海底重燃油对海胆繁殖及其子代发育的影响
2018-12-29段美娜刘泳江张欣欣熊德琪
段美娜,刘泳江,白 雪,高 翔,张欣欣,熊德琪*
海底重燃油对海胆繁殖及其子代发育的影响
段美娜1,刘泳江1,白 雪1,高 翔1,张欣欣2,熊德琪1*
(1.大连海事大学环境科学与工程学院,辽宁 大连 116026;2.中科海创环境科技(大连)有限公司,辽宁 大连 116000)
利用室内流水式粘油砾石柱模拟实际环境中的海底重燃油,研究了重燃油污染的孔隙水对成年海胆繁殖力、配子质量及子代胚胎发育的影响.结果表明,暴露结束后(7d),暴露组海胆的排配子率显著降低(=0.033),同时雌海胆繁殖力也显著降低(=0.036,(1957917±811471)个卵细胞);卵细胞的直径和精子的受精能力并未受到海底重燃油的影响.子代继续暴露48h,发现亲代暴露加剧了子代胚胎畸形程度,表明亲代暴露的毒性可传递给子代.进一步利用整合毒性指数(ITI)检测毒性传递的性别差异发现,与母本效应相比(24和48h子代的ITI分别为0.54~1.45和1.1~2.57),父本效应(24和48h子代的ITI分别为0.82~1.95和1.89~4.04)在毒性传递过程中起着关键作用.
海底重燃油;孔隙水;亲本效应;海胆;早期发育
1970~2016年间,世界范围内,发生了超过1000起船舶溢油事故,其中绝大多数事故(81%)为小型船舶溢油事故(即溢油量<7t)[1].中小型船舶溢油事故泄漏的油品往往是重燃油(HFO),其导致的HFO溢出量占HFO总溢出量的80%以上[2].HFO是一种原油精炼产品.HFO黏度大(15℃运动黏度5000~ 30000mPa·s),分散和自然降解十分困难.在风浪作用下,HFO可以出现在距溢油事故发生地点相当远的地方,使海岸线和敏感海域受到污染[3].此外,HFO密度较高(0.92~1.02g/cm3),在碎波带与泥沙混合后,更易于沉降.沉降后,在波浪和潮汐的作用下HFO可以在水体和砂砾海滩之间循环,源源不断地释放多环芳烃(PAHs)对水生生物产生毒害作用,这个过程就是孔隙水毒性假说[4].
已有学者在实验室条件下利用装填粘油砾石的容器模拟近岸处海底HFO,发现鱼类胚胎的畸形率和死亡率与流经粘油砾石的水体中PAHs浓度相关[5-9].事实上,海洋底栖生物,由于栖息在海底,不仅会暴露在有毒孔隙水中,还可能与海底HFO直接接触,加之其活动能力低下难以逃出污染区,势必会受到海底HFO的胁迫,因此海底HFO对海洋底栖生物的毒性影响亟待研究.
海胆主要栖息于浅海的岩礁、砾石、砂石等海底,对浅海生物群落的组成、结构和多样性起着关键的作用[10-13].海胆生活史包括浮游幼体和底栖成体两个主要阶段.不仅早期浮游幼体对污染敏感性高[14-21],其成年阶段也被视作海洋生态毒理学研究和环境监测的有效生物模型[22-26].成年动物的繁殖能力对整个物种的延续起着至关重要的作用,海底HFO对成年海胆的毒害作用势必会影响到其体内配子的生成、排放、受精过程,进一步影响子代发育,从而对整个种群的生存产生影响[27-34].
本文以装填粘油砾石的容器模拟海底HFO,选择海洋底栖模式生物海胆为受试生物,研究海底HFO对亲代成年海胆繁殖能力、配子质量及其子代胚胎早期发育的影响.以期为评估溢油对海胆种群数量的影响提供参考数据,同时为海洋生态风险评估、自然资源损害评估以及船舶溢油索赔提供重要科学依据.
1 材料与方法
1.1 材料
1.1.1 实验油品 HFO380,运动黏度729800mPa·s (50℃),密度0.9821g/cm³,由大连海洋燃油有限公司提供.
1.1.2 实验砾石 直径为10~50mm的表面平整的砾石,取自大连银沙滩,洗净烘干后备用.
1.1.3 实验海水 取自大连市星海湾,盐度(34±1) PSU,pH=(8.0±0.03).
1.1.4 实验海胆驯化繁殖期成年海胆购自大连海宝渔业有限公司.每只海胆注射0.5mL的KCl,排黄色配子的为雌海胆,排白色配子的为雄海胆.将成功排配子的海胆在实验室海水循环系统(大连汇锌钛设备)中驯化2周,海水温度为(18±1)°C,光照周期为12h:12h.期间每3d投喂海胆体重5%的新鲜海带.暴露实验开始前3d停止喂食.驯化期间未出现自发排配子和死亡个体.
1.2 方法
1.2.1 粘油砾石制备[5,7,9]将一定量的HFO380与1.8kg洁净砾石放入混合容器中剧烈晃动2min,使油尽可能的均匀的覆盖在砾石表面.为避免相互干扰,按粘油量由低至高依次制备不同浓度的粘油砾石.然后将粘油砾石置于避光通风处24h,用于暴露实验.实验设置粘油砾石浓度为0,400,800,1600,3200和6400μg油/g砾石(下文简写为μg/g).0μg/g为对照组.Zhadan和Vaschenko的研究[33]表明暴露于被柴油污染的海水中(总石油烃(TPH)浓度约为300μg/L)50d的雌海胆所产子代的畸形率增加;在此基础上,通过预实验本文选择400μg/g浓度组为最低浓度组,该组暴露液中TPH浓度从618.5μg/L降至308.6μg/L.进一步预实验发现,暴露于16000μg/g浓度组的雌海胆2d后出现自发排卵现象.暴露于8000和4000μg/g浓度组的雌海胆,在7d暴露期内未出现自发排卵现象,但是8000μg/g浓度组的雌海胆排卵数目少.不足以用于后续子代的暴露实验,而4000μg/g组的雌海胆产卵数量足以用于后续实验.因此本文最高浓度组的粘油量应介于4000~ 8000μg/g之间.因此本文选择400,800,1600,3200和6400 μg/g,以此保证可以观察到亲代效应,并保证有足够的卵细胞用于后续暴露实验.
1.2.2 动态暴露装置 装置为上下加盖的聚氯乙烯(PVC)柱(d=10.8cm,H=35cm),距底部3cm处有一进水管(d=1.2cm),出水管(d=1.2cm)位于对面距顶部3cm处,底部配备一个由PVC支柱(H=3.5cm)支撑的PVC网孔圆板放置砾石(图1).进水流速以针阀控制.粘油砾石制备完成后,转移至该装置,海水流速20mL/min由下至上流经粘油砾石柱,24h后盛接流出液进行暴露实验.
1.2.3 亲代海胆暴露方法每个浓度组设置3个重复,每个重复包括6只雌海胆和6只雄海胆,雌雄海胆分开暴露(图1).暴露容器上部开孔,保证暴露溶液体积为6L.暴露时间为7d,暴露期间不充气不喂食,并虹吸出容器底部排泄物.
1.2.4 子代胚胎获取及暴露方法 亲代海胆暴露结束后,用0.45μm滤膜过滤海水(FSW)清洗体表,然后经围口膜向海胆体腔内注射1mL现配的KCl溶液(0.5M).雌海胆口面向上置于盛满FSW锥形瓶口处,雄海胆口面向上,擦干体表后置于干燥的培养皿上.30min后将雌海胆取下,将每重复组6只雌海胆所产卵细胞混合,置于18℃的FSW中暂放待受精.每重复组的6只雄海胆所排精子混合后置于4℃待受精.取10μL干精子稀释于5mL的FSW中,加入到500mL密度为100个/mL的卵细胞溶液中,轻轻晃动进行受精[35].胚胎亲本组合有4种(如图2所示):对照组(对照组卵细胞和对照组精子受精),母本暴露组(暴露组卵细胞和对照组精子受精),父本暴露组(对照组卵细胞和暴露组精子受精)和双本暴露组(暴露组卵细胞和同组精子受精).受精15min后,虹吸法洗卵3次以去除多余的精子.
来自于暴露亲本的胚胎暴露浓度与其亲代暴露浓度相同.对照组胚胎均分为6份,分别于FSW和粘油砾石柱流出液中培养.因此对于子代暴露实验,共有5个暴露浓度,每个浓度组包括4种亲本组合的胚胎.胚胎于黑暗处(18±1)℃的水浴中培养,期间无需换水和喂食.
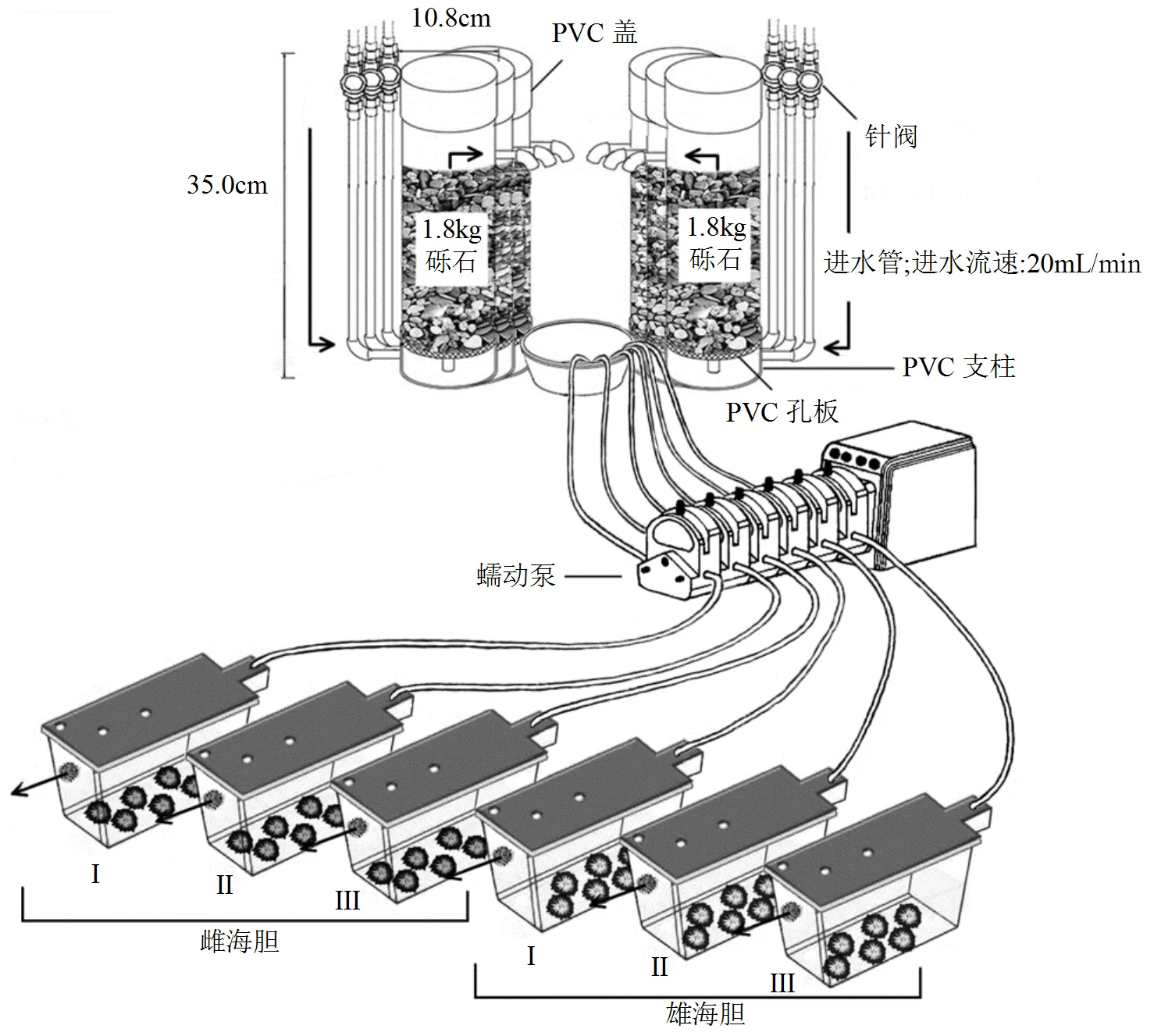
图1 粘油砾石柱和成年海胆动态暴露方法示意
该示意图为6个粘油砾石浓度组(0,400,800,1600,3200和6400μg/g)之一的模式图;I、II和III指亲代暴露实验的3个重复组;水流动方向在图中以黑色箭头标注
1.2.5 亲代海胆相关参数测定 排配子率指排配子的雌或雄海胆个数与对应性别的海胆总数的比值.繁殖力指雌海胆开始排卵的30min内排出的卵的个数[36],其中将未排卵的雌海胆繁殖力记为0.卵细胞计数使用0.1mL的浮游生物计数框进行,每只海胆重复计数4次并取平均值作为一只海胆的繁殖力.
1.2.6 配子质量 将每重复组6只雌海胆的卵细胞混合,取3个1mL重复样品,滴入几滴40%福尔马林,置于4℃待测.显微镜(OLYMPUS IX73)观测并拍照,使用Cell Standard软件测量卵细胞直径,每个样品至少测量100个卵细胞.于受精15min后取3个1mL重复样品,加入几滴40%福尔马林置于4℃待观察受精率.以受精膜鼓起为受精成功标志,每个样品至少观察100个受精卵.
1.2.7 子代海胆胚胎早期发育观察 分别于受精后24h(原肠胚期)和48h(长腕幼虫期)取样,每重复组各取4个2mL重复样品并滴入40%福尔马林放于4 ℃,在24h内完成观察,并记录畸形率.正常发育的胚胎形态应满足4个条件[37-38]:(1)胚胎在受精后24h进入原肠胚时期,受精后48h进入长腕幼虫期;(2)胚胎呈现左、右和背、腹侧对称;(3)原肠期具备发育良好的原肠,长腕幼虫期具备结构完整的消化道(口、胃、肠);(4)长腕幼虫期具备发育良好的骨针和腕.
1.2.8 综合毒性指数(ITI)[35]根据发育是否延迟和形态是否畸形,每个胚胎赋予从0~10不同分值.24h正常晚期原肠胚为0分,原肠胚、囊胚和桑椹胚各得1,3和4分;当胚胎出现畸形形态时,原肠胚、囊胚和桑椹胚各得5,7和10分.48h正常长腕幼虫为0分,早期长腕幼虫、棱柱幼虫、原肠胚、囊胚和桑椹胚各得2,3,4,5和5.5分;当胚胎出现畸形形态时,长腕幼虫、早期长腕幼虫、棱柱幼虫、原肠胚、囊胚和桑椹胚各得6,7,7.5,8,9和10分.ITI根据以下公式计算:

式中:S为每类畸形胚胎得分;F为这类胚胎出现的频率(=10).
图2 不同亲本组合的子代海胆暴露方法示意
Fig.2 Schematic exposure regime of offspring derived from different parental crosses of sea urchins
I,II和III指子代暴露实验的3个重复组.步骤(1):对照组海胆的配子平均分成11份;步骤(2):各暴露组海胆的配子均分2份;步骤(3):受精过程.另有一组来自于对照双亲的子代胚胎在FSW中培养
1.2.9 暴露溶液组分分析方法暴露期间每隔24h取水样分析暴露液TPH浓度和PAHs浓度. TPH采用紫外法测定(BIOTEK EPOCH2)[39].各浓度组各取3个混合流出液重复样品,正己烷萃取,并于225nm下正己烷调零测定吸光度.根据以下标准曲线公式计算TPH浓度:
y
= 0.051
x
-0.0033 (2)
式中:为样品萃取液吸光度与正己烷吸光度差值;为萃取液TPH浓度,mg/L;2=0.9996.
PAHs浓度采用气相色谱/质谱联用法(GC/MS)测定.样品前处理步骤参照GB/T 21247-2007《海面溢油鉴别系统规范》[40].仪器型号GC(HP 6890GC)- MS(5975),选择SIM模式,内标法进行定量分析.色谱柱为DB-5MSUI,长30m,内径0.32mm,膜厚度0.25μm.载气为高纯氮气,流量1mL/min.升温程序:50℃保持2min,以8℃/min的速度升温至150℃,保持3min;再以3℃/min速度升温至300℃,保持15min.分析16种PAHs:萘,苊烯,苊,芴,菲,蒽,荧蒽,芘,苯并[a]蒽,苯并[b]荧蒽,苯并[k]荧蒽,苯并[a]芘,茚并[1,2,3-cd]芘,二苯并[a,h]蒽,苯并[ghi]芘.
1.2.10 数据统计分析 所有生物测定结果均以3个重复组测定值的(平均值±标准差)表示.采用SPSS 19.0软件进行数据的正态性检验(Shapiro–Wilk法)和方差同质性检验(Levene法).若数据满足以上条件则进行方差分析,事后检验方法采用Tukey HSD,若方差不齐则事后检验采用Dunnett法.若数据不满足以上2个条件,则采用非参数检验的Kruskal Wallis法和Mann Whitney法进行显著性差异分析.不同发育时期之间的差异采用配对T检验法.<0.05表示差异显著.TPH浓度为3个重复样品的(平均值±标准差),使用OriginPro软件对TPH浓度和PAHs浓度进行拟合.
2 结果与讨论
2.1 暴露溶液组分分析
2.1.1 TPH浓度随时间和砾石粘油量的变化 粘油砾石柱是一种模拟近岸处沉降溢油的动态系统[41].结果表明随着海水流经粘油砾石柱,所有浓度组的流出液中的TPH浓度呈指数形式降低,并具有良好相关性(2>0.95)(见表1).这与溢油事故发生后实际测得的近岸处海水TPH浓度变化趋势一致[42].此外,根据拟合公式可对毒性进行定量表征,克服了动态暴露系统中暴露溶液浓度难以预测的缺点.
分析不同粘油量砾石柱在经海水冲洗相同时间后流出液中TPH浓度变化,发现TPH浓度与砾石粘油量呈正相关(2>0.84)(见表2).
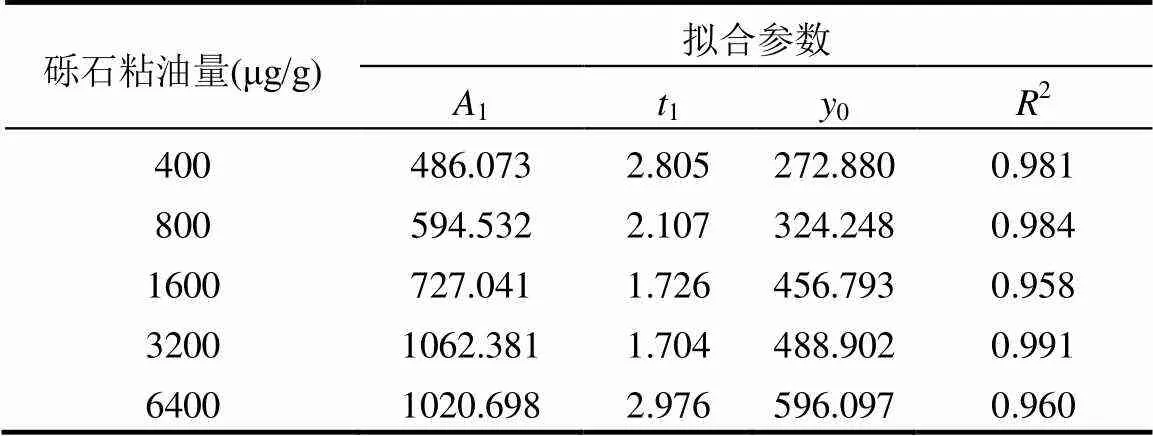
表1 流出液中TPH浓度随时间变化拟合参数
注:拟合公式:=1exp(/1) +0,式中为流出液中TPH浓度,μg/L;为暴露时间,d.
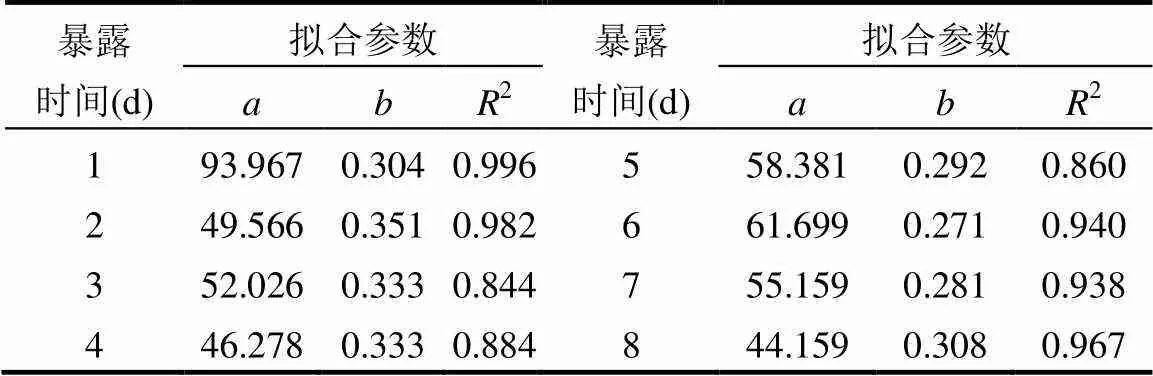
表2 流出液中TPH浓度随砾石粘油量变化拟合参数
注:拟合公式:=x,式中为流出液中TPH浓度,μg/L;为砾石粘油量,μg/g.
2.1.2 PAHs随时间的变化及其与TPH的关系 亲代海胆暴露期间溶液中PAHs浓度逐渐减小,并且各种组分之间的相对含量也有所变化.例如,6400μg/g组暴露溶液中的PAHs含量在亲代暴露期间由最初的13.140μg/L降低到结束时的3.530μg/L.开始暴露时,暴露溶液中最主要的PAHs为萘(84.4%),其次为菲(7.3%).萘的分子量相对低且易挥发,暴露结束时萘的含量仅占了38.2%,菲的相对含量增加到了27.5%[7].6400μg/g组粘油砾石上的PAHs浓度由9.806μg/g降低到了6.408μg/g,各组分的相对含量也发生改变,萘相对含量由28.4%降低到15.8%,而菲的相对含量由23.1%升高到28.2%.
通过分析6400μg/g组暴露溶液中PAHs浓度及其对应的TPH浓度在7d内的变化趋势,发现尽管PAHs的各种组分相对含量有所不同,但是PAHs浓度和TPH浓度在暴露期间呈现良好相关性(2= 0.866).考虑到TPH浓度与砾石粘油量呈良好相关性,因此本文中毒性均以砾石粘油量进行对比分析.
2.2 对亲代海胆生长繁殖的影响
暴露在粘油砾石柱流出液中7d后,海胆排配子率显著降低(Kruskal-Wallis,=0.033).粘油砾石对海胆排配子率的影响无性别差异性(Mann Whitney,>0.05).另外,与对照组相比((5551667±714587)个卵细胞),6400μg/g组雌海胆排卵数量减少((1957917± 811471)个卵细胞),繁殖力显著降低(1-way ANOVA,=0.036),表明粘油砾石流出液7d暴露影响了海胆的繁殖状态.这可能与压力环境下海胆性腺内性细胞再吸收作用相关,性细胞的再吸收可以使海胆将更多的能量分配到细胞解毒和保护过程中.
2.3 对配子质量的影响
暴露组卵细胞的大小与对照组相比无显著性差异(1-way ANOVA,>0.05).结果表明,粘油砾石暴露对卵细胞的尺寸无显著效应,说明卵细胞的大小不适合作为监测短期粘油砾石暴露对成年海胆繁殖状态影响的指标.已有研究发现海胆种群密度大的区域雌海胆所排卵细胞较小,而种群密度小的区域雌海胆所排卵细胞较大,说明卵细胞的大小具有可塑性[43].Schäfer等[44]的研究发现菲的浓度为500μg/L暴露20d,抑制海胆性腺内未成熟卵细胞的生长.据此推断本研究中卵细胞尺寸未发生变化可能是因为暴露时间较短,雌海胆未能及时调整卵细胞大小.
亲代海胆暴露在粘油砾石柱流出液中7d对受精率并无显著影响(2-way ANOVA,>0.05),最大受精率为800μg/g组的双本暴露受精卵((99.23± 0.69)%).结果表明,雄海胆短期暴露于粘油砾石流出液中并未影响精子的受精能力,与其他学者的研究结果一致[28,46].
2.4 对子代海胆胚胎早期发育的影响
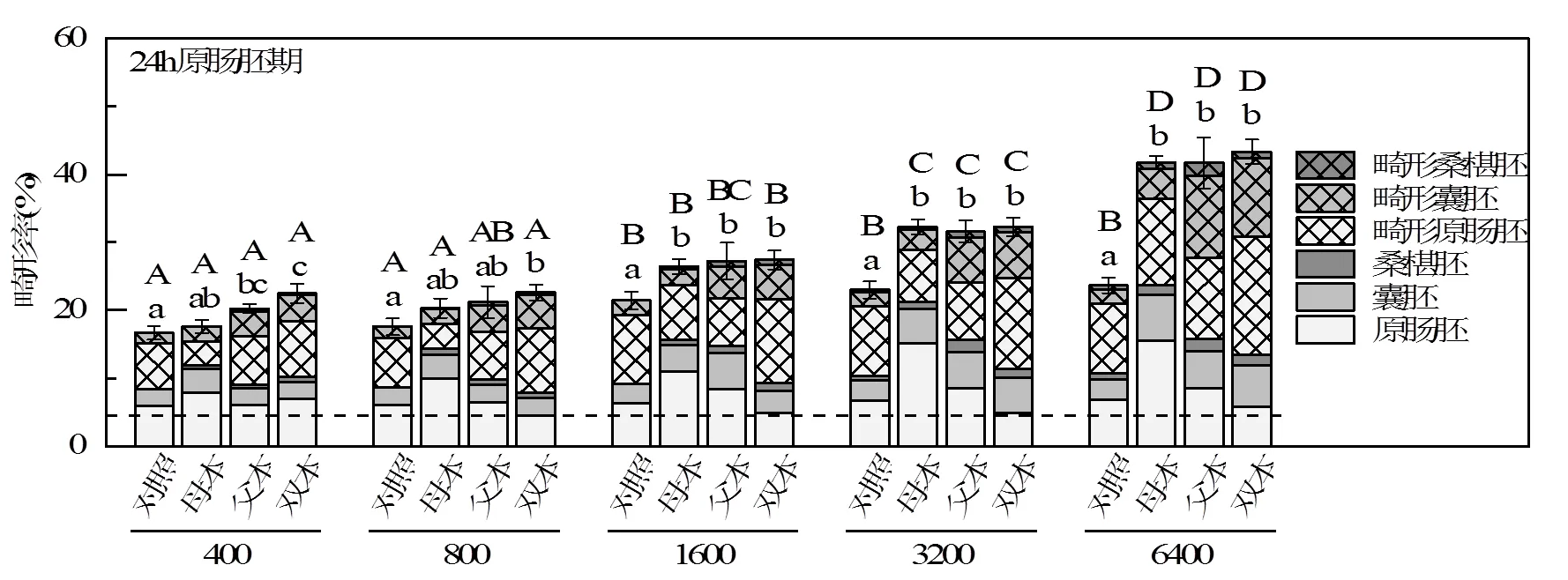
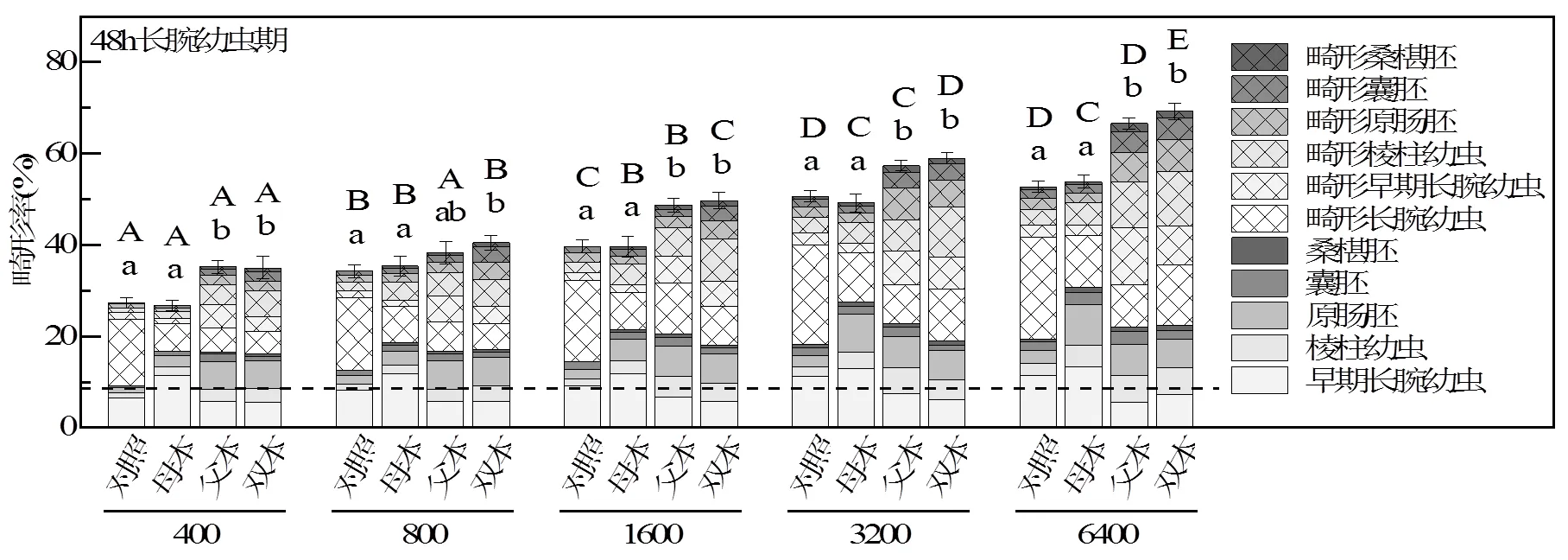
将各组子代继续暴露在相同浓度的流出液中培养48h,并在胚胎受精后24h原肠期(图3)和48h长腕幼虫期(图4)观测子代畸形率,并计算综合毒性指数(ITI,表3).生长在FSW中的对照组双亲子代畸形率始终低于10%.
砾石粘油量(μg/g)
不同字母表示具有显著性差异,大写字母表示同一亲本组合不同浓度组之间存在显著性差异,<0.05;小写字母表示同一浓度不同亲本组合之间存在显著性差异,<0.05.对照指双亲为对照组海胆的子代,母本、父本和双本指来自暴露亲本的子代.虚线指来自对照组双亲的子代在FSW中培养的畸形率.下同
砾石粘油量(μg/g)
2.4.1 亲代暴露史对子代的影响 由图3和图4可知,受精后24和48h,对于来自于对照组双亲的胚胎,随着子代暴露浓度增加,畸形率逐渐升高(ITI分别为0.58~0.92和1.38~2.76)(1-way ANOVA,<0.05).为了研究亲代海胆暴露于海底HFO对子代影响中的母本效应和父本效应,暴露组的卵细胞和精子分别与对照组的精子和卵细胞受精,胚胎继续暴露于其亲本的暴露浓度.随着亲本暴露浓度和子代暴露浓度的增加,母本暴露(ITI分别为0.54~1.45和1.1~ 2.57)、父本暴露(ITI分别为0.82~1.95和1.89~4.04)和双本暴露(ITI分别为0.88~2.08和1.91~4.14)的子代的畸形率显著增加(1-way ANOVA,<0.05).受精后24h,高浓度组(1600,3200和6400μg/g)的母本暴露、父本暴露和双本暴露的子代畸形率均显著高于对应组对照双亲的子代畸形率(1-way ANOVA,<0.05).表明亲代海胆暴露在粘油砾石流出液7d后,对子代胚胎发育产生毒性.Zhadan等[33]将海胆暴露于0.04~0.3mg/L的柴油水溶液中50d,并测定了卵细胞内钙浓度和子代发育情况,发现长期暴露于低浓度石油烃中雌海胆的卵细胞内具有显著升高的钙离子浓度,并且由这种卵细胞发育而来的子代胚胎畸形率也相应升高,这与本研究结果一致.
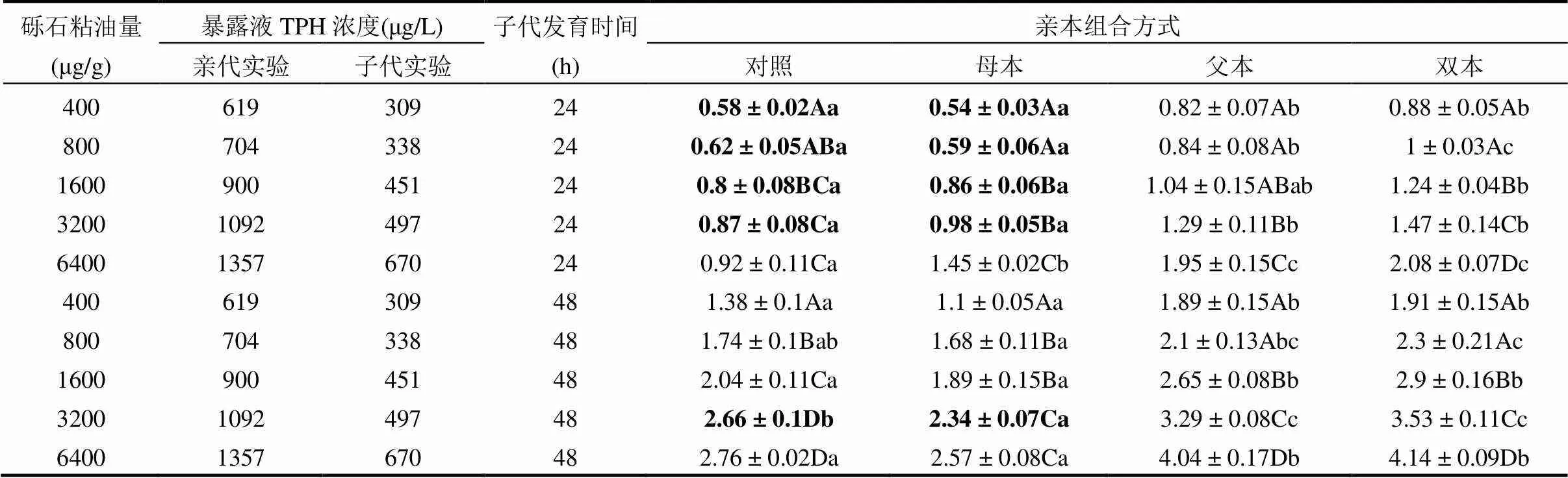
表3 亲代海胆暴露于粘油砾石柱产生的子代胚胎继续暴露24和48h的综合毒性指数
注:加粗字体为综合毒性指数与畸形率变化趋势不同的组别.
杜青平等[47]的研究结果表明,成年斑马鱼暴露在三氯苯中引起子代发育畸形.而Carls等[48]的研究结果表明,母本暴露在油中并没有对鱼的子代胚胎早期发育产生影响.这种差异可能与物种对污染物的敏感性不同有关[19].另外,本研究结果表明雄海胆的暴露对其子代发育也会产生影响.Lewis等[46]同样发现暴露在苯并芘中72h的雄贻贝所产子代的畸形率增加.
2.4.2 亲代暴露对子代影响的性别差异 随着胚胎发育的进行,48h时双本和父本暴露的子代畸形率显著高于对应浓度组的母本暴露子代和对照组子代的畸形率(图3,1-way ANOVA,<0.05).母本暴露子代的畸形率与对照组子代的畸形率无显著差异(图3,1-way ANOVA,>0.05).当暴露亲代为雌海胆时,对子代发育的影响最小,这可能与雌海胆具有较高的抗氧化能力有关.Schäfer等[44]报道雌海胆性腺与雄海胆相比具有更高的抗坏血酸浓度和较低的氧化损伤程度.Schäfer等[45]研究菲对雌海胆性腺损伤时发现主要受到影响的是未成熟的卵细胞,而已成熟的卵细胞并未遭受氧化损伤.Lister等[29]在研究膳食PAHs暴露对海胆繁殖损伤和子代的影响时,发现暴露组海胆产生的卵细胞的氧化损伤程度与对照组无显著性差异.据此作者推断,亲代海胆暴露在粘油砾石柱流出液中7d,由于雌海胆具有较高的抗氧化能力和对已成熟卵细胞具有保护作用,因此与精子相比,卵细胞受到的损伤更小,对子代的影响也较小.
海胆属体外受精动物,在受精之前精子和卵细胞被排放到海水中,精子和卵细胞对子代的发育具有同等的重要性[49].然而大多数野外和室内的毒理研究侧重于雌海胆暴露对子代胚胎发育的影响[29,31-34].本研究通过对来自父本暴露和对照组双亲的海胆子代胚胎畸形率比较,发现暴露在HFO中的父本对子代具有不可忽视的效应.这种父本效应可能来源于3种机制.其一,父本的生活环境对精子的形成具有选择性作用[50],属于环境诱导的父本效应.其二,父本受到的环境压力会使精子的基因表达情况发生改变,表观遗传突变不仅可以稳定的遗传给子代并且可以在子代中表达[51].其三,在环境压力的胁迫下父本的DNA受到损伤[46],也会引起子代畸形率的增加.事实上,在海洋无脊椎动物种群动态研究中发现精子是主要的限制因素[52-53].综合上述结果,本研究表明粘油砾石柱所模拟的海底HFO对雄海胆的精子损伤将会严重威胁到其子代胚胎的发育和种群的生存.
2.4.3 子代不同发育时期畸形率的差异 对比同一组胚胎在不同发育时期畸形率的差异,发现48h畸形率显著高于24h畸形率(图3和4,配对T检验,<0.05),表明随着暴露时间延长,致畸效应更加明显.随着胚胎发育的进行,48h母本暴露对子代的致畸作用逐渐降低,而父本暴露对子代的致畸作用越加明显.这可能与海胆早期胚胎发育过程中基因表达模式有关.有研究证明,囊胚期之前的胚胎发育过程由卵细胞中的母源mRNA控制,随着发育的进行受精卵的基因开始表达并逐步占据主导地位[54-55].因此父本效应随着胚胎发育而逐步显现出来.
2.4.4 子代综合毒性指数ITI 暴露海胆亲本产生的子代ITI见表3.结果表明除最高浓度组(6400μg/g),母本暴露组24h子代与来自于对照组双亲24h子代的ITI无显著性差异(表3,1-way ANOVA,>0.05).而畸形率在这两类亲本组合的24h子代之间存在显著性差异(图3,1-way ANOVA,<0.05).导致畸形率与ITI变化趋势不同的原因是母本子代胚胎的畸形类型中延迟发育的胚胎较多,致使ITI降低.母本暴露组48h子代的ITI与来自对照组亲本的48h子代无显著性差异(表3,1-way ANOVA,>0.05),这与48h畸形率变化趋势相同,除了3200μg/g组.在3200μg/g组,母本暴露的子代延迟发育较多,使其ITI显著低于对照组子代ITI.上述结果表明,与父本暴露相比,母本暴露对子代的影响较小,主要表现为胚胎畸形程度较轻.
3 结论
3.1 本文采用粘油砾石柱模拟海底HFO,在7d内间隙水中TPH浓度呈现指数式衰减,与溢油事故发生后实时监测的近岸TPH浓度变化趋势一致,因此该暴露装置能有效的模拟实际环境中HFO污染情况.
3.2 试验浓度下(400~6400μg/g)海底HFO对近岸底栖生物海胆产生毒性效应,雌雄海胆的排配子率和雌海胆繁殖力在最高浓度组(6400μg/g)受到显著抑制.
3.3 暴露于被海底HFO污染的孔隙水中7d,海胆卵细胞的大小和精子的受精能力并未受到显著影响.
3.4 繁殖期海胆受到HFO胁迫会显著增加子代胚胎发育的畸形率,HFO对亲代海胆的毒性传递给了子代.
3.5 与母本暴露相比,父本暴露对子代的影响较大,主要表现为胚胎畸形程度较重,并且ITI较高,即在HFO毒性由亲代传递给子代的过程中,父本效应起着关键作用.
[1] International Tanker Owners Pollution Federation. Oil Tanker Spill Statistics 2016 [EB/OL]. 2017: http://www.itopf.com/filea- dmin/data/ Documents/Company_Lit/Oil_Spill_Stats_2016_low_revised_Sep17.pdf.
[2] Spills of nonfloating oils: Risk and response [M]. Washington, DC: The National Academies Press, 1999:88.
[3] Ansell D V, Dicks B, Guenette C C, et al. A review of the problems posed by spills of heavy fuel oils [J]. International Oil Spill Conference Proceedings, 2001,2001(1):591-596.
[4] Murphy M L, Heintz R A, Short J W, et al. Recovery of pink salmon spawning areas after the Exxon Valdez oil spill [J]. Transactions of the American Fisheries Society, 1999,128(5):909-918.
[5] Marty G D, Heintz R A, Hinton D E. Histology and teratology of pink salmon larvae near the time of emergence from gravel substrate in the laboratory [J]. Canadian Journal of Zoology-Revue Canadienne de Zoologie, 1997,75(6):978-988.
[6] Marty G D, Hinton D E, Short J W, et al. Ascites, premature emergence, increased gonadal cell apoptosis, and cytochrome P4501A induction in pink salmon larvae continuously exposed to oil-contaminated gravel during development [J]. Canadian Journal of Zoology, 1997,75(6):989-1007.
[7] Carls M G, Rice S D, Hose J E. Sensitivity of fish embryos to weathered crude oil: Part I. Low-level exposure during incubation causes malformations, genetic damage, and mortality in larval pacific herring () [J]. Environmental Toxicology and Chemistry, 1999,18(3):481-493.
[8] Heintz R A, Short J W, Rice S D. Sensitivity of fish embryos to weathered crude oil: Part II. Increased mortality of pink salmon () embryos incubating downstream from weathered Exxon valdez crude oil [J]. Environmental Toxicology and Chemistry, 1999,18(3):494-503.
[9] Martin J D, Adams J, Hollebone B, et al. Chronic toxicity of heavy fuel oils to fish embryos using multiple exposure scenarios [J]. Environmental Toxicology and Chemistry, 2014,33(3):677-687.
[10] Furman B, Heck K L. Differential impacts of echinoid grazers on coral recruitment [J]. Bulletin of Marine Science, 2009,85(2):121-132.
[11] Hernández J C, Clemente S, Sangil C, et al. The key role of the sea urchinin controlling macroalgae assemblages throughout the Canary Islands (eastern subtropical Atlantic): An spatio-temporal approach [J]. Marine Environmental Research, 2008, 66(2):259-270.
[12] Rose C D, Sharp W C, Kenworthy W J, et al. Overgrazing of a large seagrass bed by the sea urchinin Outer Florida Bay [J]. Marine Ecology Progress Series, 1999,190:211-222.
[13] Pearse J S. Ecological role of purple sea urchins [J]. Science (New York, N.Y.), 2006,314(5801):940-941.
[14] Rial D, Radović J R, Bayona J M, et al. Effects of simulated weathering on the toxicity of selected crude oils and their components to sea urchin embryos [J]. Journal of Hazardous Materials, 2013,260: 67-73.
[15] Rial D, Vázquez J A, Murado M A. Toxicity of spill-treating agents and oil to sea urchin embryos [J]. Science of the Total Environment, 2014,472:302-308.
[16] Stefansson E S, Langdon C J, Pargee S M, et al. Acute effects of non-weathered and weathered crude oil and dispersant associated with the Deepwater Horizon incident on the development of marine bivalve and echinoderm larvae [J]. Environmental toxicology and chemistry, 2016,35(8):2016-2028.
[17] Bellas J, Saco-Álvarez L, Nieto Ó, et al. Evaluation of artificially- weathered standard fuel oil toxicity by marine invertebrate embryogenesis bioassays [J]. Chemosphere, 2013,90(3):1103-1108.
[18] Beiras R, Saco-Álvarez L. Toxicity of seawater and sand affected by the Prestige fuel-oil spill using bivalve and sea urchin embryogenesis bioassays [J]. Water, Air, and Soil Pollution, 2006,177(1):457-466.
[19] Bellas J, Saco-Álvarez L, Nieto Ó, et al. Ecotoxicological evaluation of polycyclic aromatic hydrocarbons using marine invertebrate embryo-larval bioassays [J]. Marine Pollution Bulletin, 2008,57(6): 493-502.
[20] Saco-Álvarez L, Bellas J, Nieto Ó, et al. Toxicity and phototoxicity of water-accommodated fraction obtained from Prestige fuel oil and Marine fuel oil evaluated by marine bioassays [J]. Science of the Total Environment, 2008,394(2):275-282.
[21] Lukyanova O N, Zhuravel E V, Chulchekov D N, et al. Sea urchin embryogenesis as bioindicators of marine pollution in impact areas of the Sea of Japan/East Sea and the Sea of Okhotsk [J]. Archives of Environmental Contamination and Toxicology, 2017,73(2):322-333.
[22] Bielmyer G K, Brix K V, Capo T R, et al. The effects of metals on embryo-larval and adult life stages of the sea urchin,[J]. Aquatic Toxicology, 2005,74(3):254-263.
[23] Cunha I, Garcia L M, Guilhermino L. Sea-urchin () glutathione S-transferases and cholinesterase activities as biomarkers of environmental contamination [J]. Journal of Environmental Monitoring, 2005,7(4):288-294.
[24] Yang B, Xiong D. Bioaccumulation and subacute toxicity of mechanically and chemically dispersed heavy fuel oil in sea urchin () [J]. Scientia Marina, 2015,79(4):497-505.
[25] Borges J C S, Branco P C, Pressinotti L N, et al. Intranuclear crystalloids of Antarctic sea urchins as a biomarker for oil contamination [J]. Polar Biology, 2010,33(6):843-849.
[26] Flammang P, Warnau M, Temara A, et al. Heavy metals in(Echinodermata, Echinoidea) from Singapore coral reefs [J]. Journal of Sea Research, 1997,38(1):35-45.
[27] Vashchenko M A. Effects of oil pollution on the development of sex cells in sea urchins [J]. Helgoländer Meeresuntersuchungen, 1980, 33(1):297-300.
[28] Lister K N, Lamare M D, Burritt D J. Pollutant resilience in embryos of the Antarctic sea urchinreflects maternal antioxidant status [J]. Aquatic Toxicology, 2015,161:61-72.
[29] Lister K N, Lamare M D, Burritt D J. Dietary pollutants induce oxidative stress, altering maternal antioxidant provisioning and reproductive output in the temperate sea urchin[J]. Aquatic Toxicology, 2016,177:106-115.
[30] Lister K N, Lamare M D, Burritt D J. Maternal antioxidant provisioning mitigates pollutant-induced oxidative damage in embryos of the temperate sea urchin[J]. Scientific Reports, 2017,7:1954.
[31] Migliaccio O, Castellano I, Cirino P, et al. Maternal exposure to cadmium and manganese impairs reproduction and progeny fitness in the sea urchin[J]. PLOS ONE, 2015,10(6): e0131815.
[32] Schweitzer L E, Bay S M, Suffet I H. Dietary assimilation of a polychlorinated biphenyl in adult sea urchins () and maternal transfer to their offspring [J]. Environmental Toxicology and Chemistry, 2000,19(7):1919-1924.
[33] Zhadan P M, Vaschenko M A. Effect of diesel fuel hydrocarbons on embryogenesis and45Ca2+uptake by unfertilized eggs of sea urchin,[J]. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology, 1993,105(3):543-548.
[34] Roepke T A, Chang E S, Cherr G N. Maternal exposure to estradiol and endocrine disrupting compounds alters the sensitivity of sea urchin embryos and the expression of an orphan steroid receptor [J]. Journal of Experimental Zoology. Part A, Comparative Experimental Biology, 2006,305(10):830-841.
[35] Morroni L, Pinsino A, Pellegrini D, et al. Development of a new integrative toxicity index based on an improvement of the sea urchin embryo toxicity test [J]. Ecotoxicology and Environmental Safety, 2016,123:2-7.
[36] Rahman M A, Uehara T, Rahman S M. Effects of egg size on fertilization, fecundity and offspring performance: A comparative study between two sibling species of tropical sea urchins () [J]. Pakistan Journal of Biological Sciences, 2002, 5(1):114-121.
[37] Pinsino A, Matranga V, Trinchella F, et al. Sea urchin embryos as an in vivo model for the assessment of manganese toxicity: Developmental and stress response effects [J]. Ecotoxicology (London, England), 2010,19(3):555-562.
[38] Henry J J. The development of dorsoventral and bilateral axial properties in sea urchin embryos [J]. Seminars in Cell & Developmental Biology, 1998,9(1):43-52.
[39] GB 17378.4-2007 海洋监测规范第4部分:海水分析 [S].
[40] GB/T 21247-2007 海面溢油鉴别系统规范 [S].
[41] Mark G C, Robert E T, Michael R L, et al. Mechanism for transport of oil-contaminated groundwater into pink salmon redds [J]. Marine Ecology Progress Series, 2003,248(3):245-255.
[42] Kim M, Hong S H, Won J, et al. Petroleum hydrocarbon contaminations in the intertidal seawater after the Hebei Spirit oil spill-Effect of tidal cycle on the TPH concentrations and the chromatographic characterization of seawater extracts [J]. Water Research, 2013,47(2):758-768.
[43] Levitan D R. Desity-dependent selection on gamete traits in three congeneric sea urchins [J]. Ecology, 2002,83(2):464-479.
[44] Schäfer S, Abele D, Weihe E, et al. Sex-specific biochemical and histological differences in gonads of sea urchins (Psammechinus miliarist) and their response to phenanthrene exposure. Marine Environmental Research, 2011,71(1):70-78.
[45] Schäfer S, Köhler A. Gonadal lesions of female sea urchin () after exposure to the polycyclic aromatic hydrocarbon phenanthrene [J]. Marine Environmental Research, 2009, 68(3):128-136.
[46] Lewis C, Galloway T. Reproductive consequences of paternal genotoxin exposure in marine invertebrates [J]. Environmental Science& Technology, 2009,43(3):928-933.
[47] 杜青平,刘伍香,袁保红,等.1,2,4-三氯苯对斑马鱼生殖和胚胎发育毒性效应[J]. 中国环境科学, 2012,32(4):736-741.
[48] Carls M G, Hose J E, Thomas R E, et al. Exposure of pacific herring to weathered crude oil: Assessing effects on ova [J]. Environmental Toxicology and Chemistry, 2000,19(6):1649-1659.
[49] Crean A J, Dwyer J M, Marshall D J. Adaptive paternal effects? Experimental evidence that the paternal environment affects offspring performance [J]. Ecology, 2013,94(11):2575-2582.
[50] Mazer S J, Gorchov D L. Parental effects on progeny phenotype in plants: distinguishing genetic and environmental causes [J]. Evolution; international journal of organic evolution, 1996,50(1):44-53.
[51] Curley J P, Mashoodh R, Champagne F A. Epigenetics and the origins of paternal effects [J]. Hormones and Behavior, 2011,59(3):306-314.
[52] Levitan D R, Petersen C. Sperm limitation in the sea [J]. Trends in Ecology & Evolution, 1995,10(6):228-231.
[53] Yund P O. How severe is sperm limitation in natural populations of marine free-spawners? [J]. Trends in Ecology & Evolution, 2000, 15(1):10-13.
[54] Gildor T, Malik A, Sher N, et al. Mature maternal mRNAs are longer than zygotic ones and have complex degradation kinetics in sea urchin [J]. Developmental Biology, 2016,414(1):121-131.
[55] Tu Q, Cameron R A, Davidson E H. Quantitative developmental transcriptomes of the sea urchin[J]. Developmental Biology, 2014,385(2):160-167.
Exposure of adult sea urchins to sunken heavy fuel oil affects the reproductive status and the development of their offspring.
DUAN Mei-na1, LIU Yong-jiang1, BAI Xue1, GAO Xiang1, ZHANG Xin-xin2, XIONG De-qi1*
(1.Department of Environmental Science and Engineering, Dalian Maritime University, Dalian 116026, China;2.Technology of Oily Sludge Eco-Treatment, Dalian 116000, China)., 2018,38(12):4720~4729
The present study investigated effects of exposure to sunken heavy fuel oil (HFO) on the fecundity, gamete quality and development of the offspring in the sea urchin. Adult sea urchins were exposed to effluents from HFO-oiled gravel columns for 7days to simulate an oil contaminated gravel shore. The spawning ability of adults and the fecundity ((1957917±811471) eggs) of females significantly decreased (=0.033 and=0.036, respectively). No effect was observed on the egg size and fertilization success. However, a significant increase in the percentage of abnormality of the offspring were observed, demonstrating that parental exposure (especially paternal exposure) had adverse effects on the offspring. The offspring from exposed fathers showed higher ITI values (ITI values of 24 and 48h offspring were 0.82~1.95 and 1.89~4.04, respectively) with a higher number of malformed embryos compared to maternal exposure (ITI values of 24 and 48h offspring were 0.54~1.45 and 1.1~2.57, respectively), indicating that detrimental effects of sunken HFO on the early development of sea urchin embryos may be largely attributed to paternal effects.
sunken heavy fuel oil;interstitial water;parental effects;sea urchins;early development
X55
A
1000-6923(2018)12-4720-10
段美娜(1991-),女,黑龙江七台河人,大连海事大学博士研究生,主要从事溢油毒理相关研究.发表论文7篇.
2018-05-10
国家自然科学基金资助项目(41276105)
* 责任作者, 教授, xiongdq@dlmu.edu.cn
