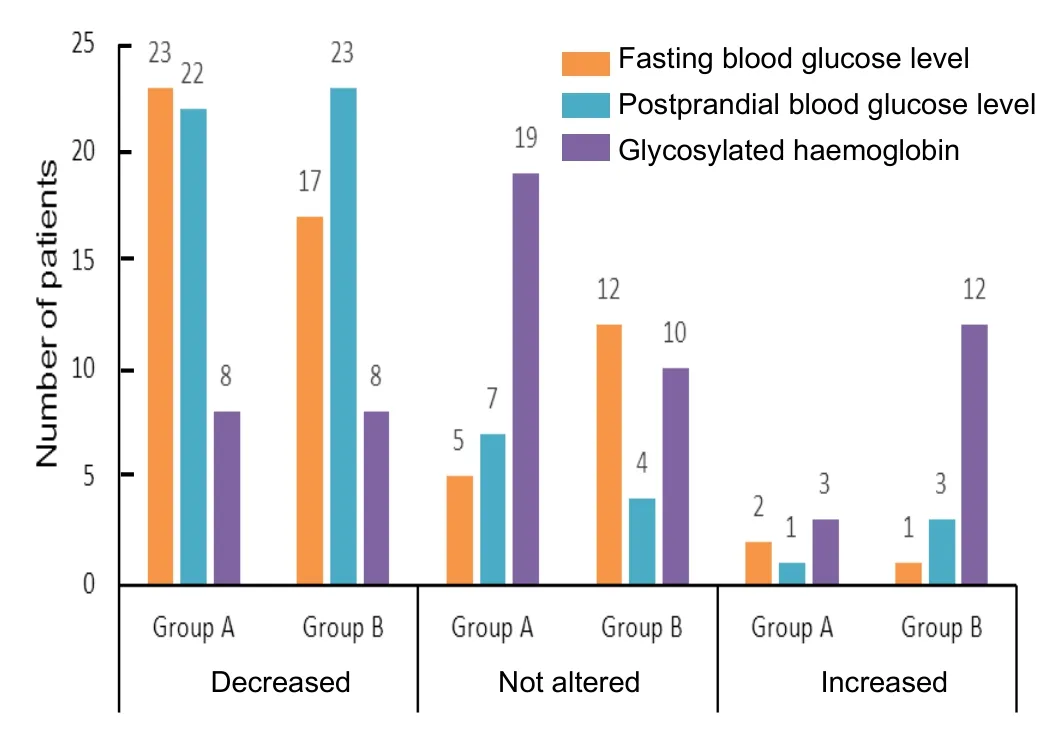
Figure 3:Blood glucose level and glycosylated haemoglobin in patients with type 2 diabetes mellitus following Vidangadi Yoga treatment.
Effect of Vidangadi Yoga on lipid profile of patients with T2DM
After Vidangadi Yoga treatment, total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) levels were not significantly changed as compared to baseline in both groups.In fact, there were no significant differences in the mean values of lipid profile parameters between before and after treatment and between Vidangadi Yoga group and metformin group (Table 5).
Effect of Vidangadi Yoga on clinical symptoms of patients with T2DM
The symptoms mentioned in Table 2 were assessed in patients from both groups at each follow-up visit.In both Vidangadi Yoga and metformin groups, all symptoms showed significant reductions on day 90 as compared to baseline (P< 0.001).There were no significant differences in these symptoms between the two groups.This suggests that both Vidangadi Yoga and metformin exhibit similar efficacy in reducing the symptoms of T2DM (Table 6).
Safety of Vidangadi Yoga treatment
Safety variables observed in this study were haemogram,hepatic and renal function tests done at baseline and the end of the study.Significant decrease was seen in levels of haemolobin and serum creatinine in the metformin (B) group (P< 0.05).But all values of both parameters were within normal reference range and were clinically insignificant.There were no significant differences in other safety parameters between Vidangadi Yoga and metformin groups (Table 7).
Other safety parameters were adverse events, serious adverse events, and adverse drug reaction.Four patients in the Vidangadi Yoga group and seven patients in the metformin group had adverse events such as fever with chills (n= 2),upper respiratory tract infection (n= 4), hypertension (n=3), knee joint pain (n= 1) and abscess on the left foot (n=1).The adverse events observed were found unrelated with study medicines.No serious adverse events or adverse drug reactions were reported in the study.
DISCUSSION
High degree of genetic predisposition, high body mass index(BMI), high body fat percentage and insulin resistance are attributed to the upsurge.12,13Increased prevalence of DM affects the life of a diabetics because of possible complications, directand indirect economic burdens.DM persists as the focus of many researches around the world.Though a lot of oral hypoglycemic agents and insulin are available, many patients fail to achieve adequate glycemic control.Oral hypoglycemic agents have many side effects, such as gastric disturbances, weight gain, and hypoglycemia.14,15They may create negative impact on overall treatment adherence and achieve normoglycemia.Current treatment modalities help correct hyperglycemia, but the basic pathology of DM remains untouched.Hence, there is a large scope for improving DM treatment.It is justified to evaluate the efficacy and safety of traditional herbal medicine in the treatment of DM.
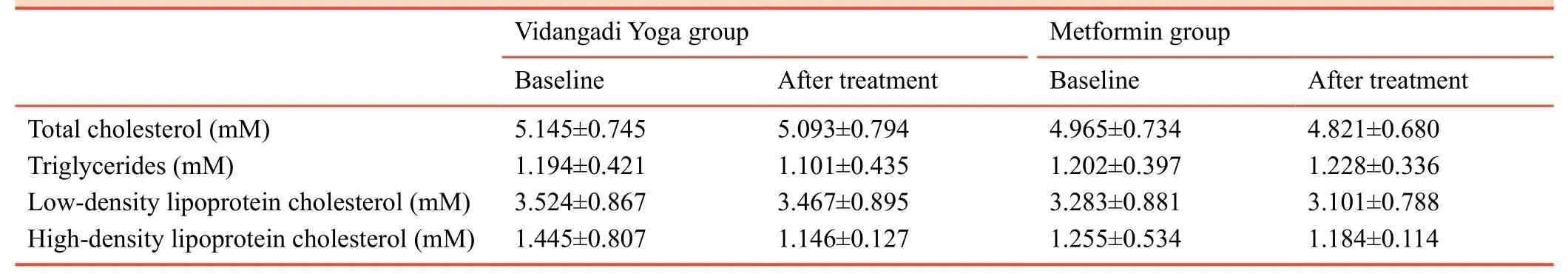
Table 5:Lipid profile of patients with type 2 diabetes mellitus following Vidangadi Yoga treatment

Table 6:Clinical symptoms of patients with type 2 diabetes mellitus following Vidangadi Yoga treatment
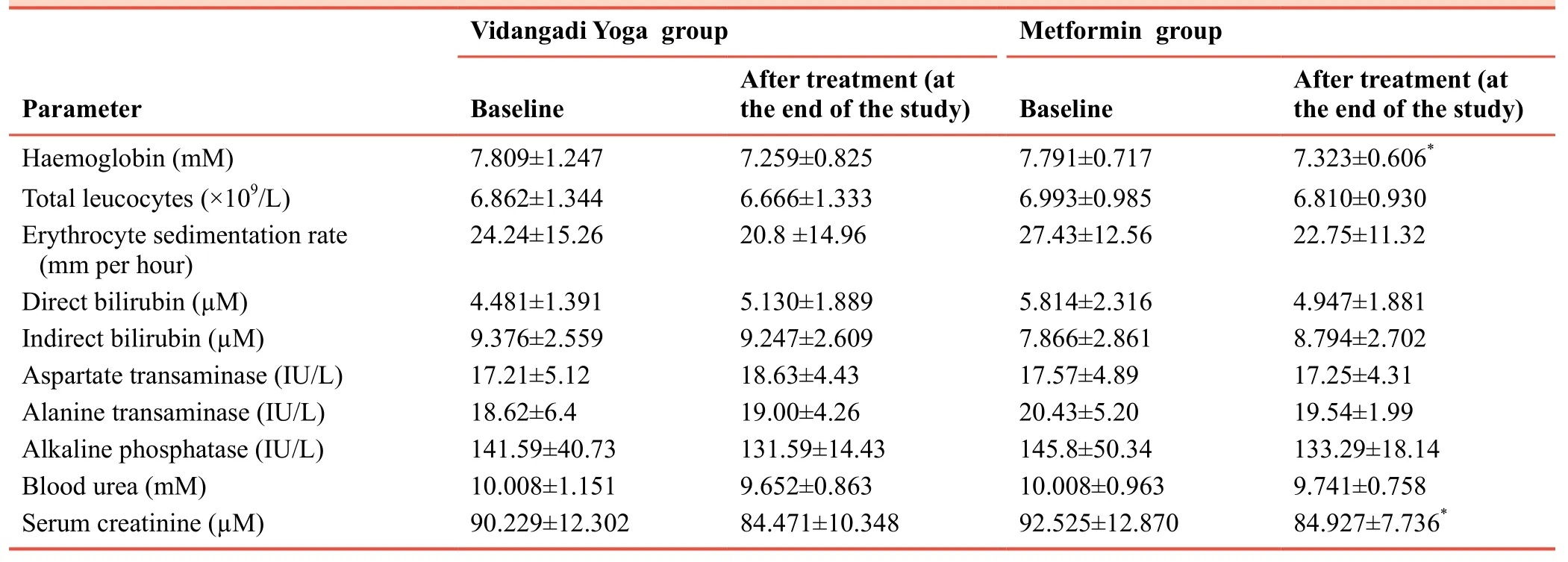
Table 7:Safety parameters of patients with type 2 diabetes mellitus following Vidangadi Yoga treatment
In this study, Vidangadi Yoga, which is mentioned in classical ayurvedic text and has been widely used in clinical practice,was selected.Though it is expected to use as freshly prepared decoction, in view of palatability, uniform dosage and easy administration, it was decided to use the same combination in tablet form.Metformin was selected as the control medicine and its dose was determined after consultation with in house physicians.
Results from this study revealed that patients receiving the herbal combination showed significant reductions in fasting and postpradial blood glucose levels as compared with the baseline.The number of patients showing reduced blood glucose level is also comparable between two groups.The effect lowering blood glucose level was comparable between Vidangadi Yoga and metformin.Therefore, it can be claimed that Vidangadi Yoga has similar therapeutic potential to metformin, however, this should be further validated by more studies.
The mean values of glycosylated haemoglobin were decreased in both groups, but they failed to achieve statistical significance within group.A previous study16shows that the levels of glycosylated haemoglobin may show false increase in cases of iron deficiency anemia.In this study, mean hemoglobin levels in both groups indicate mild anemia, which may be a reason behind the fact.
Though Vidangadi Yoga is conventionally used in the treatment of T2DM, its efficacy has not been documented.All the ingredients in Vidangadi Yoga are individually evaluated for antidiabetic or hypoglycemic effects in animal models and are found useful.P.marsupiumis widely studied and found to increase serum insulin levels in streptozotocin induced diabetic rats.17It is also believed thatP.marsupiumcan control metabolic alterations related to diabetes, apart from controlling only blood glucose level.18E.ribes, a fruit extract, has shown a positive effect in protecting pancreatic beta cells in animal models.19T.arjunahas also shown an anti-diabetic effect in alloxan-induced diabetic rats, along with decreasing activities of enzymes such as glucose-6-phosphatase and aldolase.20But exact mechanism of action of this medicine has not been evaluated.
With respect to secondary efficacy variables in both groups,the mean values of parameters in lipid profile, failed to show any statistical significance before and after the treatment.However, variation seen in the mean values of parameters in lipid profile before and after treatment was within normal range.There were no significant differences in the mean values of parameters in lipid profile between groups.
Safety variables showed no significant difference between before and after treatment in both groups.No adverse drug reactions were observed.It suggests that the drug is safe for internal use; however, safety data from larger, multi-centric studies are necessary to confirm the safety.Metformin has been reported to have gastric disturbances such as nausea, vomiting,stomach upset, diarrhea, weakness, or a metallic taste in the mouth.21,22No such side effects were seen in any patient after consuming Vidangadi Yoga.Therefore, it can be concluded that Vidangadi Yoga has better tolerability than metformin.
There are several limitations to this study.This study was a single-center study involving a small sample size and study duration is short.Larger multi-center studies with a larger sample size and longer duration are needed to further study Vidangadi Yoga in the treatment of DM.
To conclude, Vidangadi Yoga exhibited more significant effects on lowering blood glucose level and alleviating other symptomatic parameters studied in this study than metformin.Vidangadi Yoga has better tolerability than metformin and is considered safe.However, findings from this study need to be further validated.
Additional files
Additional file 1:Report of ethics committee.
Additional file 2:Informed consent document.
Additional file 3:CONSORT checklist.
Author contributions
Study concept, definition of intellectual content, literature retrieval,clinical studies, data acquisition, statistical analysis, manuscript preparation and editing, and study guarantors:SVD and KSJ.Study design, data analysis, and manuscript review:SVD.Both authors approved the final version of this manuscript.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Financial support
None.
Institutional review board statement
This study was approved by the institutional ethics committee PDEA's College of Ayurved and Research Centre, India (approval number 6833) on March 22, 2014 and performed in accordance with theDeclaration of Helsinki.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.In the form the patients have given their consent for their images and other clinical information to be reported in the journal.The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement
This study followed the CONsolidated Standards Of Reporting Trials(CONSORT) statement.
Biostatistics statement
The statistical methods of this study were reviewed by the biostatistician of PDEA's College of Ayurved and Research Centre, India.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices) will be available.Study protocol, informed consent form will be available immediately after publication.Data will be available for investigations whose proposed use of the data has been approved by an independent review committee identified for this purpose for individual participant data meta-analysis.Proposals should be directed to corresponding author (dr.shaileshd@gmail.com).Anonymized trial data will be available indefinitely at http://www.clinicaltdd.com.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
