An Evaluation on the Ratio of Plant to Animal Protein in the Diet of Juvenile Sea Cucumber (Apostichopus japonicus): Growth, Nutrient Digestibility and Nonspecific Immunity
2018-12-20BAOPengyunLIXiaoyuandXUYongping
BAO Pengyun, LI Xiaoyu, and XU Yongping, *
An Evaluation on the Ratio of Plant to Animal Protein in the Diet of Juvenile Sea Cucumber (): Growth, Nutrient Digestibility and Nonspecific Immunity
BAO Pengyun1), 2), LI Xiaoyu1), 3), 4), and XU Yongping1), 3), 4), *
1) School of Life Sciences and Biotechnology, Dalian University of Technology, Dalian 116024, China 2)School of Fisheries and Life Sciences, Dalian Ocean University, Dalian 116023, China 3) Ministry of Education Center for Food Safety of Animal Origin, Dalian 116620, China 4)Liaoning Food Safety of Animal Origin Innovation Team, Dalian 116024, China
This study was conducted to evaluate the effects of plant/animal (P/A) protein ratios (viz.1:4, 1:3, 1:2, 1:1,2:1, 3:1, 4:1) on growth performance, body composition, apparent digestibility of diets, and nonspecific immunity of juvenile sea cucumber (). Sea cucumbers were divided into 21 plastic tanks, and each tank was stocked with 15 individuals (initial weight: about 23.73g). Each feed was allocated to three replicates of sea cucumbers. The feeding experiment lasted for 50 days. Results indicated that weight gain rate (WGR) and body wall weight (BWW) significantly increased as dietary ratio of P/A increased from 1:4 to 3:1, and then decreased significantly with further increase of this ratio (< 0.05). The body wall coefficient (BWC) showed a similar tendency to WGR and BWW, but no significance was detected among dietary treatments (> 0.05). The apparent digestibility of dry matter, protein and lipid increased with ratio of P/A increasing from 1:4 to 2:1 (< 0.05), and then decreased with further increase of this ratio. Correspondingly, activities of trypsin and amylase were significantly increased as P/A increased from 1:4 to 2:1 (<0.05). The activities of SOD and CAT showed a similar trend with WGR, with the highest value observed in the ratio of 1:2 and 1:1, respectively. Results above showed that moderate or relatively higher ratio of P/A protein (1:1-3:1) significantly increased the growth performance, apparent digestibility, and nonspecific immunity of sea cucumber. This will contribute to improving the feed formulation for juvenile cucumbers.
; plant protein; animal protein; growth; digestibility; nonspecific immunity
1 Introduction
Sea cucumbers (Echinodermata: Holothuroidea) have been used as functional food and traditional medicine in some Asian countries for a long time (Toral-Granda., 2008). In China, the sea cucumber,, is an traditionally edible and pharmaceutical marine product (Chen, 2003). It is widely believed that their nutritional and medicinal benefits attribute to high value-added compounds, riterpene glycosides, carotenoids, bioactive peptides, vitamins, minerals, fatty acids, collagens, gelatins, chondroitin sulfates, amino acids (Pangestuti and Arifin, 2017). Among all sea cucumbers,have showed outstanding advantages in the capacity of healing, neuro-protective, antitumor, anticoagulant, antimicrobial, and antioxidative (Sugawara., 2006; Pérez-Vega., 2013). During the last decades, the increasing demand has caused unsustainable exploit-ing of sea cucumbers. This resulted in the decline and even extinction of natural sea cucumber resources (Liao., 2015). Therefore, the farming ofhas seen a rapid expansion since 2000, especially with the progress in the seed production technology, to meet the rapidly increasing demand of sea cucumbers in China (Chen, 2005).
It is known that sea cucumbers are omnivorous invertebrates (Liu., 2009a; Li., 2015). They usually ingest large amounts of benthic biogenic sediments, including bacteria, fungi, protozoa, flagellates, diatoms, detrital organic matter (Yingst, 1976; Slater., 2011; Yokoyama, 2013; Shi., 2015a), and other benthic inorganic matter, such as particles of sea mud, soil or shell fragments from mollusks, crustaceans, and barnacles, and echinoderm (Liu., 2009a; Shi et al., 2015b). Preliminary studies showed that the optimal protein and lipid requirement for juvenileis about 16%-36% and 2%-5%, respectively (Zhu et al., 2005; Wang., 2009; Seo and Lee, 2011; Li., 2012; Wu., 2012). However, limited research has been conducted to investigate the ratio of plant to animal (P/A) protein in this species. In a previous study, the highest weight gain weight (WGR) of juvenilewas found when they were fed the diet with 25% fish meal (FM) substituted by a commercial spirulina (Tan., 2009). Fan. (2010) reported that the maximal WGR was obtained injuveniles fed a diet with 40% FM replaced by soybean meal (SBM). In another study, the highest WGR ofjuveniles was found when 60% FM was replaced by SBM with a total protein at 14% dry diet (Liao., 2015). However, as far as we know, no information is available about effects of replacing fish meal with plant protein blend (PPB) on the growth, digestibility and nonspecific immunity in this species. Different plant protein sources vary in the nutrient profile, such as protein, amino acid, mineral and vitamin, as well as palatability and digestibility. Replacement of FM by a blend of PPB sources in fish feeds is presently a major trend in aquaculture (Cabral., 2011).
Thus, the objective of this study was to evaluate the growth, body composition,digestibility, as well as nonspecific immunity of juvenile sea cucumber in response to graded dietary ratio of P/A protein by adjusting the percentage of FM and PPB (SBM, corn gluten and).
2 Materials and Methods
2.1 Experimental Design and Diets
The ingredients and approximate composition of the experimental diets are shown in Table 1. Seven isonitrogen (16% dry diet) and isolipidic (3.8% dry diet) experimental diets are formulated with different ratios of P/A (1:4, 1:3, 1:2, 1:1, 2:1, 3:1, and 4:1) by adjusting the levels of FM and PPB (SBM, corn gluten and). Soybean oil was used as the sole lipid source in the diets.
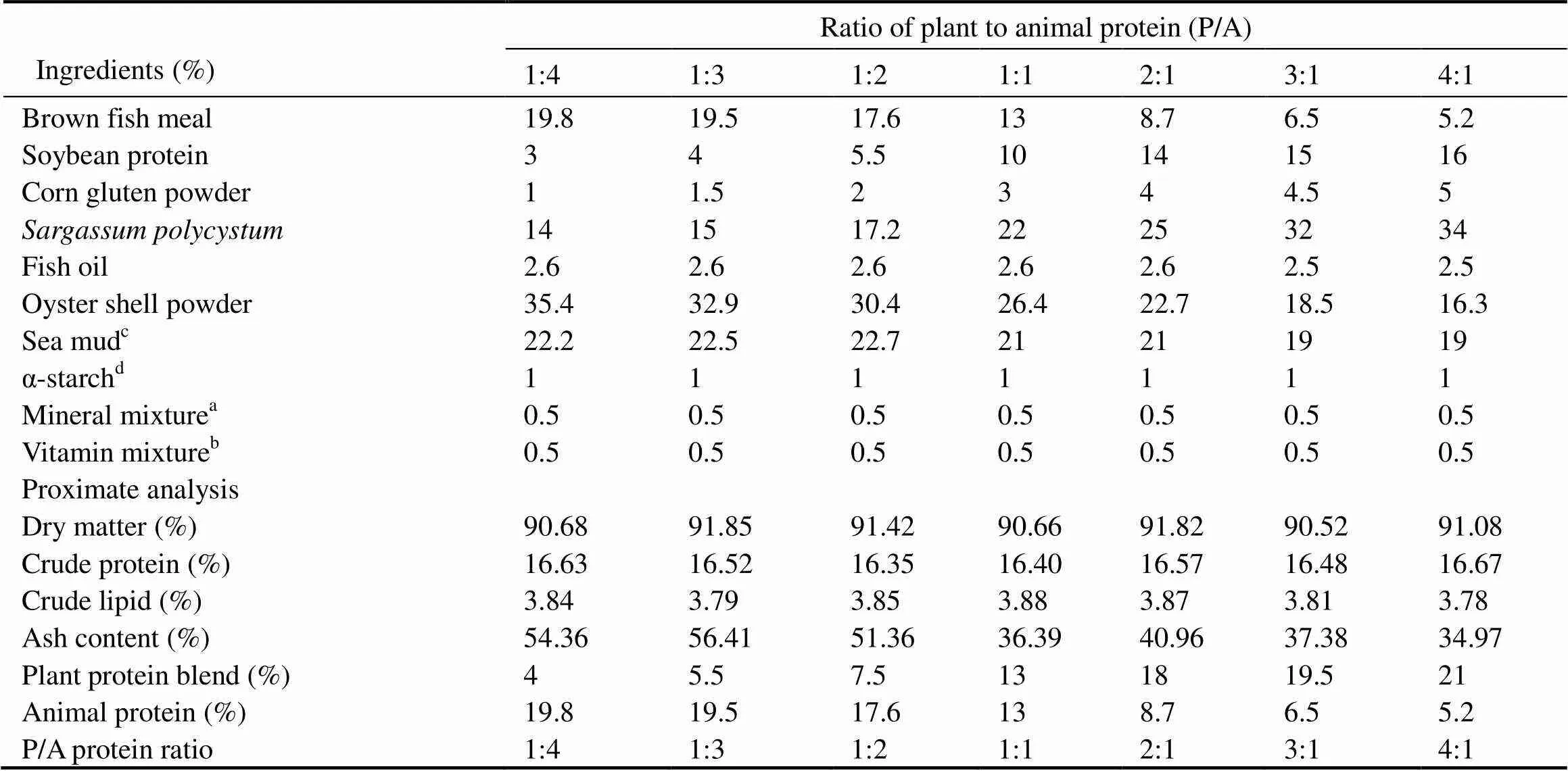
Table 1 Ingredients and proximate analysis of experimental feeds
Notes:aMineral mixture (gkg−1premix): NaCl, 8.0; MgSO4·7H2O, 2.22; Ca(H2PO4)2·2H2O,85.00; Fecitrate, 82.38; Calactate, 100.08; ZnSO4·7H2O, 7.90; CuSO4, 0.30; MnSO4·7H2O, 2.22; Ca(IO3)2, 0.50; KCl, 0.9; Na2SeO3, 0.02.bVitamin mixture (gkg−1premix): L-ascorbic acid, 22.0; retinyl acetate, 2.5; alpha-tocopheryl acetate, 70.0; pyridoxine hydrochloride, 8.0; niacin, 48.0; thiamin hydrochloride, 13.0; riboflavin, 10.0; calcium-D-pantothenate, 16.2; folic acid, 1.3; menadione, 9.8; cholecalciferol, 0.6; cyanocobalamin, 0.02; myoinositol, 150.0; D-biotin, 1.0.cSea mud (% dry weight): crude protein, 0.18±0.02%; crude lipid, 0.23±0.03%; ash, 96.24± 0.12%.dα-starch: potato starch.
Ingredients were completely ultramicro-porphyrized into particles of about 120 mesh size, and then mixed in designated proportions with clean fresh water. The pelleted feed was extruded by a moist pelleting machine, then dried at 45℃ for 24 h in a thermostatic oven. The dried feeds were stored at 4℃ in a freezer until use. The size of the final feed pellets was about 5×5mm. In addition, chromic oxide (0.5% total dry weight) was added in experimental diets as an inert tracer for determining apparent digestibility of nutrients.
2.2 Experimental Animal and Feeding Trial
Sea cucumbers () were purchased from a local culture farm (Dalian, China), and thereafter stocked in large temperature-controlled tanks. The sea cucumbers were adapted to the experimental environment for 15d by assigning a commercial feed (Lanhai feed mill, Dalian, China). Then, sea cucumbers with similar size were randomly allocated to 21 groups and placed in plastic boxes (80 × 50 × 40 cm). Each box was stocked with 15 individuals with an initial wet weight of about 23.73g. After acclimatization, individuals in each box were fed one of the seven feeds (3% wet body weight) once a day. The feeding experiment lasted for 50d. During the experiment, water temperature was maintained at 12±1℃; oxygen saturation exceeded 5.0mgL−1; the content of ammonia was lower than 0.20mgL−1; pH ranged from 7.8 to 8.2; the salinity values were within the normal range (28 to 30), and a photoperiod of 13:11 h (light/dark) was applied. One-third of the water in the box was exchanged every day, and uneaten food and feces were siphoned off. After 30d feeding experiment, the sea cucumbers began to be fed the corresponding experimental diets with chromic oxide.
2.3 Enzyme Activity
At the end of the feeding experiment, sea cucumbers in all treatments were starved for 24h, and then weighed individually after blot dried with filter paper. The alimentary tract between esophagus and cloaca was quickly removed and collected on ice. Then, samples from one tank were homogenized immediately in cold physiological saline water (4℃) in the proportion of 1:10 (w/v), followed by centrifugation (12000rmin−1, 20min, 4℃). The supernatants were removed into new centrifuge tubes, which were kept at 4℃ for later analysis of digestive enzyme activities. Then, coelomic fluid from ten sea cucumbers in each tank was collected. The upper fluid was obtained from the coelomic fluid by centrifugation (3000 r/min, 4℃, 5min), frozen in liquid nitrogen and stored at –80℃ until subsequent analysis for superoxide dismutase (SOD) and catalase (CAT) activities.
Activities of all digestive enzymes were assayed using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Amylase activity was assayed according to the method of Wang and Xu (2006), and one unit was defined as one milligramprotein that hydrolysed substrate at 37℃ for 30min. Trypsin activity was analyzed using the method described by Minekus. (2014), and one unit was defined asthe value that yields an increase in absorbance of 0.003min−1mg−1protein in intestine materials at 37℃ and pH 8.0. Lipase activity was assayed according to methods reported in Shihabi and Bishop (1971), and one unit of lipase activity was defined as 1μmol substrate consumed per min per gram protein in intestine materials at 37℃. Activities of glutamic-oxalacetic transaminase (GOT) and glutamic-pyruvic transaminase(GPT) in intestine of sea cucumber were determined colorimetrically according to Cheng. (2010), using an assay kit (Jiancheng Bioengineering Institute, Nanjing, China).
Activities of catalase (CAT) and superoxide dismutase (SOD) in the coelomic fluid were assayed using commercial kits (Jiancheng Bioengineering Institute, Nanjing, China). CAT was assayed according to Wang. (2008), and one unit was defined as the amount of enzyme required to decompose 1μmol of hydrogen peroxide per second at 37℃, pH 6.8. SOD was analyzed using a method as described by Wang. (2008), and one unit was defined as the amount of xanthine and xanthine oxidase required to yield a 50% inhibition of the xanthine reduction rate measured at 550nm.
2.4 Chemical Analysis
Samples of the feeds and body wall of sea cucumbers were stored at −20℃, and the approximate composition was analyzed according to the standard methods of the Association of Official Analytical Chemists (AOAC, 1990), with the results shown in Tables 1 and 3. Crude protein was examined using an Auto Kjeldahl system (Xinjia Electron Co. Ltd, Shanghai, China) with the Kjeldahl method after acid digestion. Crude lipid levels were determined according to ethyl ether extraction (Soxhlet technique). Moisture was tested by drying in an oven at 105℃ for 20h. The ash content was determined by combustion at 550℃ in a muffle furnace for 8h. Chromic oxide contents in diets and feces were determined by acid digestion with sulfuric acid and perchloric acid, following the method describled by Cruz-Suárez. (2009).
2.5 Calculation of Growth and Apparent Digestibility
At the beginning and the end of the feeding trial, sea cucumbers were starved for 24h, and then the initial and final wet weight of each individual was measured to calculate the specific growth rate (SGR), weight gain rate (WGR), and body wall coefficient (BWC) as follows:
SGR (% day−1) = 100 × (ln final weight −ln initial weight)/days,
WGR = 100 × (final body weight − initial body weight)/initial wet weight,
BWC = 100 × body wall weight (BWW)/ final body weight,
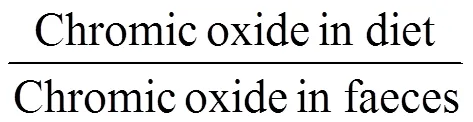
2.6 Statistical Analysis
Data were tested in SPSS 13.0 for windows (SPSS, Chicago, IL, USA). Data were analyzed for the homogeneity of variances before significance (<0.05) by using a one-way analysis of variance (ANOVA). Multiple comparisons among mean values were conducted with a Duncan’s multiple range test when significance of means were detected. Differences were considered to be significant at a 5% probability level.
3 Results
3.1 Growth performance
The weight gain rate (WGR), body wall weight (BWW), and body wall coefficient (BWC) of sea cucumbers are shown in Table 2. As the ratio of P/A increased from 1:4 to 3:1, WGR significantly increased from 81.53% to 123.58%, and then decreased significantly to 93.31% with further increase of P/A (<0.05). WGR of sea cucumbers fed diets with P/A at 2:1 (118.78 %) was comparable to that with P/A at 3:1 (123.58%) (>0.05), but was significantly higher than that with P/A at 1:4 (81.53%), 1:3 (88.15%), 1:1(88.44%) and 4:1 (93.31%) (<0.05). The BWW showed a similar tendency to WGR with the increase of P/A. The highest BWW was observed in sea cucumbers fed diets with P/A at 3:1, which was comparable to that with P/A at 2:1 (>0.05), but was significantly higher than that in the other groups (<0.05). BWC ranged from 61.15% to 63.67%, but no sig- nificance was detected among dietary treatments (> 0.05).
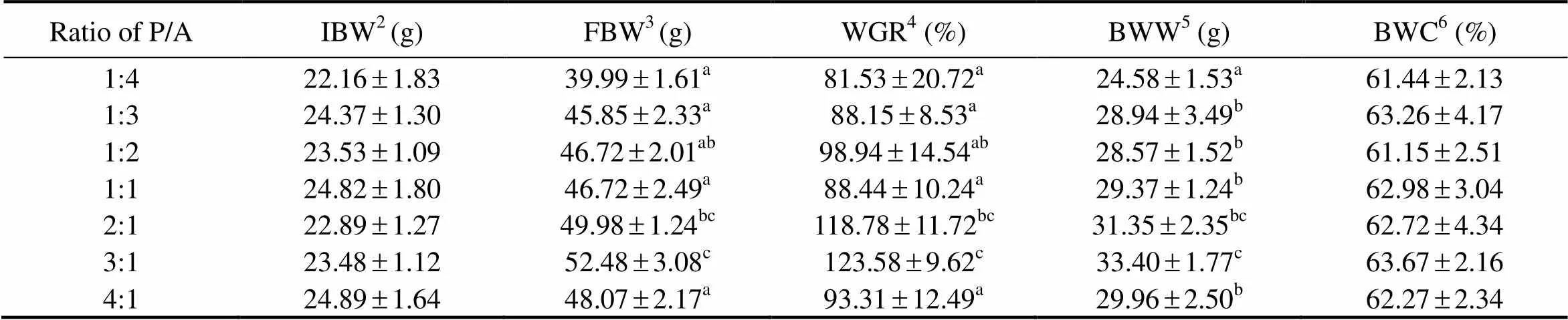
Table 2 Growth performance of sea cucumbers in response to graded ratios of plant to animal (P/A) protein*
Notes:*Value is given as mean ±SE (n=3), with different superscript letters indicating significant difference between dietary treatments (<0.05).2IBW: initial body weight;3FBW: final body weight;4WGR: weight gain rate;5BWW: body wall weight;6BWC: body wall coefficient.
3.2 Body Composition
The approximate composition of the whole body of sea cucumbers fed the experimental diets is shown in Table 3. Dry matter, crude protein, crude lipid, and ash content of sea cucumbers were 7.69%-8.12%, 39.84%-42.71%,8.12%-7.83%, and 42.71%-40.80% with the increase of dietary P/A. However, no significant differences were detected among dietary treatments (>0.05).
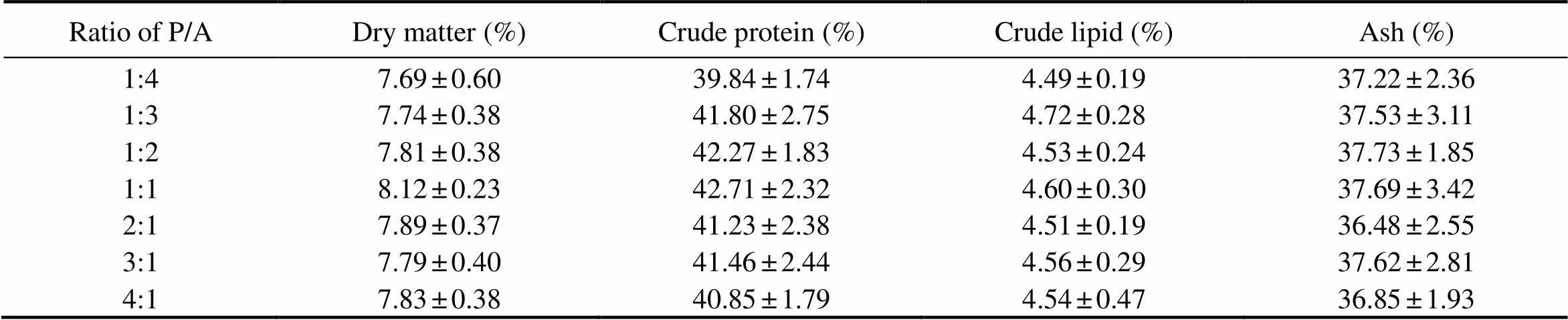
Table 3 Body composition of sea cucumbers (dry matter) in response to graded ratios of plant to animal (P/A) protein*
Notes:*Value is given as mean ±SE (n=3), with different superscript letters indicating significant difference between dietary treatments (<0.05).
3.3 Apparent Digestibility of Diets
Apparent digestibility coefficients (ADC) for dry matter (ADCD), ADC for protein (ADCP) and ADC for lipid (ADCL) for juvenile sea cucumber are listed in Table 4. As the ratio of P/A increased from 1:4 to 2:1, ADCD significantly increased from 36.54% to 63.34% (<0.05), and then decreased to 56.41% with further increase of P/A (>0.05). ADCP and ADCL showed a similar tendency to ADCD with the increase of dietary P/A. The highest values was observed in the diet with the P/A at 2:1, which were significantly higher than that in the diets with P/A equal to or lower than 1:1 (<0.05).
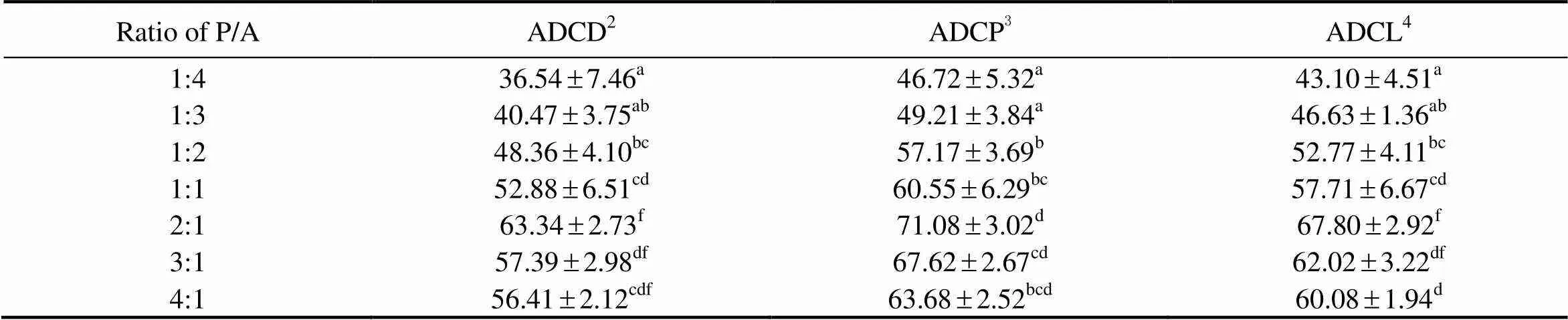
Table 4 Apparent digestibility coefficients for dry matter, crude protein and lipid of sea cucumber in response to graded ratios of plant to animal (P/A) protein*
Notes:*Value is given as mean ± SE (n = 3), with different superscript letters indicating significant difference between dietary treatments (< 0.05).2ADCD: apparent digestibility coefficient for dry matter;3ADCL apparent digestibility coefficient for lipid;4ADCP apparent digestibility coefficient for protein.
3.4 Activities of Digestive Enzyme and Protein Metabolism Related Enzymes
The intestinal digestive enzyme activities of sea cucumber are shown in Table 5. The P/A ratios of the diets had a significant influence on trypsin and amylase activities in the intestine (>0.05), whereas lipase activity was not significantly affected (>0.05). As the ratio of P/A increased from 1:4 to 1:1, trypsin activity significantly increased from 1042.33 to 1359.67 U/mg/prot (<0.05), and then decreased significantly increased to 1064.33 U/mg/prot with further increase of this ratio (<0.05). As the ratio of P/A increased, amylase activity significantly increased from 18.35 to 27.27 U/mg/prot (<0.05).
As the ratio of P/A increased from 1:4 to 2:1, GOT activity in the intestine of sea cucumbers significantly increased from 13.76 to 25.67 U/g prot (<0.05), and then decreased significantly increased to 22.37 U/g prot with further increase of this ratio (<0.05). As the ratio of P/A increased from 1:4 to 4:1, GPT activity increased from 11.03 to 14.63 U/g prot (>0.05) (Table 5).
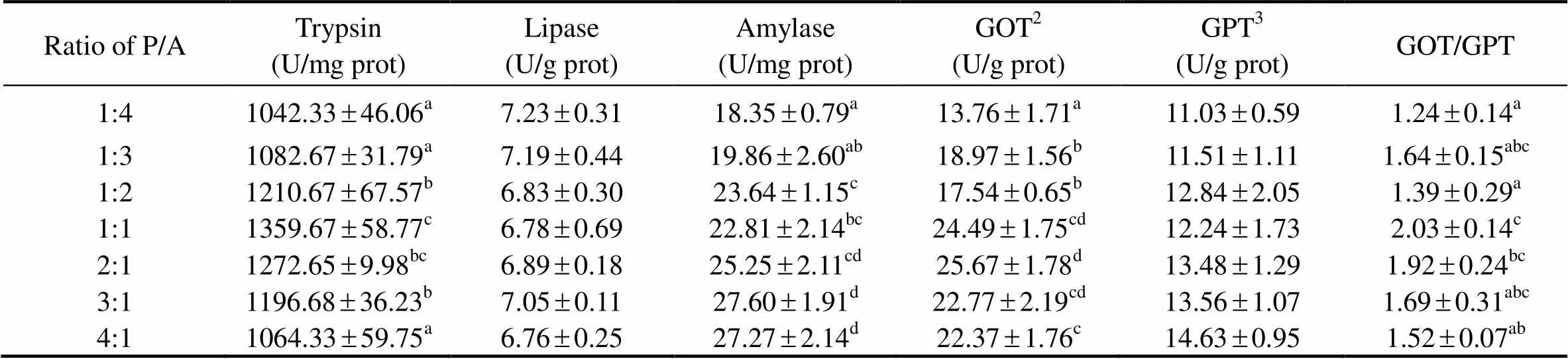
Table 5 The activities of digestive enzyme and protein metabolism related enzymes in the intestine of sea cucumbers in response to graded ratios of plant to animal (P/A) protein*
Notes:*Value is given as mean ±SE (n=3), with different superscript letters indicating significant difference between dietary treatments (<0.05).2GOT: glutamic-oxalacetic transaminase;3GPT: glutamic-pyruvic transaminase.
3.5 Activities of Immune Related Enzymes
As the ratio of P/A increased from 1:4 to 1:2, SOD activity in the coelomic fluid of sea cucumbers significantly increased from 39.13 to 67.35Ug−1prot (<0.05), and then decreased significantly increased to 41.42Ug−1prot with further increase of this ratio (<0.05). As the ratio of P/A increased from 1:4 to 1:2, CAT activity in the coelomic fluid of sea cucumbers significantly increased from 21.16 to 32.77Ug−1prot (<0.05), and then decreased significantly increased to 19.23Ug−1prot with further increase of this ratio (<0.05) (Table 6).
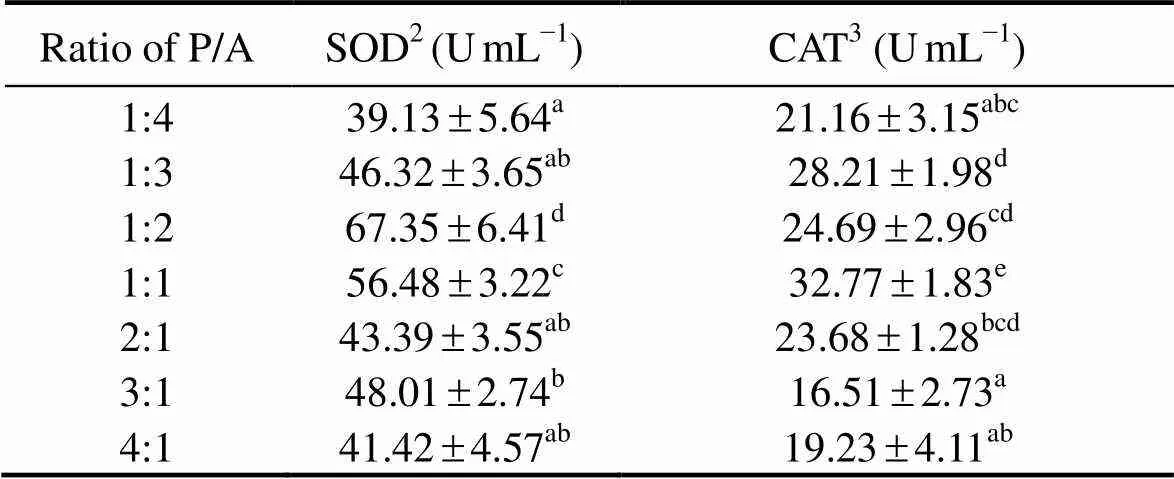
Table 6 The activities of immune related enzymes of sea cucumber in response to graded ratios of plant to animal P/A) protein*
Notes:*Value is given as mean ±SE (n=3), with different superscript letters indicating significant difference between dietary treatments (<0.05).2SOD: superoxide dismutase;3CAT: catalase.
4 Discussion
In this study, the highest WGR of sea cucumbers were found when the ratio of P/A protein was at 3:1. This was higher than the findings of some previous studies which found that 25% FM could be replaced by a commercial spirulina (Tan., 2009), or 40% FM replaced by SBM (Fan, 2010) and 60% FM replaced by SBM in the diets of sea cucumbers (Liao., 2015). The discrepancy could be due to the more balanced amino acid profile for the combination of several plant protein sources in this study. Furthermore, the SBM content was lower than 16% dry diet in the present study, and this means that anti-nutritive factors provided by SBM were relatively lower. Anti-nutritive factors, such as trypsininhibitor and phytic acid, have been found to exert negative effects on the structure of intestine, and thus decrease the growth rate of aquatic animals including fish species and shrimp (Ehsani., 2014; Li., 2014).
In the present study, the protein levels in the body wall of experimental organisms first showed an increasing trend then decreasing trend as the P/A protein ratio increased, with no statistical differences observed among dietary treatments. This was consistent with the findings of some previous studies, which have reported that body composition was not significantly affected for rainbow trout and Atlantic salmon with the change of dietary P/A protein ratio (Davies and Morris, 1997; Refstie., 2001). However, this was inconsistent with the findings of Fan. (2010) who found crude protein of sea cucumbers were significantly elevated when 40% SBM was replaced by FM. The discrepancy was probably due to the plant protein sources used in the two experiments. In this study, blend of three plant protein was included in the diet, while only SBM was used to replace FM in that study of Fan. (2010). It is known that there is a big variation in the amino acid pattern, especially essential amino acid, between SBM and FM. In addition, the initial body weight of experimental animals in this study (23.18g) was much larger than that in Fan. (2010). It seems that the larger in initial body weight of experimental animals, the less change in body composition when they are fed different diets.
Digestibility was usually used as an index to assess the feed utilization in aquatic animals. Digestibility could be affected by different species, ingredients, nutritional value and manufacturing methods. As the fish meal has seen a great shortage with the development of feed industry, more and more attention has been paid to the plant protein sources. In the present study, the digestibility of dry matter, crude protein and crude lipid were first increased and then decreased as the ratio of P/A increased. Previous studies found that higher animal protein increased the feed digestibility in tilapia () (Liu., 2009b), rainbow trout () (Yang., 2011), Pacific white shrimp () (Xie., 2016), and red sea bream(Hossain and Koshio, 2017). The discrepancy could be due to the relatively higher requirement for animal protein in most fish and shrimp species compared to sea cucumber. It has been known that sea cucumbers mainly feed on large algae and deposited matters, which are abundant in fiber and some anti-nutritional factors (Liao., 2015; Michio., 2003; Shi., 2015a). Thus, higher resistance to anti-nutritional factors and higher utilization of fibers could be responsible for the high feed digestibility by sea cucumbers that were fed diets with moderate ratio of animal protein to plant protein.
In thecurrent study, a moderate dietary P/A ratio (1:1) contributed to the highest activity of trypsin in the intestine of juvenile sea cucumbers. The findings of some previous studies on sea cucumber juveniles and tilapia have concluded that the protease activity was significantly affected by SBM content in the diet, with highest activity of protease observed in animals fed diets with moderate level of SBM (Fan., 2010; Lin., 2011; Liao., 2015). However, no significance was detected in the activity of protease of giantfreshwaterprawn () in response to graded level of SBM (25, 50, 75, and 100%) (Dong and Niu, 2000).Anti-nutritionfactors(ANFs)inthe SBM, such as trypsininhibitor and phytic acid, have been verified to exert inhibitoryeffects on the activity of protease (Ehsani., 2014; Li., 2014). In response to ANFs, animals may have corresponding mechanism to remove the negative effects by increasing the activity of protease. However, excess ANFs could combine large percentage of protease, and therefore animals showed decreased activity of this digestive enzyme. Similarly, as P/A ratio increased, the increasing activity of amylase could be the adaptive response to the increasing content of starch from corn gluten powder and SBM, as well as seaweed polysacchride from.
Glutamic-oxalacetic transaminase (GOT) and glutamic-pyruvic transaminase(GPT)are the two critical enzymes during transamination reactions(Song., 2016). In this study, the activity of GOT was significantly elevated as P/A increased from 1:4 to 2:1. This could be due to the relatively lower content of essential amino acids in the plant proteins, especially methionine and lysine.Thus, it is possible that sea cucumbers needed to synthesize more essential amino acids through transamination to fulfill their requirement. Indeed, the findings of some previous studies have showed that the increased ratio of GOT to GPT resulted in the increased amino acid synthesis, and thus increased the nitrogen retention (Deng., 2006; Leng., 2013). However, the activities of GOT and GPT were not significantly affected as the plant protein varied in the diets of large yellow croaker () (Wang., 2017) and juvenile Japanese flounder () (Pham., 2007; Wang., 2017). This could be due to different species and diet formulation. Thus, following studies are needed to remove this confusion.
SOD and CAT are generally considered to be cellular antioxidants scavenging superoxide anion and hydrogen peroxide, which has been extensively studied for a variety of animal species (Campa-Córdova., 2002; Hermes-Lima., 1998; Pörtner, 2002). They were taken as the indicators of non-specific immune capacity in sea cucumber (Bai., 2016). In the present study, sea cucumbers fed the diet with moderate ratio of P/A (1:2 and 1:1) contributed to the relatively higher activities of SOD and CAT. This was similar to the findings of Wang. (2017) who found that SOD activity was significantly decreased when FM was totally replaced by soybean protein concentrate (SPC). On one hand, it could be more balanced amino acid profile of the experimental diets when certain FM was replaced by the blend of several plant protein. However, activities of SOD and CAT were not significantly affected in juvenile Ussuri catfish () when FM was replaced by cottonseed meal up to 60% (Bu., 2017).
In conclusion, moderate or relatively higher ratio of P/A protein (1:1-3:1) significantly increased the growth performance, apparent digestibility, and nonspecific immunity of sea cucumber. Following studies are needed to elucidate mechanisms involved in this process.
Acknowledgements
This study was financially supported by the Natural Public Sciences and Technology Research Funds Projects of Ocean (201405003-3). The authors are grateful to Jianfeng Ding for the editorial advice during the manuscript preparation.
Association of Official Analytical Chemists (AOAC), 1990. Official methods of analysis. In:, 15th ed. Helrich, K., ed., Arlington, VA, USA, 1298pp.
Bai, Y., Zhang, L., Xia, S., Liu, S., Ru, X., Xu, Q., Zhang, T., and Yang, H., 2016. Effects of dietary protein levels on the growth, energy budget, and physiological and immunological performance of green, white and purple color morphs of sea cucumber,., 450 (2015): 375-382.
Bu, X. Y., Chen, A. J., Lian, X. Q., Chen, F. Y., Zhang, Y., Muhammad, I., Ge, X. P., and Yang, Y. H., 2017. An evaluation of replacing fish meal with cottonseed meal in the diet of juvenile Ussuri catfish: Growth, antioxidant capacity, nonspecific immunity and resistance to., 479 (10): 829-837.
Cabral, E. M. Bacelar, M., Batista, S., Castro-Cunha, M., Ozório, R.O. A., and Valente L. M. P., 2011. Replacement of fish meal by increasing levels of plant protein blends in diets for Senegalese sole () juveniles., 322-323 (12): 74-81.
Campa-Córdova, A., Hernández-Saavedra, N., De Philippis, R., and Ascencio, F., 2002. Generation of superoxide anion and SOD activity in haemocytes and muscle of American white shrimp () as a response to β-glucan and sulphated polysaccharide., 12 (4): 353-366.
Chen, J., 2005. Present status and prospects of sea cucumber industry in China., 25-38.
Cheng, Z., Ai, Q., Mai, K., Xu, W., Ma, H., Li, Y., and Zhang, J., 2010. Effects of dietary canola meal on growth performance, digestion and metabolism of Japanese seabass,., 305 (1): 102-108.
Cruz-Suárez, L. E., Tapia-Salazar, M., Villarreal-Cavazos, D., Beltran-Rocha, J., and Nieto-López, M. G., Lemme, A., Ricque-Marie, D., 2009. Apparent dry matter, energy, protein and amino acid digestibility of four soybean ingredients in white shrimpjuveniles., 292 (1): 87-94.
Davies , S.I., and Morris , P.C., 1997. Influence of multiple amino acid supplementation on the performance of rainbow trout,(Walbaum), fed soya based diets., 28 (1): 65-74.
Deng, J., 2006. Effects of animal and plant protein sources on feed intake, growth and protein and lipid metabolism of Japanese flounder,. Dissertation for the Doctoral Degree. Qingdao: Ocean University of China, 99-109 (In Chinese with an English abstract).
Dong, Y., and Niu, C., 2000. Effects of dietary protein source on growth and activities of digestive enzymes of giant freshwater prawn ().(). 36: 260-263 (In Chinese with an English abstract).
Ehsani, J., Azarm, H. M., Maniat, M., Ghabtani, A., and Eskandarnia, H., 2014. Effects of partial substitution of dietary fish meal by fermented soybean meal on growth performance, body composition and activity of digestive enzymes of juvenile yellowfin sea bream., 5: 99-107.
Fan, Y., Li, X., Luo, Z., and Sun, Z., 2010. Effects of replacement of dietary fish meal by soybean meal on growth,body composition and digestive enzyme activities in sea cucumberjuveniles., 25 (1): 71-75 (In Chinese with an English abstract).
Hermes-Lima, M., Storey, J. M., and Storey, K. B., 1998. Antioxidant defenses and metabolic depression. The hypothesis of preparation for oxidative stress in land snails., 120 (3): 437-448.
Hossain, M. S., and Koshio, S., 2017. Dietary substitution of fishmeal by alternative protein with guanosine monopho- sphate supplementation influences growth, digestibility, blood chemistry profile, immunity, and stress resistance of red sea bream,., 43 (11): 1-16.
Li, J., Dong, S., Tian, X., Shi, C., Wang, F., Gao, Q., and Zhu, C., 2015. Effects of the diatom Cylindrotheca fusiformis on the growth of the sea cucumberand water quality in ponds., 23 (4): 955-965.
Li, Y., Ai, Q., Mai, K., Xu, W., Deng, J., and Cheng, Z., 2014. Comparison of high‐protein soybean meal and commercial soybean meal partly replacing fish meal on the activities of digestive enzymes and aminotransferases in juvenile Japanese seabass,(Cuvier, 1828)., 45 (6): 1051-1060.
Li, S., Liang, M., Sun, H., and Jingping, Y., 2012. Optimum dietary protein and animo acid levels for the growth of juvenile sea cucumber., 33: 59-63 (In Chinese with an English abstract).
Liao, M., Ren, T., He, L., Han, Y., and Jiang, Z., 2015. Optimum dietary proportion of soybean meal with fish meal, and its effects on growth, digestibility, and digestive enzyme activity of juvenile sea cucumber., 81 (5): 915-922.
Liu, Y., Dong, S., Tian, X., Wang, F., and Gao, Q., 2009a. Effects of dietary sea mud and yellow soil on growth and energy budget of the sea cucumber(Selenka)., 286 (3): 266-270.
Liu, Y., Leng, and X., Li, X., 2009b. Effects of replacing fish meal with soybean meal on growth, digestibility and immunity of tilapia (Oreochromis niloticus× O. aureus)., 24: 95-100 (In Chinese with an English abstract).
Michio, K., Kengo, K., Yasunori, K., Hitoshi, M., Takayuki, Y., Hideaki, Y., and Hiroshi, S., 2003. Effects of deposit feederon algal bloom and organic matter contents of bottom sediments of the enclosed sea., 47 (1): 118-125.
Minekus, M., Alminger, M., Alvito, P., Ballance, S., Bohn, T., Bourlieu, C., Carriere, F., Boutrou, R., Corredig, M., and Dupont, D., 2014. A standardised static in vitro digestion method suitable for food–an international consensus., 5 (6): 1113-1124.
Pangestuti, R., and Arifin, Z., 2017. Pangestuti R, Arifin Z, Medicinal and health benefit effects of functional sea cucumbers,, http://dx.doi.org/10.1016/j.jtcme.2017.06.007.
Pérez-Vega, J. A., Olivera-Castillo, L., Gómez-Ruiz, J. Á., and Hernández-Ledesma, B., 2013. Release of multifunctional peptides by gastrointestinal digestion of sea cucumber ()., 5 (2): 869-877.
Pham, M. A., LEE, K. J., LIM, S. J., and Park, K. H., 2007. Evaluation of cottonseed and soybean meal as partial replacement for fishmeal in diets for juvenile Japanese flounder., 73 (4): 760-769.
Pörtner, H. O., 2002. Climate variations and the physiological basis of temperature dependent biogeography: Systemic to molecular hierarchy of thermal tolerance in animals., 132 (4): 739-761.
Purcell, S. W., Mercier, A., Conand, C., Hamel, J. F., Toral-Granda, M. V.n., Lovatelli, A., and Uthicke, S., 2013. Sea cucumber fisheries: Global analysis of stocks, management measures and drivers of overfishing., 14 (1): 34-59.
Refstie, S., Storebakken, T., Baeverfjord, G., and Roem, A. J., 2001. Long-term protein and lipid growth of Atlantic salmon () fed diets with partial replacement of fish meal by soy protein products at medium or high lipid level., 193 (1-2): 91-106.
Seo, J. Y., and Lee, S. M., 2011. Optimum dietary protein and lipid levels for growth of juvenile sea cucumbers., 17 (2): 56-61.
Shi, C., Dong, S., Pei, S., Wang, F., Tian, X., and Gao, Q., 2015a. Effects of diatom concentration in prepared feeds on growth and energy budget of the sea cucumber(Selenka)., 46 (3): 609-617.
Shi, C., Dong, S., Wang, F., Gao, Q., and Tian, X., 2015b. Effects of the sizes of mud or sand particles in feed on growth and energy budgets of young sea cucumber ()., 440 (4): 6-11.
Shihabi, Z. K., and Bishop, C., 1971. Simplified turbidimetric assay for lipase activity., 17 (12): 1150- 1153.
Slater, M. J., Jeffs, A. G., and Sewell, M. A., 2011. Organically selective movement and deposit-feeding in juvenile sea cucumber,determined in situ and in the laboratory. J, 409 (1-2): 315-323.
Song, Z., Li, P., Wang, J., Huang, B., Li, B., Wang, S., Zhang, Y., Gong, X., Li, X., and Tan, Q., 2016. Effects of seaweed replacement by hydrolyzed soybean meal on growth, metabolism, oxidation resistance and body composition of sea cucumber., 463 (10): 135-144.
Sugawara, T., Zaima, N., Yamamoto, A., Sakai, S., Noguchi, R., and Hirata, T., 2006. Isolation of sphingoid bases of sea cucumber cerebrosides and their cytotoxicity against human colon cancer cells., 70 (12): 2906-2912.
Tan, X., Luo, Z., Li, X., Zhang, S., and Sun, Z., 2009. Effects of dietary fishmeal replacement by different levels of alga Spirulina meal on growth performance and body composition of sea cucumber ()., 24 (1): 559-562 (In Chinese with an English abstract).
Toral-Granda, V., Lovatelli, A., Vasconcellos, M., Conand, C., Hamel, J. F., Mercier, A., Purcell, S.W., and Uthicke, S., 2008. Sea cucumbers. A global review on fishery and trade.. 28: 4-6.
Wang, P., Zhu, J., Feng, J., He, J., Lou, Y., and Zhou, Q., 2017. Effects of dietary soy protein concentrate meal on growth, immunity, enzyme activity and protein metabolism in relation to gene expression in large yellow croaker., 477 (8): 15-22.
Wang, F., Yang, H., Gao, F., and Liu, G., 2008. Effects of acute temperature or salinity stress on the immune response in sea cucumber,., 151 (4): 491-498.
Wang, J., Song, Z., Wang, S., Huang, b., Li, p., and Zhang, l., 2009. Study on dietary protein requirement for sea cucumber at different stages of growth., 36: 229-233 (In Chinese with an English abstract).
Wang, Y., and Xu, Z., 2006. Effect of probiotics for common carp () based on growth performance and digestive enzyme activities., 127 (4): 283-292.
Wu, Y., Wang, Q., Feng, Z., Li, B., and Zhu, W., 2012. The effect of dietary protein on the enzymes and intestinal structure of., 36 (1): 36-41 ( In Chinese with an English abstract).
Xie, S. W., Liu, Y. J., Zeng, S., Niu, J., and Tian, L. X., 2016. Partial replacement of fish-meal by soy protein concentrate and soybean meal based protein blend for juvenile Pacific white shrimp,., 464 (11): 296-302.
Yang, Y. H., Wang, Y. Y., Lu, Y., and Li, Q. Z., 2011. Effect of replacing fish meal with soybean meal on growth, feed utilization and nitrogen and phosphorus excretion on rainbow trout ()., 19 (3): 405-419.
Yingst, J.Y., 1976. The utilization of organic matter in shallow marine sediments by an epibenthic deposit-feeding holothurian., 23 (1): 55-69.
Yokoyama, H., 2013. Growth and food source of the sea cucumbercultured below fish cages-Potential for integrated multi-trophic aquaculture., 372-375 (1): 28-38.
Zhu, W., Mai, K., Zhang, B., Wang, F., and Xu, G., 2005. Study on dietary protein and lipid requirement for sea cucumber,., 29 (3): 54-58 (In Chinese with an English abstract).
November 6, 2017;
March 21, 2018;
March 29, 2018
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2018
. Tel: 0086-411-84709800 E-mail: xyping@dlut.edu.cn
(Edited by Ji Dechun)
杂志排行
Journal of Ocean University of China的其它文章
- Comparative Evaluation of Toleration to Heating and Hypoxia of Three Kinds of Salmonids
- Optimization of Hydrolysis Conditions for the Isolation of Angiotensin-I Converting Enzyme (ACE) Inhibitory Peptides from Rhopilema hispidum
- Trophic Interaction in a Portunus rituberculatus Polyculture Ecosystem Based on Carbon and Nitrogen Stable Isotope Analysis
- The Effect of Hydrolysis with Neutrase on Molecular Weight, Functional Properties, and Antioxidant Activities of Alaska Pollock Protein Isolate
- Weighted Correlation Network Analysis (WGCNA) of Japanese Flounder (Paralichthys olivaceus) Embryo Transcriptome Provides Crucial Gene Sets for Understanding Haploid Syndrome and Rescue by Diploidization
- Lead Induces Different Responses of Two Subpopulations of Phagocytes in the Holothurian Eupentacta fraudatrix
