Optimization of Hydrolysis Conditions for the Isolation of Angiotensin-I Converting Enzyme (ACE) Inhibitory Peptides from Rhopilema hispidum
2018-12-20SUNZhenliangQINHuanlongCAODuoYANXuebingLIHaoHUANGLinshengQUXiaoKONGChengandWANGMan
SUN Zhenliang, QIN Huanlong, CAO Duo, YAN Xuebing, LI Hao, HUANG Linsheng, QU Xiao, KONG Cheng, and WANG Man
Optimization of Hydrolysis Conditions for the Isolation of Angiotensin-I Converting Enzyme (ACE) Inhibitory Peptides from
SUN Zhenliang1), 2), #, QIN Huanlong1), #, CAO Duo3), YAN Xuebing1), LI Hao1), HUANG Linsheng1), QU Xiao1), KONG Cheng1), and WANG Man2), *
1),,,200072,2),,201499,3),,710069,
To obtain the maximum angiotensin-I converting enzyme (ACE) inhibitory activity, the protein hydrolysis conditions of the jellyfishwere optimized using response surface methodology (RSM). Trypsin was selected to produceprotein hydrolysates (RPH) with ACE inhibitory activity. The optimal parameters for producing protein hydrolysates with the highest ACE inhibitory activity were as follows: hydrolysis time 5h, hydrolysis temperature 50℃, and the enzyme-to-substrate ratio 6%. Under these conditions, the ACE inhibitory rate of RPH could reach 64.28%±5.72%. In addition, RPH contained high levels of Gly, Glu, Pro, Ala, Asp and Arg, with a molecular weight distribution range of 0.32–6.84kDa. The following three novel ACE inhibitory peptides were isolated and identified: Ile-Gly-Glu-Thr-Gly-Pro, Gly-Ala-Thr-Gly-Pro-Ala-Gly-Tyr-Val and Gly-Ala- Phe-Gly-Pro-Gly-Gly-Leu-Val-Gly-Arg-Pro. The IC50values of the ACE inhibitory activity of these three purified peptides were 19.07, 27.42 and 31.26μmolL−1, respectively. These results suggested that proteins and peptides isolated fromcould be utilized as antihypertensive functional food sources.
; ACE inhibitory peptide; response surface methodology; protein hydrolysate
1 Introduction
Hypertension is a major health issue affecting about 20% of the world’s adult population (Lee., 2009). Angiotensin-I-converting enzyme (ACE) is one of the primary regulators of blood pressure (BP) by converting angiotensin-I (Ang I) into the potent vasoconstrictor Ang II, and catalyzing the degradation of the potent vasodilator bradykinin (Liu., 2012). Inhibition of ACE activity can decrease the concentration of Ang II and consequently reduce BP (Skeggs., 1957). Natural ACE-I inhibitory peptides can control hypertension with minimum adverse effects, whereas synthetic ACE-I inhibitors cause adverse effects such as cough, dysgeusia and rash (Torruco-Uco., 2009). Consequently, several bioactive peptides with ACE inhibitory activities have been isolated from natural food protein sources such as soy, ribbonfish, shrimp, sandworm (Sun., 2017), and mushroom (Wu and Ding, 2002; Wang., 2011; Ais-saoui., 2017; Zhang., 2017). Among the various sources, marine organisms have been widely used in the search for ACE inhibitory peptides. The development of food protein hydrolysates with ACE inhibitory effects is considered as a safe alternative for synthetic ACE inhibitors in the management of hypertension.
is a species of the order, belonging to the class. It is an important species in Asian jellyfish fisheries and widely distributed in the South China Sea and the Pakistani coast. It has long been recognized as an effective medicine against asthma, bronchitis, hypertension and arthritis (Sun., 2015).is also a valuable raw material for producing bioactive peptides in disease treatments due to its rich protein content. In the present study, we optimized the hydrolysis conditions including hydrolysis time, temperature and the enzyme-to-substrate ratio (E/S) to obtain peptides with the maximum ACE inhibitory activity by response surface methodology (RSM). In addition, we isolated three novel ACE inhibitory peptides fromby ultrafiltration, gel chromatography and high performance liquid chromatography (HPLC), and finally identified them by the Edman degradation method.
2 Materials and Methods
2.1 Materials
Fresh samples of the jellyfishwere collected from the Beibu Gulf near Beihai city (Guangxi Province, China) in May 2015. The internal organs and impurities were immediately removed, washed with fresh water, and stored at −80℃ for further use. Pepsin, papain, flavorzyme, alcalase, trypsin, and protamex were purchased from Novo Nordisk A/S (Bagsvaerd, Denmark). Hippuric acid, hippuryl-l-histidyl-l-leucine (HHL) and ACE were purchased from Sigma (St. Louis, MO, USA). Other reagents were of analytical grades.
2.2 Screening of Commercial Proteinases
was hydrolyzed using six different commercial proteinases at the recommended pH and temperature as suggested by the manufacturer, and the proteinases include pepsin (pH 2, 37℃), papain (pH 6, 37℃), flavorzyme (pH 7, 50℃), alcalase (pH 8.5, 50℃), trypsin (pH 8, 50℃) and protamex (pH 7, 50℃). Enzymatic hydrolysis was carried out at a constant enzyme dosage, with an E/S ratio of 5%, and the same hydrolysis time of 4h. At the end of the hydrolysis, the enzyme was inactivated in a water bath at 100℃ for 10min, cooled to room temperature, and centrifuged at 2688for 15min. The supernatant was lyophilized for the determination of ACE inhibitory activity.
2.3 Determination of ACE Inhibitory Activity
The ACE inhibitory activity was assayed by measuring the concentration of hippuric acid liberated from HHL as described in a previous study (Liu., 2013). For each assay, 25mL ACE solution (10mUmL−1) was pre-incu- bated at 37℃ for 10min and then incubated with 10mL sample solution (1mgmL−1) and 150mL substrate (25mmolL−1HHL in 50mmolL−1sodium borate buffer containing 500mmolL−1NaCl at pH 8.3) at 37℃ for 30min. The reaction was terminated by adding 200mL 1molL−1HCl. Hippuric acid was extracted using 1500mL ethyl acetate. Then, a 1000-mL aliquot of the extract was removed by evaporation in a dry oven at 80℃. The residue was dissolved in 1mL distilled water, and its UV spectral absorbance was measured at 228nm. The ACE inhibitory activity was calculated using the following equation:
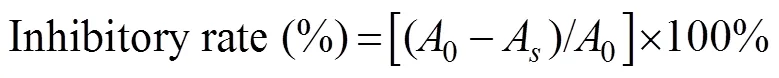
where0is the absorbance of the mixture containing ACE and HHL in the absence of the sample, andAis the absorbance of the abovementioned mixture (ACE and HHL) in the presence of the sample.
2.4 Optimization of the Hydrolysis Conditions
Based on the preliminary single-factor experiments, trypsin was selected as the hydrolysis enzyme. RSM was used to optimize the effects of the hydrolysis parameters, including the hydrolysis time, temperature, and E/S. The dependent variable was the ACE inhibitory rate.
A three-factor, three-level Box–Behnken experimental design (BBD) was used to further determine the optimal hydrolysis conditions (Table 1). Independent variables se- lected in the experimental design were as follows: hydrolysis time (X, h), hydrolysis temperature (X, ℃) and E/S (X, E/S, %w/w). A set of 17 experiments were carried out as shown in Table 2. For predicting the responses, the following second-order polynomial equation was used:
, (2)
whereis the dependent variable,βis an intercept, andβ,βandβare the coefficients of the linear, quadratic and interactive terms, respectively. Accordingly,XandXrepresent the coded independent variables. The experimental design was analyzed, and to estimate the response of the independent variables, the predicted data were calculated using the Design-Expert software (v.8.0.6.1 trial, State-East, Inc, Minneapolis, USA). The effect and re- gression coefficients of individual linear, quadratic, and interactive terms were measured according to the analysis of variance (ANOVA). The regression coefficients were subsequently applied to generate dimensional maps from the regression equation.
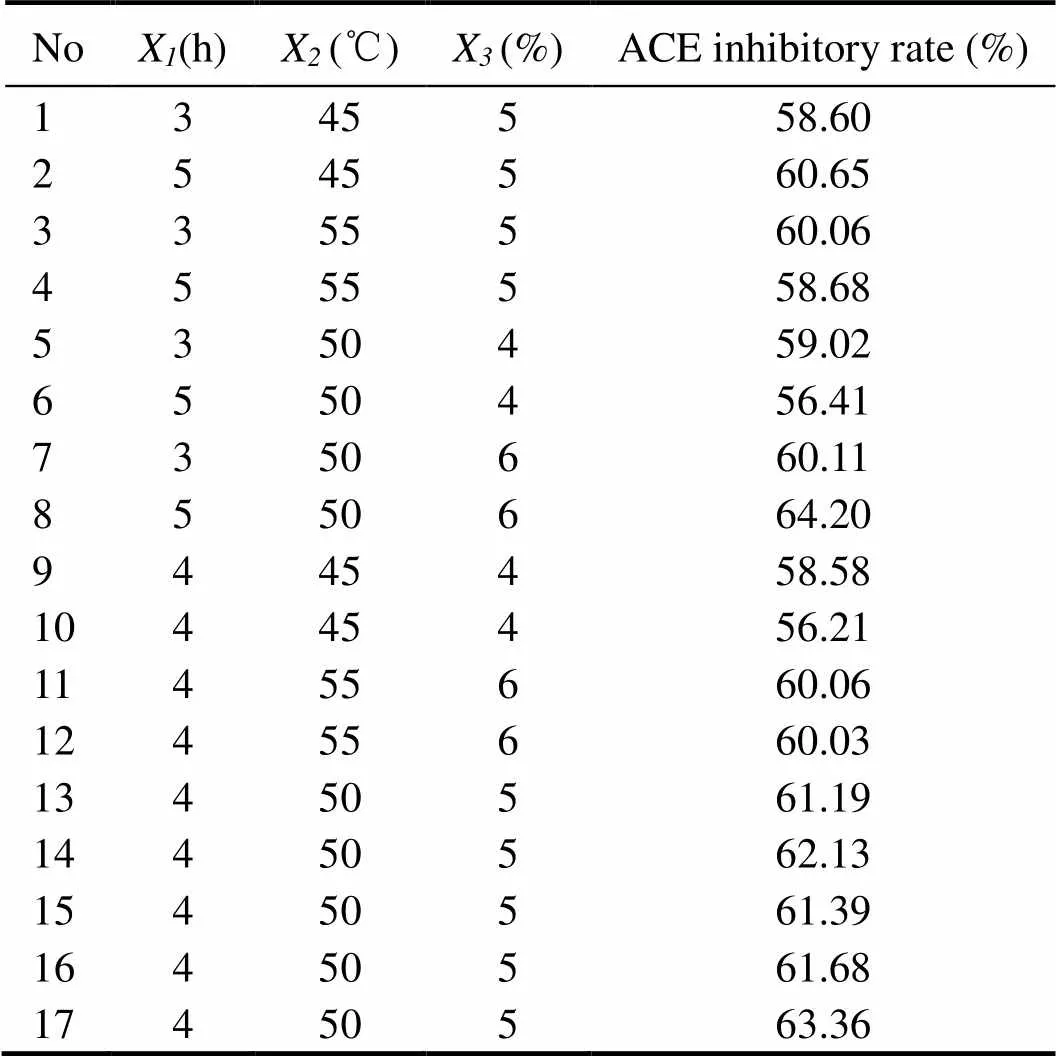
Table 1 Experimental design and results of Box–Behnken Design (BBD)
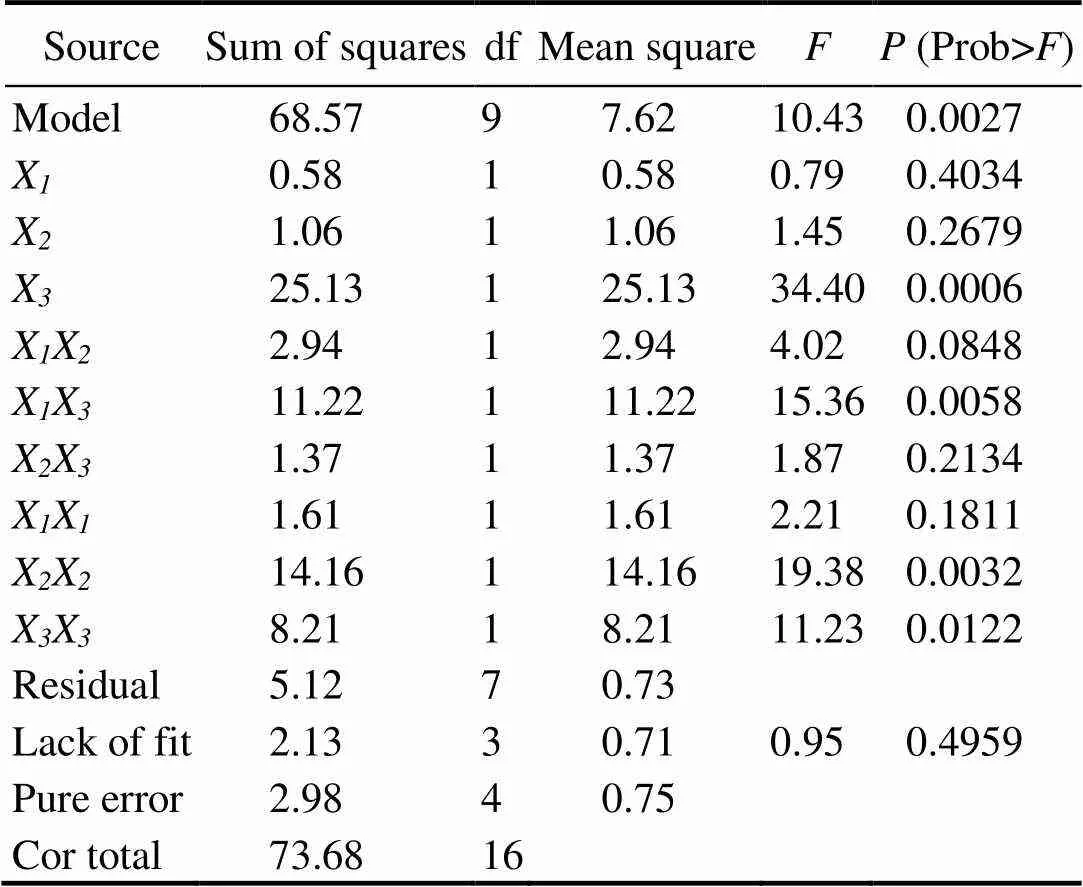
Table 2 Analysis of variance (ANOVA) of the experimental results of BBD
2.5 Amino Acid Composition and Molecular Weight Distribution Assay
Theprotein hydrolysate was ultrafiltered using a hydrophilic 5000-Da cut-off membrane. The fraction (RPH) passing through the 5000-Da membrane was lyophilized and used for further separation.
RPH (5mg) was added to glass ampoules. After addition of 5mL HCl (6molL−1), oxygen was expelled by pumping nitrogen gas into the sample. The glass ampoule was sealed using a Bunsen flame and placed in an oven at 110℃ for 12h. The hydrolysate was appropriately diluted and filtered through a 0.22-mm filter. The amino acid composition was determined using an amino acid analyzer (Hitachi, L-8000, Japan) according to the manufacturer’s instructions.
The molecular weight distribution of RPH was determined using a high performance gel-permeation chromatography (HPGPC) instrument. The sample (20mg) was dissolved in distilled water, passed through a 0.45-mm filter, and applied to a gel-filtration chromatographic co- lumn of TSK-Gel G2000PWXL(7.5×300mm). The column was maintained at 35℃ and eluted with acetonitrile:wa- ter:trifluoroacetic acid (45:55:0.1) at a flow rate of 0.5mL min−1. Column calibration was performed using standard peptides (cytochrome, 12500; aprotinin, 6500; bacitracin, 1450; Glycine-Glycine-Tyrosine-Arginine, 450; Glycine- Glycine-Glycine, 189). The calibration curve of log MW of standard peptides against their retention time (RT) was obtained (LogMW=−0.2407RT+6.9352,2=0.9812).
2.6 Purification of ACE Inhibitory Peptides
RPH was purified using a Sephadex LH-20 column (2.5×60cm) and eluted with distilled water at a flow rate of 0.6mLmin−1. The fractions were collected at 4-min intervals using a fraction collector, and the absorbance was measured at 220nm. Each fraction was assayed for the ACE inhibitory activity. The fraction from the Sephadex LH-20 column with the highest ACE inhibitory activity was further separated by RP-HPLC on a Waters Xbridge BEH C8 Prep Column (10×250mm, 5μm, Waters, Massachusetts, USA). The column was eluted by a linear gradient of acetonitrile (0%–40%) containing 0.1% trifluo- roacetic acid at a flow rate of 1.0mLmin−1. The absorbance of the eluent was monitored at 220nm. The fractions were collected according to the elution peaks and lyophilized immediately. The fraction with the highest ACE inhibitory activity was dissolved in distilled water for the second step of RP-HPLC on a Waters Atlantis T3 C18 Prep column (10×250mm, 5μm, Waters, Massachusetts, United States). The elution was conducted at a flow rate 0f 0.8mLmin−1using a linear gradient from 5% to 40% acetonitrile. The eluted peaks were detected at 220nm and lyophilized for determining the ACE inhibitory activity.
2.7 Amino Acid Sequence Analysis
The amino acid sequence of the purified peptides were determined using an automatic protein sequencer based on the Edman degradation method (PPSQ-31A, Shimadzu, Japan).
2.8 Statistical Analysis
The data presented are mean±SD of three independent experiments.
3 Results and Discussion
3.1 Screening of Proteinases
Six commercial food grade proteases were screened to hydrolyzeproteins. The hydrolysis conditions of 5% E/S and 4h hydrolysis time were the same for all enzymes. As shown in Fig.1, the ACE inhibitory rate of these hydrolysates varied substantially from 22.14% to 41.92%, of which trypsin showed the strongest ACE inhibitory activity. Therefore it was chosen as the best proteinase for the hydrolysis of.
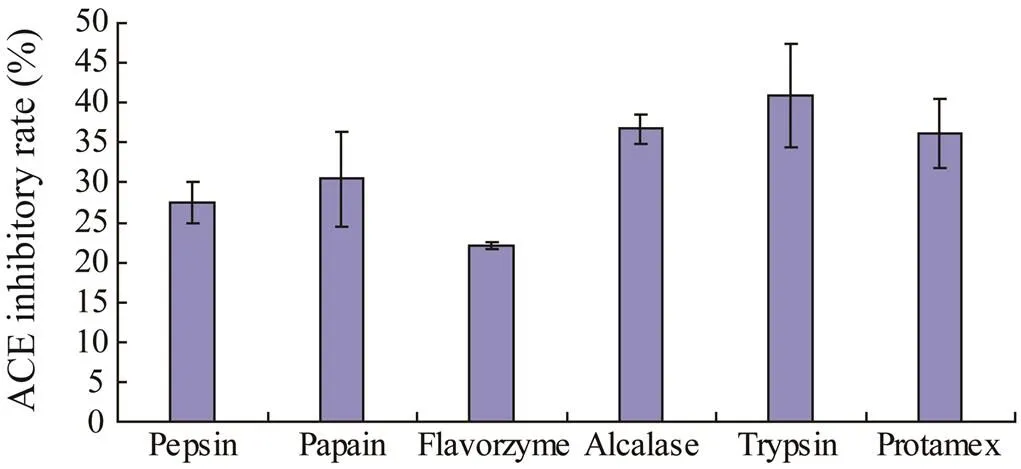
Fig.1 ACE inhibitory activities of R. hispidum protein hydrolysates derived with six commercial food grade proteases.
3.2 Response Surface Analysis
The enzymatic hydrolysis conditions were optimized to determine the optimal values of the independent variables (hydrolysis time, hydrolysis temperature and E/S) for obtaining the maximum ACE-inhibitory activity. The results obtained after 17-run trials according to BBD are presented in Table 1. The responses of these three variables to the hydrolysis conditions, the interactive terms, and the probability values (values) are shown in Table 2. The effects with<0.05 indicated the statistical validity and the significance of the ACE inhibitory rate model.
As shown in Table 2, the effect of E/S was the most significant, with a confidence interval of 99%, followed by hydrolysis temperature and hydrolysis time. Although the effect of hydrolysis time and hydrolysis temperature did not have a significant impact, the interaction termXXand the quadratic termsXXandXXwere statistically significant. According to the multiple regression analysis, the following second-order polynomial regression equation could explain the experimental data:
(3)
The significance of each factor was checked using the-test and thevalue. Table 2 shows the analysis of variance (ANOVA) results for the response surface quadratic model. The determination coefficient (2=0.9306) indicates that the model was adequate for prediction within the range of the experimental variables. The lack of fit was not significant, which further validates the model.
The relationship between the three variables was illustrated in 3D response surfaces and 2D contour plots generated by the model for the ACE inhibitory rate (%), and the two variables were depicted in a 3D surface plot while the other variable remained at zero level. Figs.2A and D depict the effect of hydrolysis time (X), hydrolysis temperature (X), and their reciprocal interaction on the ACE inhibitory rate when E/S was maintained at a constant level. The results indicated that the ACE inhibitory rate ofprotein hydrolysate increased with the increase in hydrolysis time and temperature up to an optimum point, beyond which there was a decrease in the ACE inhibitory rate with an increase in the variables. The impacts of hydrolysis time (X) and E/S (X) are illus- trated in Figs.2B and E, indicating that they had a significant mutual effect on the ACE inhibitory rate. Similarly, Figs.2C and F show that hydrolysis temperature (X) and E/S (X) had a quadratic effect on the ACE inhibitory rate when the hydrolysis time (X) was fixed at the zero level. The optimal conditions were predicted by ‘Numerical Optimization’ of the Design-Expert software. The optimal conditions of the three parameters are as follows: hydrolysis time 5h, hydrolysis temperature 49.15℃ and E/S 6%. Under these conditions, the predicted ACE inhibitory rate was 63.71%. Taking into account the practical feasibility, the parameters were adjusted as follows: hydrolysis time 5h, hydrolysis temperature 50℃, and E/S 6%. To confirm the validity of the model, the experiment was performed at optimal conditions, resulting in an ACE inhibitory rate of 64.28%±5.72%. The experimental values were in good agreement with the predicted value, suggesting that these conditions were suitable for producing ACE inhibitory peptides fromproteins.
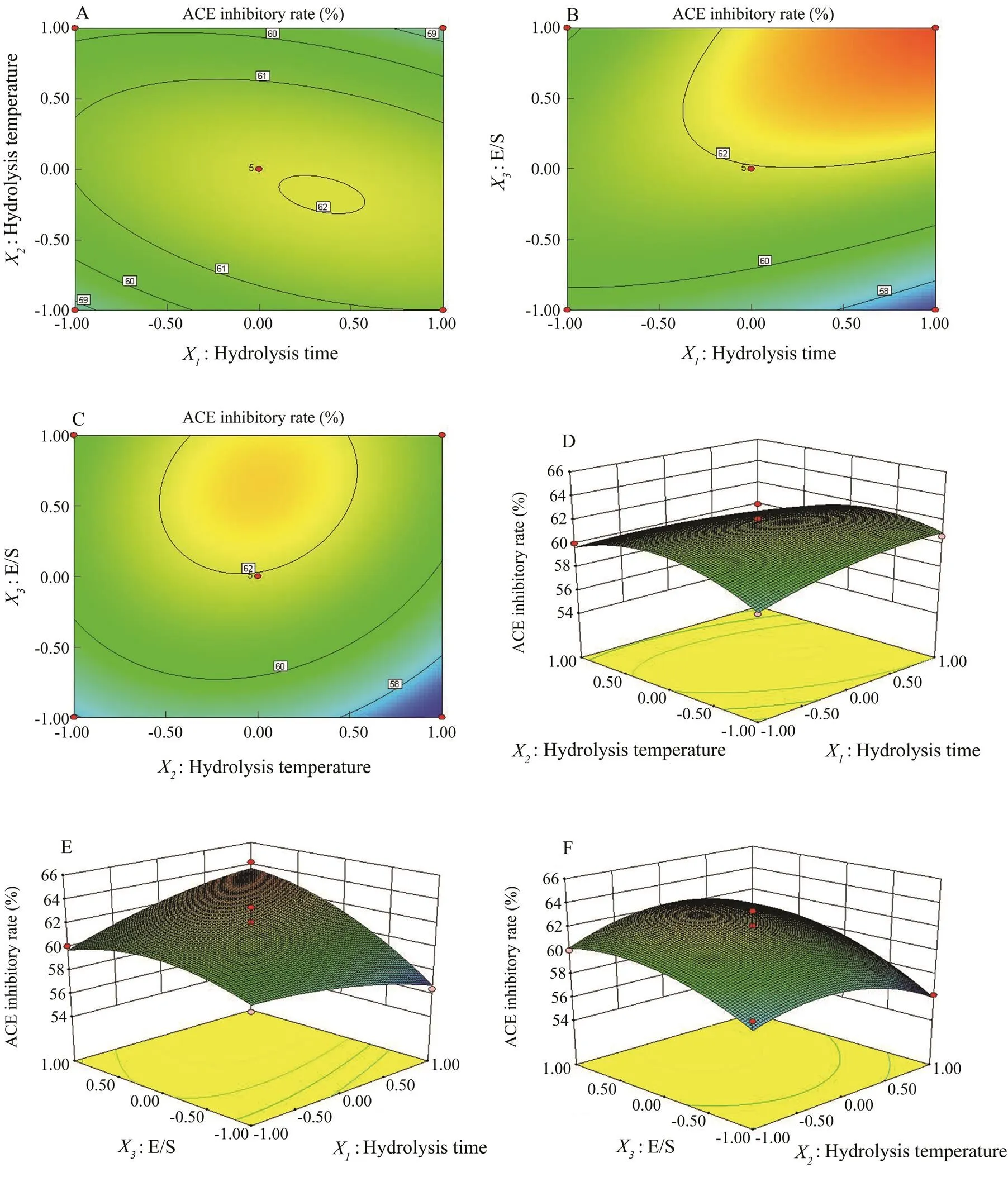
Fig.2 Contour plots (A, B, C) and response surface plots (D, E, F) showing the effects of hydrolysis time, hydrolysis temperature, and E/S on the ACE inhibitory rate (%) of the R. hispidum protein hydrolysate.
3.3 Amino Acid Composition and Molecular Weight Distribution Assay
As shown in Table 3, high levels of Gly, Glu, Pro, Ala, Asp and Arg were observed in RPH, which is consistent with the amino acid composition of protein hydrolysates from(Liu., 2012). According to the previous study, Gly, Glu, Pro, Ala, Asp and Arg from the ACE inhibitory peptides were all observed in marine species, such as sea cucumber (Zhao., 2009), lizard fish (Wu., 2012), jellyfish (Liu., 2013), and marine bivalve (Liu., 2014). The amino acid composition of the ACE inhibitory peptide has been recognized as a critical factor in ACE inhibitory activity. These results provide meaningful clues for further identification of the structures of ACE inhibitory peptides in RPH.
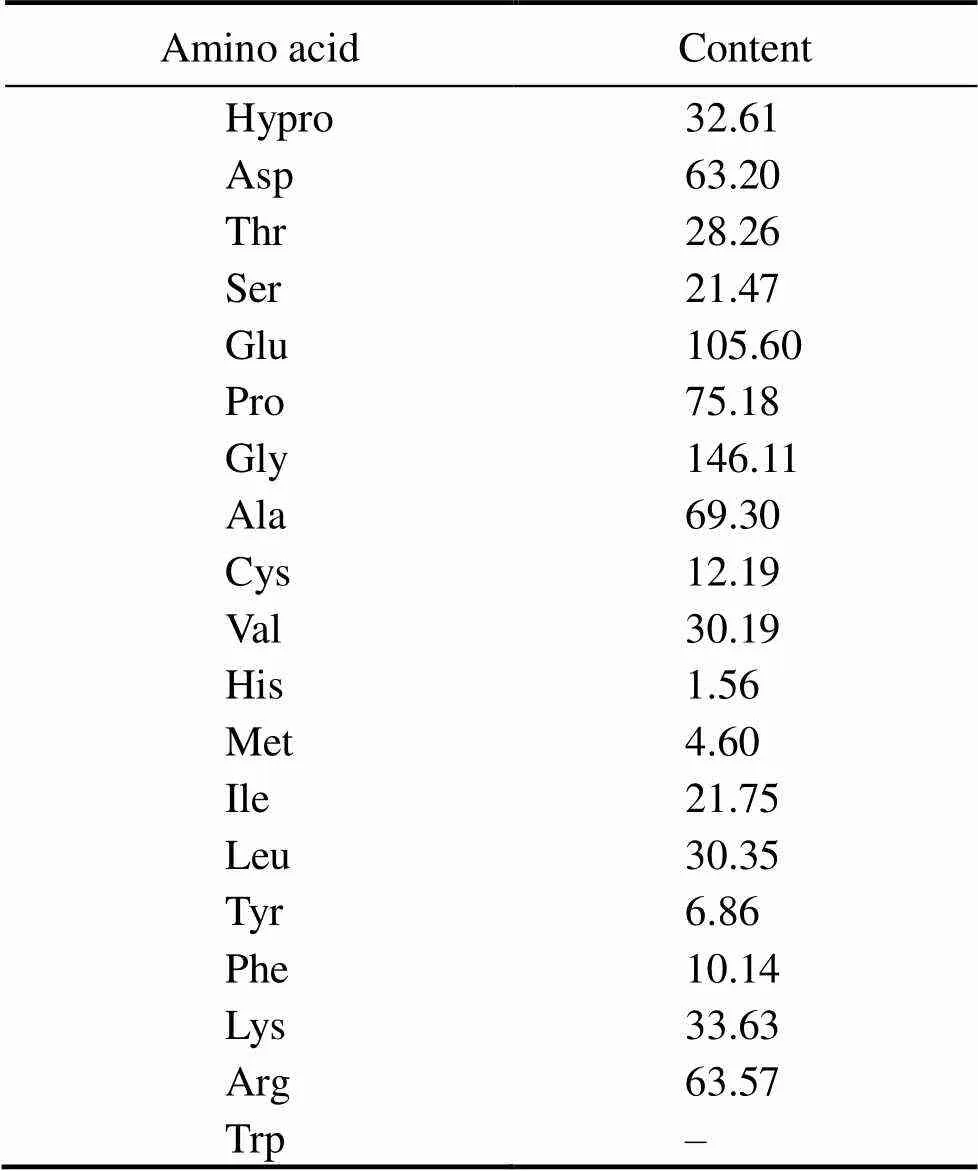
Table 3 Amino acid composition of RPH (mgg−1)
Note: ‘–’, not determined.
The molecular weight distribution of RPH was determined by HPGPC on a TSKgel G2000PWXLcolumn. As shown in Fig.3, RPH had a size distribution of 0.32–6.84kDa, and the main peaks were located at 0.87–2.0kDa. According to the previous study, most of the potent ACE inhibitory peptides had a molecular weight below or about 1000Da (Liu., 2013). Therefore, the results of the molecular weight profiles suggested that RPH may possess rich ACE peptides.
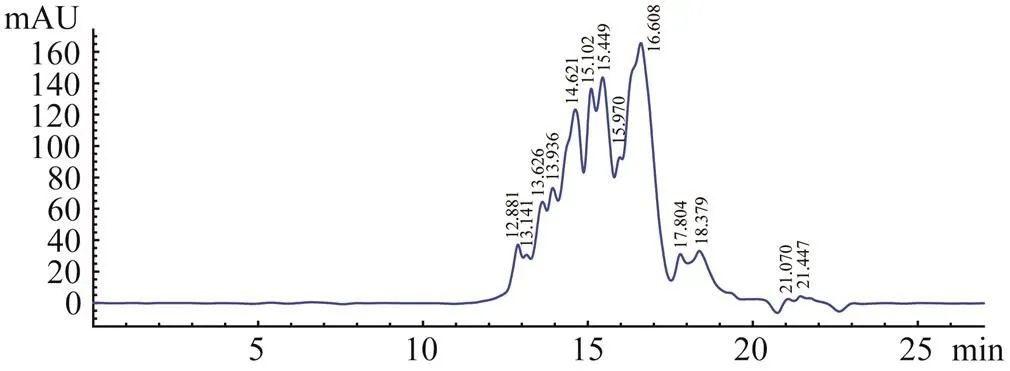
Fig.3 HPGPC chromatogram of RPH on TSKgel G2000- PWXL column.
3.4 Purification and Identification of ACE Inhibitory Peptides
As shown in Fig.4, RPH was divided by the Sephadex LH-20 column into three major fractions, namely, 1, 2, and 3. The ACE inhibitory activity of each fraction was determined, and fraction 2 displayed the strongest activity (Table 4). Fraction 2 was purified by the first step in HPLC. Four fractions (A, B, C, and D) were collected and lyophilized, while fraction B exhibited the strongest ACE inhibitory activity (Fig.5 and Table 4). Then fraction B was further purified by the second step in HPLC, resulting in three major peaks (B1, B2, and B3), which were lyophilized and identified for the amino acid sequence (Fig.6). In addition, their ACE inhibitory activities were evaluated.
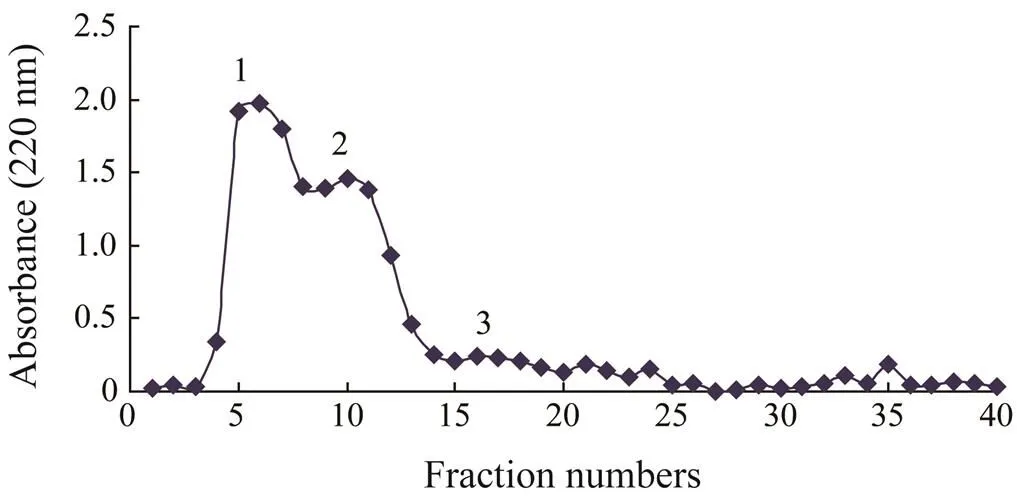
Fig.4 Sephadex LH-20 gel chromatography profile of RPH. Elution was monitored at 220nm.
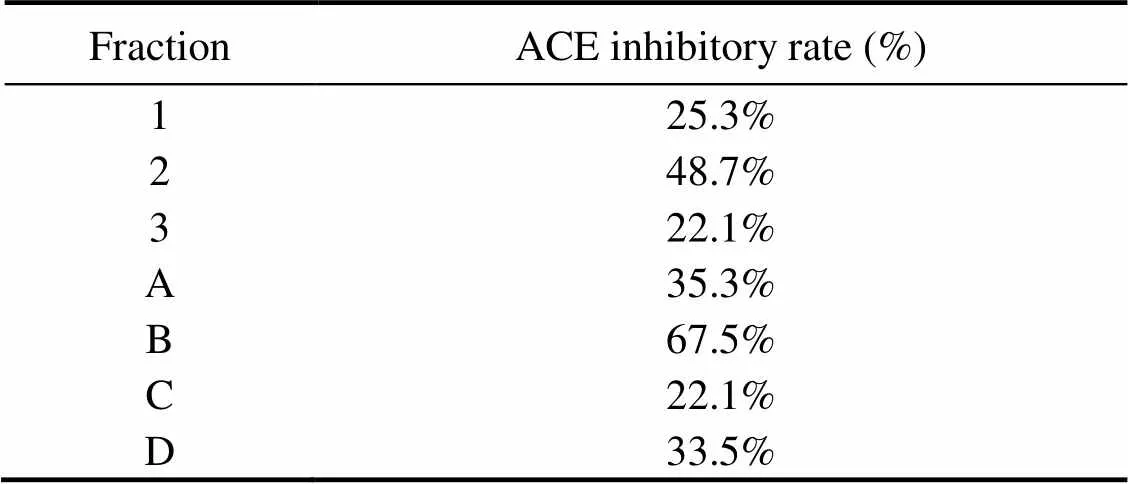
Table 4 ACE inhibitory rate (%) of the fractions obtained from each separation step
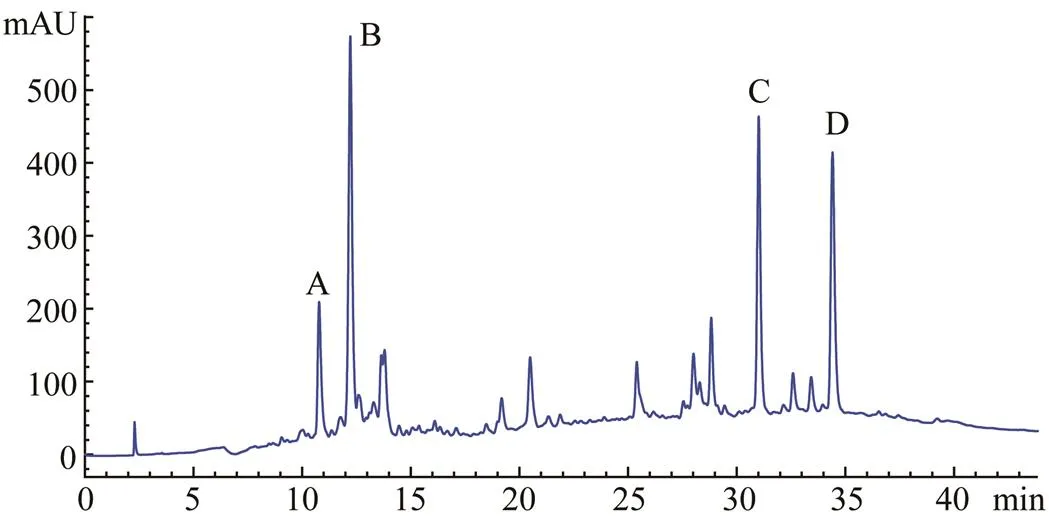
Fig.5 The first step of HPLC chromatogram profile of fraction 2, while elution was monitored at 210nm.
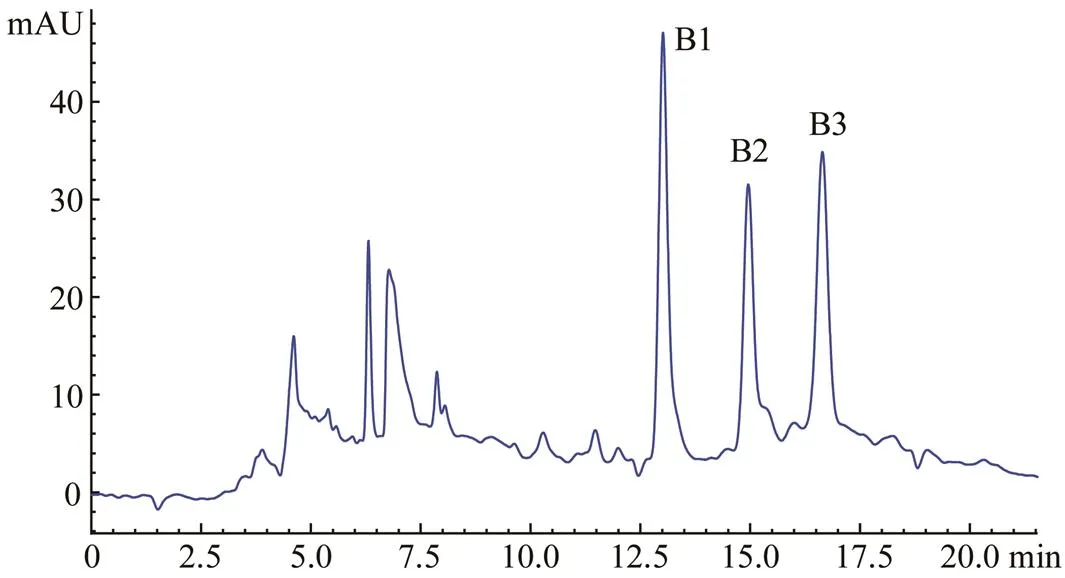
Fig.6 The second step HPLC chromatogram profile of fraction B, while elution was monitored at 210nm.
3.5 Amino Acid Sequence of the Purified Peptides
The amino acid sequences of the three peaks (B1, B2, and B3) were identified as Ile-Gly-Glu-Thr-Gly-Pro (B1, IGETGP), Gly-Ala-Thr-Gly-Pro-Ala-Gly-Tyr-Val (B2, GA TGPAGYV) and Gly-Ala-Phe-Gly-Pro-Gly-Gly-Leu-Val- Gly-Arg-Pro (B3, GAFGPGGLVGRP), respectively. The ACE inhibitory activity of IGETGP (IC50=19.07±1.82μmolL−1) was higher than those of GATGPAGYV (IC50=27.42±2.33μmolL−1) and GAFGPGGLVGRP (IC50=31.26±2.73μmolL−1).
Three peptides with high ACE inhibitory activities were isolated and identified from RPH. To our knowledge, this is the first report describing the isolation and iden- tification of ACE inhibitory peptides fromprotein hydrolysates, and these three ACE inhibitory peptides are also novel. The amino acid composition of the ACE inhibitory peptides has been recognized as an important factor in the ACE inhibitory activity. The structure-activity correlations indicated that the ACE inhibitory peptides containing a hydrophobic amino acid at each of the three C-terminal positions showed a strong ACE inhibitory activity. The C-terminal amino acid residues such as proline, tryptophan, tyrosine, valine and phenylalanine were reported to be the most favorable for ACE inhibition (Wang., 2016). The present study showed that the ACE inhibitory activity was high in the ACE inhibitory peptide IGETGP, which is known to be composed of six amino acids and have a hydrophobic amino acid (Pro) at the C-terminal. According to the pre- vious studies, several ACE inhibitory peptides with a pro- line residue at the C-terimnal have been isolated from various natural proteins such as FFVAP (6.0μmolL−1) and KVLPVP (5.0μmolL−1) from milk (Guan., 2009; Tauzin., 2002); DYGLYP (62.0μmolL−1) from bo- nito (Yokoyama., 1992); FKGRYYP (0.55μmolL−1) from chicken (Iroyukifujita., 2000), and AFVGYVLP(18.02μmolL−1), EKSYELP (14.41μmolL−1), and VELYP (5.22μmolL−1) from cuttlefish (Balti., 2015). Some reports have also demonstrated that ACE inhibitory pep- tides with a proline residue at the C-terminal and a branched hydrophobic amino residue (Leu, Ile and Val) at the N-terminal had higher ACE inhibitory activity (Zhao., 2011). This characteristic was also confirmed in IGETGP, which has a leucine residue at the N-terminal. This may account for the high ACE inhibitory activity of IGETGP compared with the other two peptides. GATGP- AGYV also has a hydrophobic amino residue at the C- terminal. ACE inhibitory peptides with valine at the C-terminal have been rarely reported. However, KLKFV (30μmolL−1) from the Antarctic krill (Kawamura., 1992) and NIPPLTQTPV (173.30μmolL−1) from bovine casein (Gobbetti., 2000) were found to possess po- tent ACE inhibitory activity. Although GAFGPGGLVGRP has a proline residue at the C-terminal, its relatively high molecluar weight compared with the other two peptides may have a negative effect on its ACE inhibitory activity. However, GAFGPGGLVGRP may be degraded into small peptides with a higher activity, and small peptides are considered to be more suitable to be absorbed in the gut.
4 Conclusions
In the present study, RSM was successfully used to optimize the process ofprotein hydrolysis for obtaining ACE inhibitory peptides. The optimal hydro- lysis conditions for generating hydrolysates with a strong ACE inhibitory activity were as follows: hydrolysis time 5h, hydrolysis temperature 50℃, and E/S 6%. The ACE inhibitory rate of 64.28%±5.72% obtained under these conditions is consistent with the predicted data. In addition, three novel ACE inhibitory peptides were isolated for the first time using gel-filtration and HPLC. These results suggest thatproteins and these ACE inhibitory peptides could be used as candidate ingredients in functional foods or food supplements for the prevention and treatment of hypertension, while further studies are required to investigatetheir antihypertensive effectsin spontaneously hypertensive rats (SHR).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 81230057, 81372615, 81472262, 41306133 and 81200264), the Emerging Cutting-Edge Technology Joint Research projects of Shanghai (No. SHDC12012106), the Tongji University Subject Pilot Program (No. 162385), and partly funded by the Shanghai Municipal Health and Family Planning Commission Project (Nos. 201540027 and 20174Y0236), the seed fund program of Shanghai University of Medicine & Health Sciences (No. HSMF-17-22-031), Excellent Young Medical Expert of Shanghai (No. 2017YQ048), Shanghai Natural Science Foundation (No. 18ZR1431700), and Chi- na Postdoctoral Science Foundation (No. 2017M610278) and the Key Research and Developing Plan of Shandong Province (No. 2015GSF115015).
Aissaoui, N., Abidi, F., Hardouin, J., Abdelkafi, Z., Marrakchi, N., Jouenne, T., and Marzouki, M.N.,2017. ACE inhibitory and antioxidant activities of novel peptides fromby-product protein hydrolysate., 23: 13-23.
Balti, R., Bougatef, A., Sila, A., Guillochon, D., Dhulster, P., and Nedjar-Arroume, N.,2015. Nine novel angiotensin I-conver- ting enzyme (ACE) inhibitory peptides from cuttlefish () muscle protein hydrolysates and antihypertensive effect of the potent active peptide in spontaneously hypertensive rats.,170: 519-525.
Gobbetti, M., Ferranti, P., Smacchi, E., Goffredi, F.,and Addeo, F., 2000. Production of angiotensin-I–converting-enzyme–inhibitory peptides in fermented milks started bysubsp.SS1 andsubsp.FT4.,66: 3898-3904.
Guan, X., Liu, J., Wang, L.,and Yao, H. Y., 2009. Preparation, purification and structure identification of angiotensin I converting enzyme inhibitory peptide with high activity from oat protein.,30: 1992- 1997.
Iroyukifujita, H., Eiichiyokoyama, K.,and Yoshikawa, M., 2000. Classification and antihypertensive activity of angiotensin I- converting enzyme inhibitory peptides derived from food proteins.,65: 564-569.
Kawamura, Y., Takane, T., Stake, M.,and Sugimoto, T., 1992. Physiologically active peptide motif in proteins-peptide inhibitors of ACE from the hydrolysates of Antarctic krill muscle protein.,26: 210- 213.
Lee, J. K., Hong, S., Jeon, J.K., Kim, S.K., and Byun, H.G.,2009. Purification and characterization of angiotensin I converting enzyme inhibitory peptides from the rotifer,.,100: 5255- 5259.
Liu, R., Zhu, Y., Chen, J., Wu, H., Shi, L., Wang, X., and Wang, L., 2014. Characterization of ACE inhibitory peptides fromhydrolysate by nano-liquid chromatography electrospray ionization mass spectrometry (Nano-LC-ESI-MS) and molecular docking.,12: 3917- 3928.
Liu, X., Zhang, M., Jia,A., Zhang, Y., Zhu, H., Zhang, C., Sun, Z.,and Liu, C., 2013. Purification and characterization of angiotensin I converting enzyme inhibitory peptides from jellyfish.,50:339-343.
Liu, X., Zhang, M., Zhang, C.,and Liu, C., 2012. Angiotensin converting enzyme (ACE) inhibitory, antihypertensive and antihyperlipidaemic activities of protein hydrolysates from.,134: 2134-2140.
Skeggs, L.T., Kahn, J.E.,and Shumway, N.P., 1957. The pre- paration and function of the angiotensin-converting enzyme., 13: 295-299.
Sun, X., Wang, M., Liu, B., and Sun, Z., 2017. Purification and characterization of angiotensin I converting enzyme inhibition peptides from sandworm., 16: 911-915.
Sun, Z., Yang, Z., Liu, X.,and Wang, C., 2015. Study on enzymatic hydrolysis ofand its biological activities., 36: 20-23.
Tauzin, J., Miclo, L.,and Gaillard, J. L., 2002. Angiotensin-I-converting enzyme inhibitory peptides from tryptic hydrolysate of bovine alphaS2-casein.,531: 369-374.
Torruco-Uco, J., Chel-Guerrero, L., Martínez-Ayala, A., Dávila-Ortíz, G.,and Betancur-Ancona, D., 2009.Angiotensin-I con- verting enzyme inhibitory and antioxidant activities of protein hydrolysates fromandseeds.,42: 1597-1604.
Wang, J. L., He, G. Q., Chen, Q.H., Feng, F.Q., Yu, G.J.,and Shoemaker, C. F., 2011. Optimization of angiotensin I-con- verting enzyme inhibitory peptide production by ribbonfish (trichiurus haumela) backbone hydrolysis using response surface methodology.,34: 2177-2190.
Wang, X., Xue, L., Hu, Z., Zhang, Q., Li, Y.,and Xia, L., 2016. Progress in research on structure-activity relationship of ACE inhibitory peptides.,38: 305-310.
Wu, J.,and Ding, X., 2002. Characterization of inhibition and stability of soy-protein-derived angiotensin I-converting enzyme inhibitory peptides.,35: 367-375.
Wu, S., Sun, J., Tong, Z., Lan, X., Zhao, Z.,and Liao, D., 2012. Optimization of hydrolysis conditions for the production of angiotensin-I converting enzyme-inhibitory peptides and isolation of a novel peptide from lizard fish (muscle protein hydrolysate.,10: 1066-1080.
Yokoyama, K.H., Chiba, H.,and Yoshikawa, M., 1992. Peptide inhibitors for angiotensin-I-converting enzyme from thermo- lysin digest of dried bonito.,56: 1541-1545.
Zhang, P., Roytrakul, S.,and Sutheerawattananonda, M., 2017. Production and purification of glucosamine and angiotensin-I converting enzyme (ACE) inhibitory peptides from mushroom hydrolysates.,36: 72-83.
Zhao, H., Gong, J., Li, Z.,and Tang, J., 2011. Research progress of ACE inhibitory peptide.,6: 44-46.
Zhao, Y.H., Li, B.F., Dong, S.Y., Liu, Z.Y., Zhao, X., Wang, J.F.,and Zeng, M.Y., 2009.A novel ACE inhibitory peptide isolated fromhydrolysate., 30: 1028-1033.
September 11, 2017;
December 27, 2017;
August 31, 2018
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2018
# The two authors contributed equally to this work.
. wangmanfx@126.com
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Comparative Evaluation of Toleration to Heating and Hypoxia of Three Kinds of Salmonids
- Morphological Change in the Northern Red River Delta, Vietnam
- Internal Facies Architecture and Evolution History of Changxing Mouth-Bar Complex in the Changjiang (Yangtze) Delta, China
- The Holocene Environmental Evolution of the Inner Hangzhou Bay and Its Significance
- Provenance of Sediments Filling a Paleo-Channel that Formed on the Western Yellow Sea Continental Shelf During the Last Glacial Period
- Distribution and Characteristics of Hazardous Geological Features in the Marine Coastal and Offshore Areas of Zhejiang Province, East China Sea
