Sacubitril/valsartan improves ejection fraction in heart failure with reduced ejection fraction:a retrospective study
2019-01-02WalidIbrahimAhmedYassinAhmedSubahiHassanMohamedAymanKhaddamMuhammadBajwaAshrafAbugrounAmirKakiMahirElderTamamMohamad
Walid Ibrahim , Ahmed S.Yassin Ahmed Subahi Hassan Mohamed, Ayman Khaddam, Muhammad Bajwa,Ashraf Abugroun, Amir Kaki, Mahir Elder, Tamam Mohamad
1 Department of Internal Medicine, Wayne State University, Detroit Medical Center, Detroit, MI, USA
2 Hearts and Vascular Institute, Detroit Medical Center, Detroit, MI, USA
3 University of Oklahoma Health Science Center, Oklahoma City, OK, USA
4 Department of Medicine, Advocate Illinois Masonic Medical Center, Chicago, IL, USA
5 Department of Cardiovascular Medicine-CCU, Wayne State University, Detroit Medical Center, Detroit, MI, USA
Abstract
Key words:sacubitril; valsartan; heart failure; reduced ejection fraction; mortality; entresto; ejection fraction; HFrEF; retrospective study
INTRODUCTION
The management of heart failure with reduced ejection fraction (HFrEF) had progressed significantly over the last two decades.Improving the quality of life and decreasing overall mortality were the main accomplishments.Since the introduction of angiotensin-converting enzyme inhibitors (ACEIs) in 1987 vivid reduction in death was shown earlier by randomized controlled studies; The CONSENSUS (Enalapril in severe heart failure [HF]) and The 1991 Studies of Left Ventricular Dysfunction SOLVD Investigators.1,2The overall mortality had dropped to 30% with optimal medical therapy with the addition of beta-blockers, and mineralocorticoid-receptor antagonists to ACEIs.3-6A further benefit has proven after the landmark study in heart failure management (PARADIGM trial), which favored the use of angiotensin receptor-neprilysin inhibitors over ACEIs.This conclusion based on the reported 20% reduction in the composite endpoint of cardiovascular death or HF hospitalization, and entitled the integration of the outcome into the 2016 American College of Cardiology/American Heart Association/Heart Failure Society of America Guidelines.7,8We studied a total of 228 patients with hHFrEF who recently started on sacubitril/valsartan and proposed the hypothesis of improving ejection fraction (EF) after treatment with sacubitril/valsartan.
SUBJECTS/METHODS
Study design
This single-center, retrospective, descriptive study was performed at the Outpatient Heart Failure Clinic in Heart and Vascular Institute, Detroit, Michigan/Wayne State University.Data were collected retrospectively from the charts of diagnosed patients with HFrEF, defined as EF less than 40%.EF was calculated based on quantitative measurement of left ventricular systolic function by the Modified Simpson's Method.The patients enrolled attended the clinic during the period from January 2017 to December 2017.The cohort was followed up for a mean of 4.7 weeks after initiation of treatment.Patients who met the criteria mentioned earlier for HFrEF and started on sacubitril/valsartan (LCZ696; EntrestoTM,Novartis, Switzerland) 200 mg twice daily, were followed by another echocardiography to estimate the EF using the same method.An increase of EF from the baseline before treatment by 10-15% is considered an appropriate response.Ethical approval of this study was obtained from the Research Committee, DMC/Wayne State University (IRB# 015618MP4X)(Additional file 1) on February 14, 2018 for the analysis and publication of this study.
Statistical analysis
Data presented as a mean and standard deviation for quantitative variables and frequencies, percentages used to summarize qualitative variables.Percentage improvement according to different risk factors was compared using the chi-square test or Fisher's exact test as appropriate.AP-value less than 0.05 was considered statistically significant.SPSS version 22 (IBM,Armonk, NY, USA) was used to analyze the data.
RESULTS
Demographic data
The cohort has a mean age of 59.2 ± 11.7 years, and mean EF before treatment of 15-20% with the New York Heart Association class (NYHA) II to IV.145 (63.6%) were males.77.6% of them were blacks, 10.1% white Caucasians, and 12.3% other ethnic groups (Table 1).

Table 1:Demographic characteristics in 228 HFrEF patients
Data on the cohort comorbid conditions and heart failure treatment
The highest percentage of the co-morbidities in the examined sample was hypertension (94.7%).However, the prevalence of diabetes, coronary artery disease, vascular heart disease,arrhythmias, and alcoholism were 43.4%, 35.5%, 19.3%,11.4%, respectively (Table 2).
Concerning the heart failure treatment, the majority of patients were on beta-blockers and aspirin 96.9% and 86.4%,respectively.Except those who could not tolerate the medicines, 29.8% were on spironolactone, only ~1.3% was on digoxin.Furosemide users were 56.6% and 36.4% wearing a defibrillator (Table 3).There is no statistically significant link between the improvement in EF and other comorbid conditions or heart failure treatment.More than 2/3 (177 out of 228) of the participants were blacks.However, the higher percentage of improvement (71%) was shown among non-blacks or non-Caucasians).Patients without a history of hospitalization showed better post-treatment EF improvement than those with one or more hospital admission (Table 4).
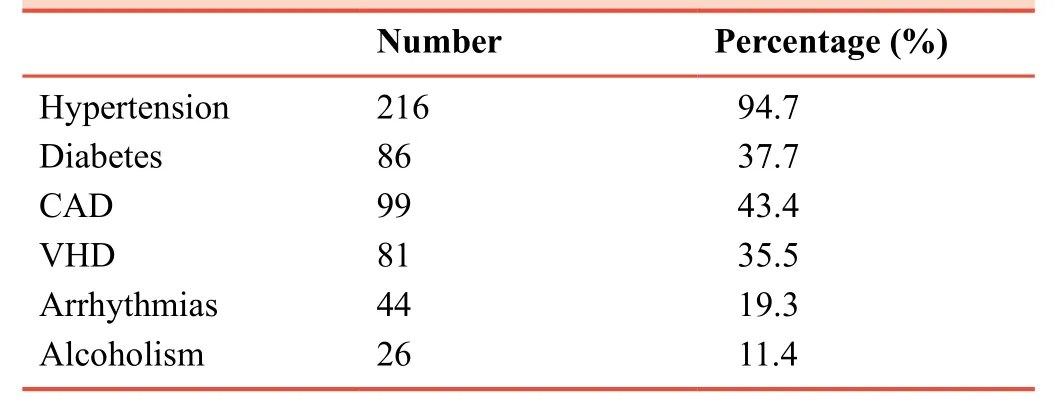
Table 2:Co-morbidities in 228 HFrEF patients
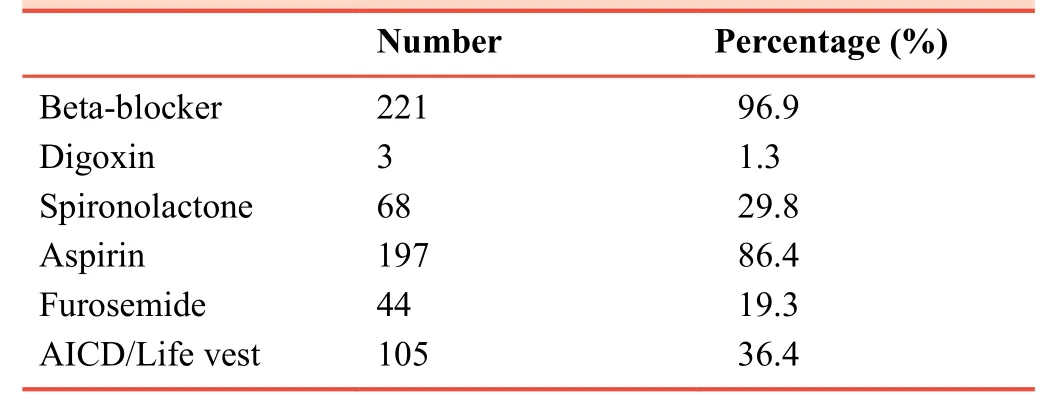
Table 3:Medications in the treatment of HFrEF in 228 patients
DISCUSSION
Congestive heart failure affects around 2% of population aged> 18 years in the developed countries, approximately five million in the US alone.1-3Chronic heart failure is associated with high morbidity and mortality.9-11In the United States alone,5-year mortality rate is 50%, and heart failure is the reason for more than one million hospitalizations each year, with high re-admissions rate.1,12For a long time, ACEIs, β-adrenergic receptor antagonists and mineralocorticoid/aldosterone receptor antagonists have been the backbone of therapy for HFrEF,because of their benefits on morbidity and mortality.3However,the introduction of sacubitril/valsartan is a new era with even more reduction in mortality, morbidity, and hospitalization among heart failure patients with reduced EF.4,5
Sacubitril is a neprilysin inhibitor, and valsartan is an angiotensin receptor blocker.The combination of sacubitril and valsartan inhibits neprilysin (neutral endopeptidase)viaLBQ657, the active metabolite of the pro-drug sacubitril, and blocks the angiotensin II type-1 receptorviavalsartan.13,14Through a multi-mechanism action and at different receptor levels, sacubitril combined with valsartan enhances the neurohormonal response of the myocardium, in the same time blocking the renin-angiotensin-aldosterone devastating effect and halting the remodeling process.15
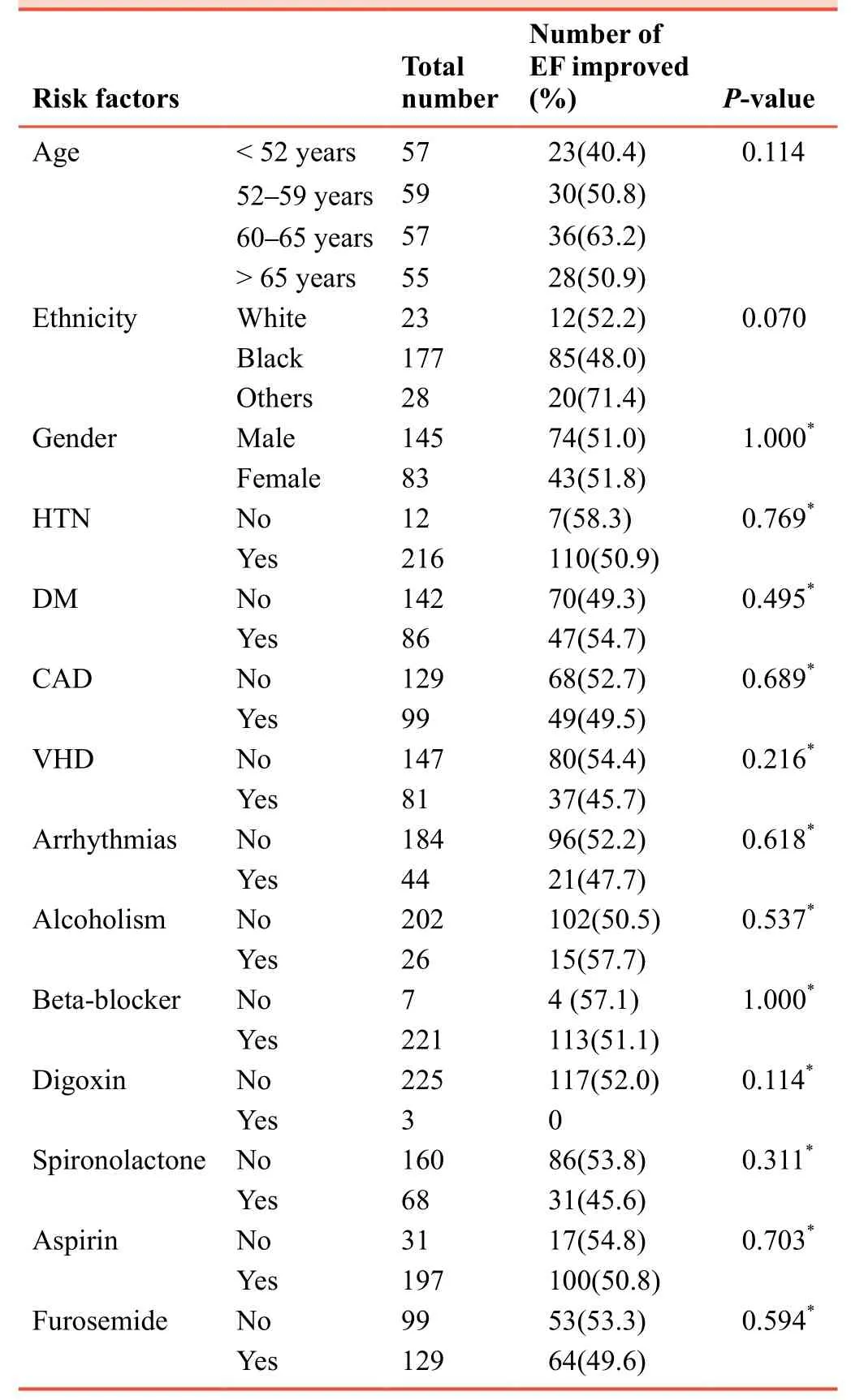
Table 4:The percentage and number of HFrEF patients showing EF improvement treated with sacubitril/valsartan according to their comorbidities and concomitant heart failure treatment
Our study highlighted the effect of the sacubitril/valsartan in the real EF value, demonstrating a calculated value to judge the efficacy of this drug.Improvement in EF was found to be associated with lower mortality in HFrEF patients.Kramer et al.16conducted a meta-analysis of over 69,000 patients and reported that improvement in left ventricular ejection fraction(LVEF) was associated with lower rates of mortality among patients with HFrEF.Of interest, there are only a few studies in the literature studying the direct effect of sacubitril/valsartan on EF, although sacubitril/valsartan effect on enhancing the mortality in HFrEF is always the reported outcome in most of the previous studies.Our study aims to document the effect of this new novel medication in the EF narrative value, and it is clinically implication.Out of the 228 of our study patients, 117 patients (51.3%) showed an objective improvement, which was defined by our study as a 10-15% increase in the baseline EF.
PARADIGM-HF trial7is the largest RCT to assess sacubitril/valsartan efficacy in comparison to the standard treatment.Patients enrolled in the trial were adults (age ≥ 18 years)with NYHA class II-IV symptoms, an EF of ≤ 40% and a plasma B-type natriuretic peptide level of ≥ 150 pg/mL (or N-terminal B-type natriuretic peptide level ≥ 600 pg/mL)or a B-type natriuretic peptide ≥ 100 pg/mL (or N-terminal B-type natriuretic peptide ≥ 400 pg/mL) if hospitalized for heart failure in the previous year to the study.However, the trial was terminated prematurely (after 1251 deaths from cardiovascular causes) because of a statistically compelling benefit with sacubitril/valsartan compared with enalapril alone for the primary endpoint (cardiovascular death or hospitalization for HF exacerbation).The PARADIGM-HF 7 concluded significantly fewer patients on sacubitril/valsartan either died from cardiovascular causes or were hospitalized for worsening heart failure (primary endpoint) than patients on enalapril, representing a 20 % reduction in the risk of the primary endpoint(21.8vs.26.5 %; hazard ratio:0.80; 95%CI, 0.73-0.87,P<0.001).However, this trial is not without criticism, especially in regards to the trial population, who were predominantly white (66%), male (78%), and NYHA functional class II HF(70%).Only 60 patients (< 1%) had NYHA functional class IV HF at baseline, and only 5% of the study population was black patients.These factors might reduce the external validity and generalizability of the data from this trial.
Around 78% of our study population is black patients, this group of patients are essential to include, so to generate data in the future might be a backbone for more significant metaanalysis studies that will consist of all population variation and produce results with high external validity and generalization.Another point to rising is that PARADIGM-HF trial population was mainly middle-aged with a mean age of 64 years.Our study includes more age groups with a mean age of 59.2 ± 11.7 years, giving the fact that HF patients are usually older.However, our study is just a descriptive study to report a noticeable EF improvement before and after sacubitril/valsartan treatment.
In the study of Almufleh et al.,1748 patients with HFrEF were treated with sacubitril/valsartan, there was an increase in the mean EF of 5.09 ± 1.36% in the medium/high sacubitril/valsartan dose cohort, and 4.03 ± 3.17% in low dose cohort, respectively.They concluded that sacubitril/valsartan was found to improve LVEF above and beyond the effect of pre-existing optimal medical therapy.It was the first study to describe improvements in LVEF after treatment with sacubitril/valsartan.Our study agrees with the study of Almufleh et al.17showing EF improvement following sacubitril/valsartan treatment.Our results regarding improved EF also go in line with the study of Suematsu et al.,18their mice-based study showed that statistically significant improvement in LVEF after sacubitril/valsartan treatment.
Our study has some limitations.First, the use of a database to quantify and compare EF before/after sacubitril/valsartan administration is prone to reporting and coding errors.Disparities in reporting and coding practice have been shown to significantly impact the reported incidence and outcome of conditions based on a database.19However, different database-based data have been used in a wide variety of medical subspecialties and considered a validated tool.Second, our study used a single center data, so the external validity of our results might not be enough to be generalized,20therefore more multicenter studies should be conducted to strengthen our study findings.Finally,most of our patients (77%) were blacks, so the results of our research should be managed with caution before applying for the general population.
In conclusion, sacubitril/valsartan has an exceptional therapeutic role in reducing mortality and rate of hospitalizations in heart failure with reduced EF patients.Our study analysis showed improvement of EF in one out of every two patients with HFrEF treated with sacubitril/valsartan, this result is independent of other risk factor and concomitant heart failure treatment.Further studies with more significant patient samples and a more diverse population are warranted to explore this subject and address our study question.
Additional file
Additional file 1:Ethical approval documentation.
Author contributions
Study concept:TM and WI; critical revision of the paper for important intellectual content:HM and AS; data collection and analysis:WI,ASY, AS, HM, AK, MB, AK, ME; literature retrieval and critical review of the manuscript:AA.All authors approved the final version of this manuscript.
Conflicts of interest
None declared.
Financial support
None.
Institutional review board statement
The study protocol has been conducted in accordance with theDeclaration of Helsinki.This trial was approved by the Research Committee, Detroit Medical Center/Wayne State University (IRB#015618MP4X) on February 14, 2018.Written informed consent will be obtained from all participants.
Biostatistics statement
The biostatisticians of Liverpool School of Tropical Medicine, Liverpool, UK, reviewed the statistical methods of this study.
Data sharing statement
Individual participant data that underlie the results reported in this article, after deidentification (text, and tables), will be available upon request.Data will be available immediately following publication, No end date; for anyone who wishes to access the data.In order to gain access, data requestors will need to sign a data access agreement.Proposals should be directed to wibrahim@med.wayne.edu
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
