Effects of a heel raise program on central hemodynamics and cognitive performance in chronic stroke:study protocol for a randomized, controlled,crossover trial
2019-01-02AndrewMitchelmoreDanielleLambrickSimonJobsonLeeStonerJamesFaulkner
Andrew Mitchelmore , Danielle Lambrick, Simon Jobson Lee Stoner, James Faulkner
1 Department of Sport, Exercise and Health, University of Winchester, Winchester, UK
2 Faculty of Health Sciences, University of Southampton, Southampton, UK
3 Department of Exercise and Sport Science, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Abstract
Key words: central blood pressure; stroke; sedentary time; training program; pulse wave velocity; randomized controlled trial
INTRODUCTION
Stroke has enormous physical and psychosocial impacts on individuals' health-related quality of life.The risk of recurrent stroke is 26% within 5 years and 39% within 10 years of initial incidence of stroke.1With the burden of disease caused by stroke expected to double by 2030, there is now good evidence to support interventions in stroke rehabilitation.2
Blood pressure post-stroke
Peripheral blood pressures are positively and continuously related to stroke incident, however central blood pressures may be more indicative of the pathophysiology associated with central organ damage.3,4These measures are particularly pertinent as around 30% of peripherally normotensive males and 10% of peripherally normotensive females may have central pressures in common with those in stage I peripheral hypertension.5Although traditionally contraindicated due to the invasive nature of the procedure, recent technological advances in oscillometric devices allow central blood pressure to be estimated using brachial arm cuffs through pulse wave analysis (PWA).6Peripheral and central blood pressures are both risk factors of cardiovascular disease, including stroke.5,7Behaviourably modifiable risk factors can be targeted through lifestyle changes.One aspect of lifestyle which is relatively easy to target post-stroke is the application of interventions that may decrease sedentary time.
Sedentary behaviour post-stroke
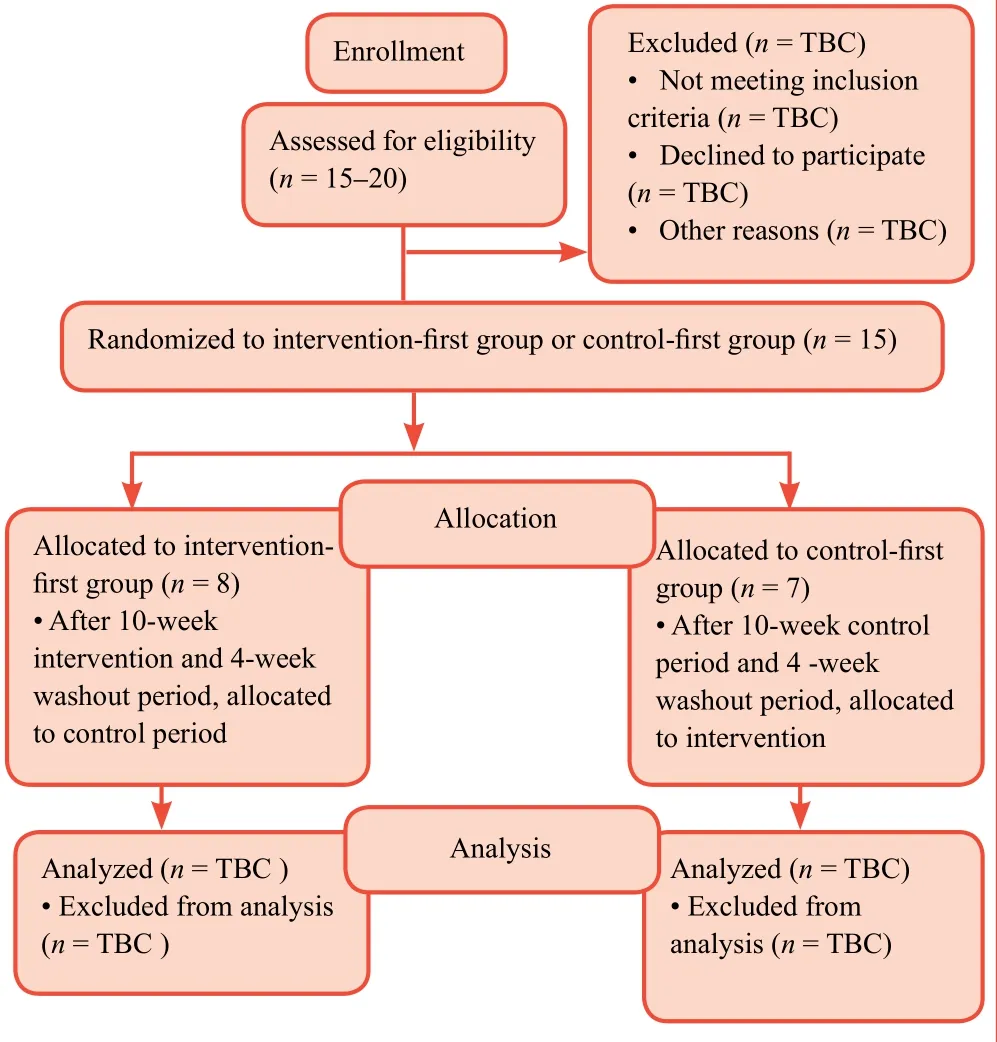
Figure 1:Study enrolment flow chart.
Physical disability and the loss of physical function following a stroke frequently leads to increased levels of sedentary time.8Stroke survivors typically spend 11 hours per day in a seated position (75% of waking hours), of which many of these hours are accumulated during prolonged periods of sitting.9Extended bouts of seated time may be particularly bad for health,10leading to decreased levels of physical activity and subsequent cardiovascular deconditioning.As sedentary behaviour has also been linked with mild cognitive impairment,11interventions to counter sedentary behaviours should be put in place.If not, the physiological and cognitive improvements that may be observed during in-patient stays may be lost in the months and years that follow hospital discharge.12
Strategies for breaking up sedentary behaviour post-stroke
This study hypothesizes that the engagement of patients in movement patterns such as heel raises may reduce the physical and cognitive damage caused by extended sedentary time.Although low-intensity movements may not increase aerobic fitness, they have the potential to reduce prolonged inactivity and, by extension, reduce the risk of future vascular events.Heel raises have been demonstrated to elicit physiological changes over the course of a prescribed program in elderly populations.Daily bouts of 100 heel raises over 40 days,136 weeks,14and 2 months15have been reported to show significant improvement in plantarflexor strength, balance, gait speed,improvements in ankle movement and decreases in postural fluctuation.The effect of resistance-free heel raises on physiological measures such as peripheral and central blood pressure and central systolic loading has not, to our knowledge, been investigated, despite each of these measures being indicative of cerebrovascular risk both before and after stroke.
Objectives
This study will investigate whether a daily, 10-week, heel raise intervention affects the peripheral and central hemodynamics,and cognitive performance of chronic stroke survivors.The study hypothesizes that a daily 10-week heel raise protocol will cause a significant decrease in peripheral and central blood pressure, measures of systolic loading and a significant increase in cognitive performance in individuals living with stroke.
METHODS/DESIGN
Study design
This is a randomized, controlled, crossover trial.The study team will recruit stroke survivors from local, community-based stroke support groups through declarations of interest and without financial incentives.The path that participants will take is outlined in Figure 1.Participants will then be randomized into an intervention-first (heel raise) group or a control-first group.All participants have previously been diagnosed with stroke by a stroke consultant from a UK National Health Service Foundation Trust and have received standard inpatient and outpatient care (physiotherapy, occupational therapyetc.)in accordance with the National Institute for Health and Care Excellence (NICE) guidelines.2
Eligibility criteria Inclusion criteria
The inclusion criteria are as follows:
· Over the age of 50 years
· Diagnosed with stroke by a stroke consultant from a UK National Health Service Foundation Trust 3 months to 5 years before the start of the study
· Patients who are cognitively aware and able to provide informed consent
· Patients who are currently medically stable
· Patients who are able to complete the full range of motion in plantarflexion when seated (heel raise)
Exclusion criteria
The exclusion criteria are as follows:
· Unresolved deep vein thrombosis
· Dementia or an inability to provide informed consent
· Type I or II diabetes or unstable glycaemic control
Randomization
A web-based randomization program will assign participants to either the intervention-first or control-first groups.
1.Intervention-first group:daily, 10-week, home-based, heel raise program.
2.Control-first group:10-week usual care; no home-based,heel raise program.
Blinding
Outcomes assessors and data analysts will be blinded to group allocation (intervention-first and control-first) and will not be aware of the randomization procedure.It is not possible to blind participants to group allocation.The fidelity of blinding will be checked at each assessment.
Baseline measures and participant schedule
Participants will be fasted (> 12 hours), refrain from caffeine consumption for more than 12 hours, and will not undertake structured moderate to strenuous physical activity in the 24hours prior to the initial baseline assessment.Pertinent outcome measures assessed in the baseline visit are presented in Table 1.During this session, and following 20 minutes of supine rest, PWA and pulse wave velocity (PWV) will be recorded on both the affected and unaffected sides using the SphygmoCor XCEL (Sydney, Australia).Both PWA and PWV are markers of cardiovascular health that have been linked to incident and recurrent stroke risk,16,17whilst a supine posture is the most reliable posture when using the SphygmoCor XCEL in an older (> 50 years) population.18Participants will also perform three maximal voluntary contractions of the medial gastrocnemius through a heel raise against maximum resistance, during which, skeletal muscle activity will be recorded using electromyography (Delsys Trigno, USA).Five Stroop tasks will be completed to provide baseline data of cognitive performance and to ensure no learning effect takes place in the 10-week follow-up sessions.After each 10-week period,a session identical to the baseline visits will be completed.Table 2 describes the schedule of enrolment and participation for control and intervention time-frames.

Table 1:Primary and secondary outcome measures
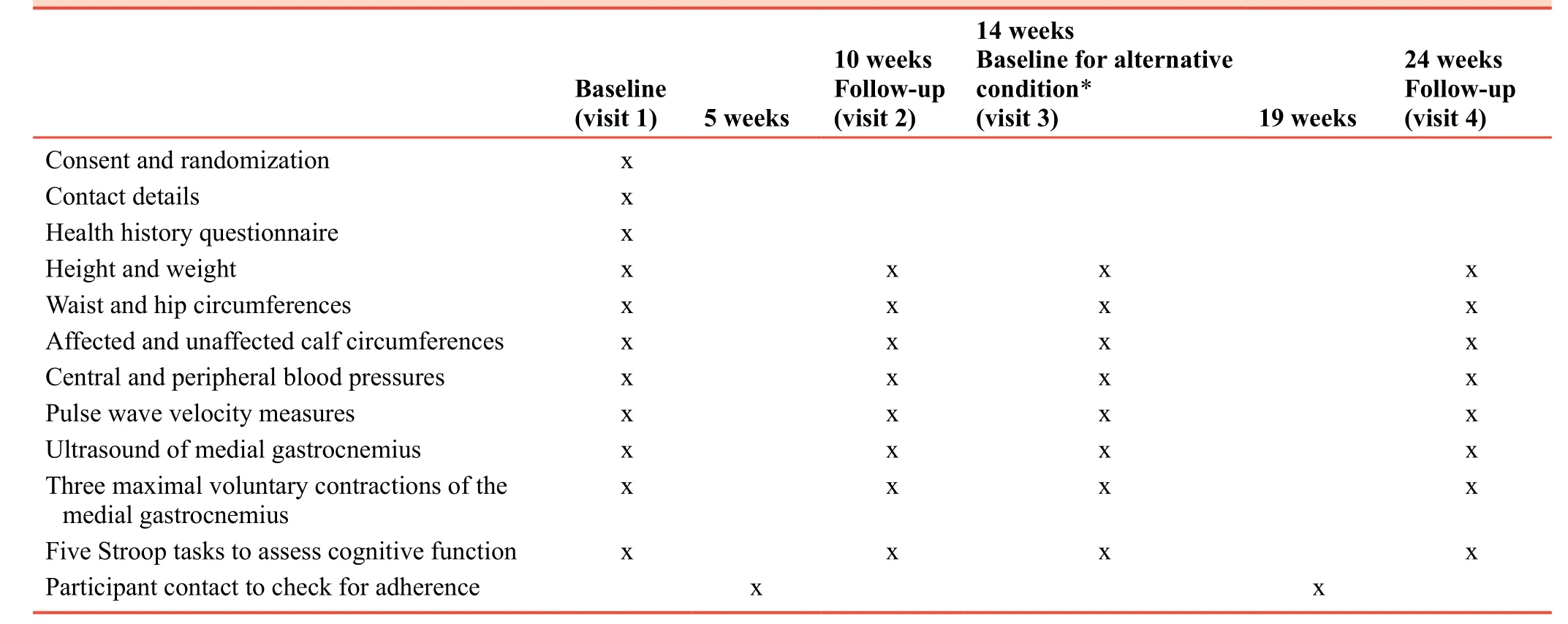
Table 2:Enrolment and participation schedule
Intervention
Following randomization, participants who are firstly assigned to the intervention group will complete 170 heel raises per day, for 10 weeks, with ten heel raises performed every 10 minutes for 2 hours and 50 minutes of seated time per day.This number is in accordance with a study investigating the acute effects of heel raises in stroke which is currently under review.Bouts of 100 heel raises have been demonstrated to be viable in elderly, non-clinical population.13,14Participants will record their daily adherence to the heel raise intervention.In the control group, participants will go about their normal lives (usual care) for ten weeks before attending the followup session.On completion of the follow-up assessment, and following a 2-week washout period, participants will complete the alternative testing group.
Sample size
A minimum sample size of 15 was calculated using G*Power 3.1 software (University of Dusseldorf, Germany) based on alpha being set at 0.05, power at 0.80, a medium F effect size of 0.5, and when accounting for a 10% dropout rate.19Participants will be recruited and randomly assigned to intervention-first or control-first groups.
Ethical approval and consent
The protocol has received approval from the University of Winchester Ethics Committee (BLS/17/11) on November 30,2017 (Additional file 1) and has been registered with Clinical-Trials.gov (NCT03423433).Participants will provide written informed consent (Additional file 2) before taking part in the study and will maintain the right to withdraw at any time.The protocol adheres to the recommendations provided by the Standard Protocol Items:Recommendations for Interventional Trials (SPIRIT) 201320(Additional file 3).An independent data monitoring committee has been established and will meet every 6 months following commencement of the trial.This study will be conducted in accordance with theDeclaration of Helsinki, formulated by the World Medical Association.
Data analysis
A mixed model analysis of variance with between- (Condition;heel raise intervention, control) and within- (Time; baseline,follow-up) subjects factors will be used to identify whether a heel raise program has a significant effect on all primary and secondary outcome measures.Statistical significance will be set atP< 0.05.All analyses will be conducted using SPSS(v.24.0, SPSS, Chicago, IL, USA).
DISCUSSION
With an ever-increasing population of individuals with stroke-related disability, it is of great importance to discover and implement appropriate lifestyle interventions to reduce this burden.One of the key lifestyle choices which raises recurrent stroke risk is elevated levels of sedentary time and physical inactivity.Stroke survivors tend to live extremely sedentary lifestyles due to physical impairments or a lack of confidence, and a large proportion of this sedentary time takes place in prolonged bouts.9,21These extended periods,of predominantly sitting time, are particularly damaging to the health of an individual.As a result, this predisposition to inactivity causes these survivors to be at an even greater risk of recurrent stroke events, placing ever greater stress on acute-treatment services which are already accommodating increased numbers of total stroke.
Simple exercises, such as heel raises, may have the potential to decrease sedentary time after stroke, improve cardiovascular health and improve cognition.Heel raises have previously been demonstrated to improve muscle strength, gait speed and balance,13-15but the effect on central and peripheral hemodynamic variables are yet to be investigated.Furthermore, interventions such as heel raises may lead individuals to feel more confident in their ability to perform physical tasks, and may attenuate impaired cognition as a result of sedentary behaviours.The consideration of a robust methodological design, such as the implementation of a randomized controlled crossover trial, is a particular strength to this study.Data from this study may demonstrate whether a heel raise program of this nature is physically demanding enough to elicit physiological changes over a 10-week period in a chronic stroke population
To better contextualize any future findings, protocol limitations should be addressed.It should be noted that the wide recruitment window (3 months to 5 years post-stroke diagnosis)could influence any potential effects from the prescribed heel raise intervention.A further limitation is that the engagement in the actual 10 week heel raise protocol is self-reported.Although participants will be asked to record their participation, the actual adherence to the program is purely based on participant feedback.
The conclusions of this study may provide a statistical rationale for the implementation of simple lower limb exercises in chronic stroke and a demonstration of the specific physiological pathways (i.e., arterial compliance, cognitive function) which may be improved using these low-intensity heel raise motions.
TRIAL STATUS
The trial status of this study is currently recruiting.It is anticipated that data analysis will be completed in January 2019 with study completion achieved by February 2019.Findings will be published thereafter.
Additional files
Additional file 1:Ethical approval documentation.
Additional file 2:Model consent form.
Additional file 3:SPIRIT checklist.
Author contributions
All authors contributed towards concept, design, literature search, and critically revising the manuscript for important intellectual content.All authors gave final approval for publication.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Financial support
None.
Institutional review board statement
This study protocol was approved by the University of Winchester Ethics Committee (approval No.BLS/17/11) on November 30, 2017 and will be performed in accordance with theDeclaration of Helsinki.
Declaration of patient consent
The authors certify that they will obtain all appropriate patient consent forms.In the form the patients will give their consent for their images and other clinical information to be reported in the journal.The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement
This study follows the Standard Protocol Items:Recommendations for Interventional Trials (SPIRIT) guidance for protocol reporting.
Biostatistics statement
The statistical analysis was decided by the research authors as all individuals on this manuscript have extensive experience in managing and analyzing studies such as the one presented here.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Anonymized individual data will be available immediately after study publication upon request from those who wish to access the data for 5 years.If anonymized data is provided, it should be done so after proposals to andrew.mitchelmore@winchester.ac.uk.Raw data (including personal information and participant codes) will be stored in a locked cabinet at the University of Winchester for this time period before being destroyed.Personal results will also be available to participants upon request.If requested, study protocols and outputs of statistical analysis will be available.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
