Novel approach to quantify the chemical stability and shelf life of modified double-base propellants
2018-12-25AshrfElghfourMostfRdwnAhmedFhdHosmMostfSherifElsuney
Ashrf M.A.Elghfour,Mostf Rdwn,Ahmed Fhd,Hosm E.Mostf,Sherif Elsuney,*
aSchool of Chemical Engineering,Military Technical College,Kobry El-Kobba,Cairo,Egypt
bBritish University in Egypt,Elshrouk City,Cairo,Egypt
Keywords:Double-base propellant Chemical stability Artificial aging Shelf life assessment
A B S T R A C T Double-base(DB)propellant is vulnerable to auto-catalytic decomposition reactions during storing with the evolution of nitrogen oxides.Modified DB propellant based on energetic nitramines(RDX)can offer enhanced thrust and action time.This study is devoted to evaluate the impact of RDX on chemical stability and shelf life of DB propellant.Extruded modified DB propellant based on RDX was manufactured by solventless extrusion process.Shelf life assessment was performed using an artificial aging at 70°C up to 120 days and employing Van't Hoff's formula.Quantification of evolved NOx gases and stabilizer depletion with aging time was conducted using Bergmann-Junk test and HPLC respectively.Modified DB formulation based on RDX 20 wt%demonstrated enhanced chemical stability and extended service life up to 46 years compared with reference formulation.This finding was ascribed to the high chemical and thermal stability of RDX as well as its compatibility with DB constituents;no side chemical reactions could take place during storing.This manuscript shaded the light on RDX as effective energetic constituent that offered DB propellants with enhanced performance,good chemical stability,and extended service life.
1.Introduction
Modified DB propellants have found wide applications in modern military and space rocketry,in view of their superior performance[1,2].Modified DB propellants are evolved by integrating energetic constituent such as HMX or RDX[3-6].RDX is one of the most commonly used energetic explosives for the development of modified DB propellants,as it can offer superior characteristics as illustrated in Table 1[7].
All these characteristics can allow RDX to be an ideal energetic constituent for DB propellants with enhanced combustion characteristics in terms of high combustion temperature,gaseous products,and specific impulse[7].Specific impulse is the thrust imparted to a vehicle per effective weight of propellants;it is expressed in second(s).Modified DB propellants based on RDX can offer wide range of specific impulse which can be varied from 220 to 270s[9-12].These characteristics can extend the application of DB propellants[12-14].Ourrecentresearch demonstrated enhanced combustion characteristics were achieved by integrating RDX into DB propellants[7,15].Fig.1 demonstrates the impact of RDX content on pressure-time curve of DB propellants using smallscale ballistic evaluation test motor.
RDX not only offered an increase in specific impulse and characteristic exhaust velocity of gaseous products but also offered an increase in the action time by 14.2%.Furthermore there was an increase in the total impulse with RDX content(Table 2).
The great impact of RDX on ballistic performance was attributed to the positive heat of formation(+417 kJ/kg).RDX is a highly effective explosive material with heat of explosion 5.647 kJ/g and gaseous product of 903 l/kg[16].RDX has also a slightly negative oxygen balance which means decomposition products of low molecular weight[16,17].These features indicate that RDX candecompose with the generation of massive amount of gaseous products at high temperature.This why enhanced specific impulse could be achieved as represented by Equation(1).
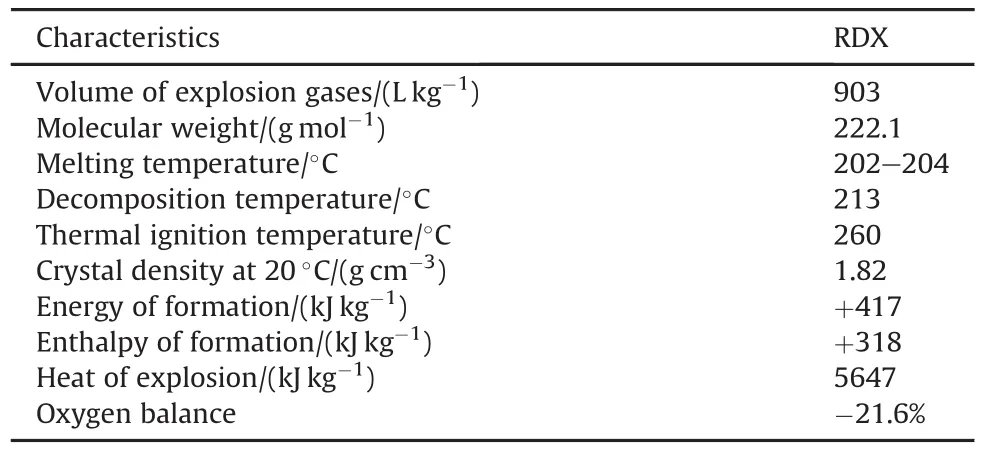
Table 1 Physical and thermal characteristics of RDX[8].
Where:Ispis the specific impulse,Tcis the combustion temperature,and M is the average molecular weight of gaseous products.
Even though,much research has been directed to investigate the combustion/ballistic characteristics of modified DB propellants[18,19];less attention has been directed to investigate the impact of different energetic additives on chemical stability and shelf life assessment[20].Chemical stability is one of the main problems of DB propellants as the main energetic constituents i.e.nitrocellulose(NC)and nitroglycerine(NG)are molecules that aren't chemically stable.While,decomposition of DB propellants is slow at ambient conditions;it becomes auto-catalytic in severe environmental conditions[10].The main mechanisms through which chemical decomposition can occur include:
1.1.Chain reactions
Chain reactions start with the homolytic breaking of the weak O-NO2bond,forming nitrogen dioxide and corresponding alkoxyl free radical[21-23].The generated reactive free radicals immediately undergo successive reactions with nearby nitrate ester molecules(Equation(2))[23].
1.2.Saponification(Hydrolysis)
Another decomposition mechanism is hydrolysis of the nitrateesters[22].This reaction is catalyzed by moisture and acidic residues(Equation(3)).
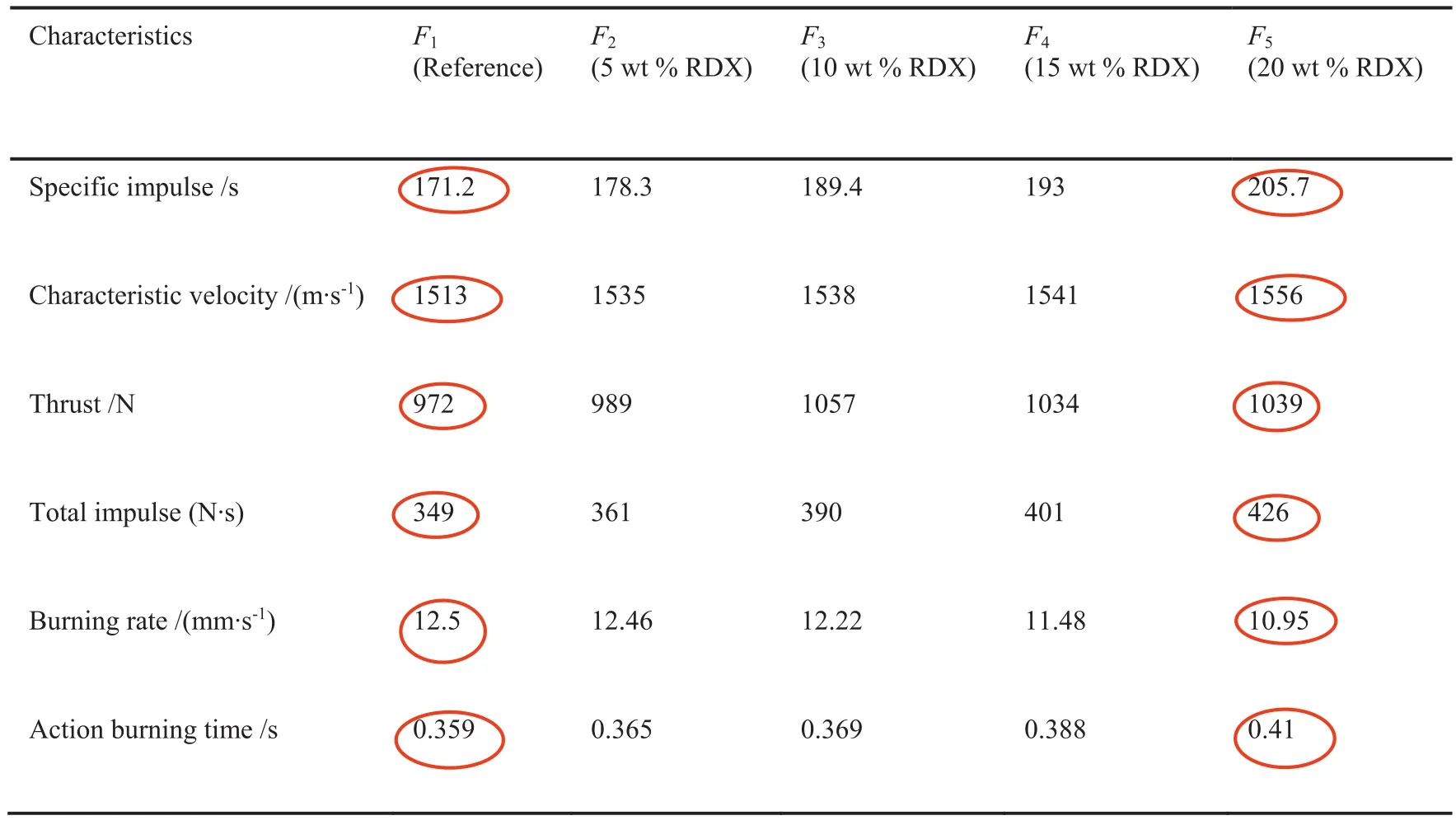
Table 2 Combustion characteristics of modified DB propellants based on RDX.
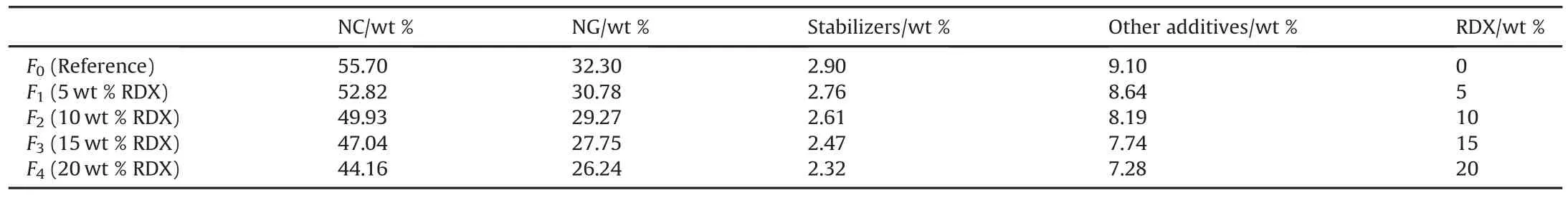
Table 3 Chemical structure of developed modified DB propellants.
A further decomposition reaction is the enhanced hydrolysis with low activation energy of 71 kJ/mol.It could be the dominant decomposition reaction at low temperatures[24].
1.3.Auto-catalytic reactions
Decomposition products of reactions(2)can further transformed in presence of moisture and oxygen as follow:
Whereas the primary hemolytic reactions(2-3)are very dif ficult being suppressed,the consecutive reactions(4-6)can be slowed down by binding or elimination of acidic residues from the system.Stabilization of DB propellants was achieved by integrating stabilizing agents[24,25].Stabilizers can bind with nitrogen oxides and neutralize the decomposition products[26].Stabilized DB propellants should offer a safe shelf life of at least 20 years[27].For modified systems containing energetic solid additives similar shelf life should be secured[28].Complete information regarding the influence of high energy ingredients on chemical stability and shelf life assessment is vital in regards of their handling,processing,transportation,and storage[29-32].
Many researchers have studied the rate of stabilizer depletion and the time to ignition of modified DB propellants[33].S.N.Asthana and R.B.Ghavate investigate the effect of high energy materials on stability and shelf life of modified DB propellant[34].The results of stability tests indicate higher stability of RDX,PETN and their combined formulations with modified double base propellants than ammonium perchlorate(AP)containing formulations.G.K.Gautarn et al.reported that energetic nitramine double base propellants are chemically stable based on data determined by Berman-Junk test and thus RDX appears to be compatible with double base matrix[16].This paper is devoted to investigate the impact of energetic nitramine(RDX)on chemical stability of DB propellants,as well as shelf life assessment.Shelf life assessment was conducted through artificial aging and Van't Hoff's formula.A novel approach to quantify the volume of decomposition gaseous products(NOx)using Pergman-Junk test and stabilizer depletion using HPLC with aging time is represented.RDX demonstrated enhanced chemical stability with aging time to reference formulation;furthermore it demonstrated extended service life of 46 years.This superior chemical stability could be attributed to the chemical/thermal stability of RDX.Great energy consumption is required to initiate RDX decomposition to that of common DB constituents mainly NC&NG.
2.Manufacture of modified DB propellants
It is widely accepted that screw extrusion technique can emphasize good mixing of different ingredients to the molecular level,high density,and dimensional stability.This technique includes different main stages including:
Blending,followed by rolling,grinding,granulation,and finally extrusion to obtain grains of desired shape and dimensions[35](as shown in Fig.2).
Different modified DB propellants based on RDX up to 20 wt%were developed by solventless extrusion technique.The chemical composition of the developed formulations is represented in Table 3.
3.Chemical stability of modified DB propellant
Chemical stability of modified DB propellants deals with the fact that the decomposition rate at normal temperature is judged from decomposition at higher temperature[36,37].Quantitative stabilitytests were employed for fast and reliable evaluation of DB propellants;they were devoted to the direct measurement of NOx evolved gasses[38].The most commonly used quantitative stability tests is Bergmann-Junk test.
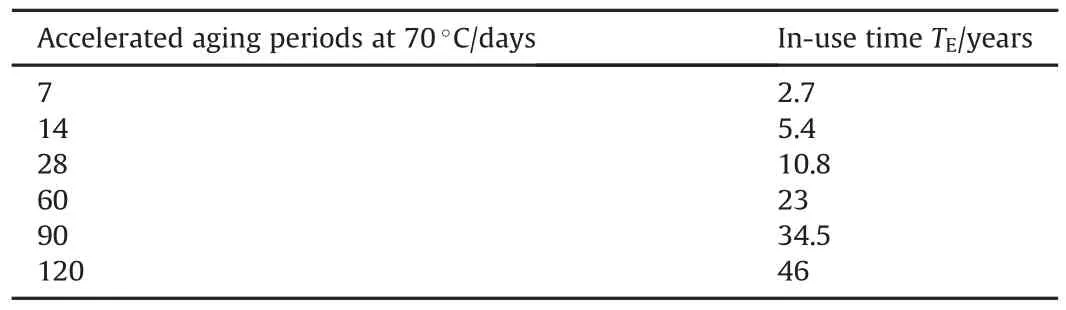
Table 4 Ageing times calculated on the basis of thermal equivalent load at TE=25°C using the generalized Van't Hoff's rule with factor F=3.
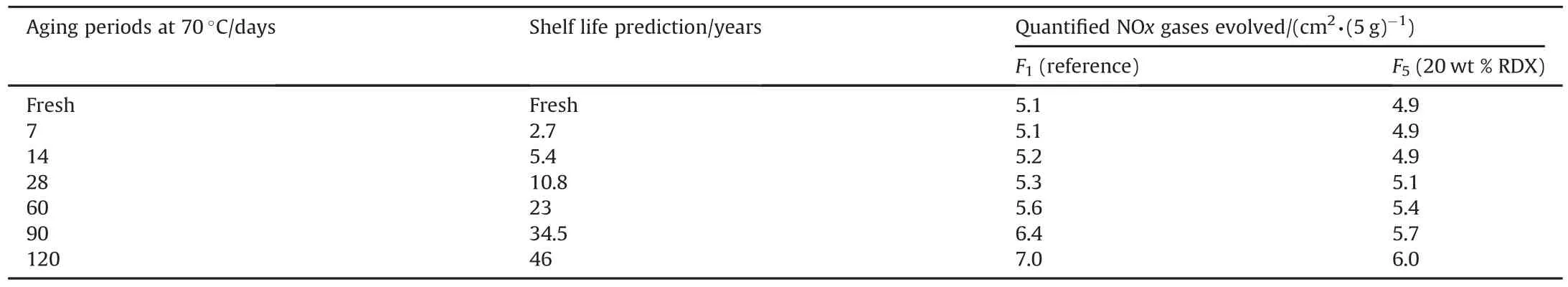
Table 5 Quantified NOx gases evolved from fresh and aged formulations by Bergmann-Junk test.
3.1.Quantitative determination of evolved NOxgases
Bergmann-Junk test is the main quantitative test for chemical stability evaluation DB propellants.In this test,5 g of the tested sample was heated at 120°C for 5 h.The evolved nitrogen oxides(NOx)were entrapped in a secondary tube containing 20 ml of potassium iodide.The evolved NOx gases were quantitatively determined by back titration with sodium thiosulphate(0.448N).The acceptable limit for Bergman-Junk test is 10ml of NOx/5g sample[8,39].
3.2.Quantitative determination of stabilizer content
Quantitative determination of stabilizer content was performed using high performance liquid chromatography(HPLC)according to STANAG 4620.Stabilizer extraction process was performed using Soxhlet extraction for 24h.Quantification of stabilizer content was conducted using HPLC.The operating conditions were,mobile phase composition(water 20%,methanol 51%,and acetonitrile 29%),column type is Zorbax SB-C18,25 cm length(Agilent),flow rate(1.6 ml/min),detector wave length(230 nm),and injection volume(100ml in methanol).
4.Shelf life assessment of DB propellants
Artificial aging was conducted to reduce the time scale by storing the propellant at elevated temperatures.This approach can offer prediction of service life in short time interval[40,41].Artifi cial aging was performed by isothermal heating at 70°C under ambient atmospheric conditions.The developed modified DB formulations were stored under isothermal heating for 7,14,28,60,90 and 120 days[42].Van't Hoff's formula(Equation(7))enabled the estimation of in-service periods at given in-storage temperatures,from the equivalent time-temperature loads during the artificial ageing.Van't Hoff's formula has been proved to be suitable to establish the time-temperature profile[43].
Where:TE,TT,F andΔTFare time in years at the in-service temperature(TEin°C),test time in days at the test temperature(TTin°C),reaction rate change factor per 10°C of temperature change(F usually between 2 and 4),and temperature interval for actual value F respectively.Factor F was determined using Arrhenius Equation(8)[43].
Where,Ea is the activation energy(kJ/mol),and R is the ideal gas constant[40].F factor was deduced by compiling and comparing reaction rates obtained at different temperatures[40].The rangefor this factor is often between 2 to 4[43].Table 4 demonstrates the accelerated ageing conditions simulating an in-use time up to 46 years at 25°C for developed modified DB propellants.
Modified DB formulation with 20 wt%RDX demonstrated the highest ballistic performance in terms of specific impulse as well as action time.The change in chemical stability of artificially aged DB formulation with 20 wt%RDX was evaluated by quantifying the volume of evolved NOx gases with aging time using Pergman-Junk test(Table 5).
It is apparent that modified DB formulation based on 20 wt%RDX demonstrated less NOx gases with aging time.Therefore this formulation not only offered enhanced ballistic performance but also enhanced chemical stability and extended service life to reference formulation.This enhanced chemical stability can be ascribed to the compatibility between RDX and DB constituents(no side reactions could take place);RDX could also offer enhanced thermal stability compared with DB constituents.It has been reported that some energetic additives such as ammonium perchlorate and potassium perchlorate are reactive toward nitrate esters(NC&NG).They deteriorate the chemical stability as they can initiate decomposition reaction[39].
Quantification of stabilizer content with aging time was conducted using HPLC.The stabilizer depletion of modified DB formulation to reference formulation was quantified with aging time as represented in Fig.3.
The stabilizer depletion rate for modified DB formulation is less than that for reference formulation.This finding confirms the obtained results from Bergman-Junk test.It is widelyaccepted that DB propellants is considered unstable if stabilizer depletion is?50%.
The superior chemical stability of modified DB propellant was correlated to the fact that the degradation of N-NO2requires great amount of activation energy than O-NO2.By contrast to high thermal stability of RDX which requires high energy to decompose conventional nitrate esters in DB propellants could take place easily by hemolytic breakdown of the weak O-NO2initiating a serious of decomposition reactions.This study shaded the light on RDX as energetic material that not only offered enhanced combustion characteristics in terms of specific impulse and total impulse but also offered enhanced chemical stability and extended shelf life up to 46 years.Novel artificial aging using Vant Hoff's equation with quantification of evolved NOx gases and stabilizer content with aging time was conducted.
5.Conclusion
Modified DB propellant based on RDX 20 wt%exhibited good chemical stability using quantitative chemical stability tests.Artifi cial aging and found to be an effective approach to facilitate the planning service life and to simulate the natural ageing of developed MDB with limited knowledge about their degradation behavior.Employing artificial aging and Vant Hoff's equation,modified DB formulation based on RDX exhibited service life up to 46 years.This novel finding confirms thatRDX has positivelyimpact the chemical stability and ballistic performance.
杂志排行
Defence Technology的其它文章
- Influence of control strategy on stability of dual-spin projectiles withfixed canards
- Study on buffering performance of thin-walled metal tube with different angles
- Path planning in uncertain environment by using firefly algorithm
- An experimental and numerical approach-characterisation of power cartridge for water-jet application
- Analysis and use of fuzzy intelligent technique for navigation of humanoid robot in obstacle prone zone
- The law of barrel wear and its application
