Chemical Defense of Soft Coral Sinularia polydactyla from the Red Sea Against Marine Biofilm-Forming Bacteria
2018-12-20ESKANDERRehabALSOFYANIAbdulmohsinELSHERBINYMohsenBAAKDAHMohammadAbdulazizandSATHEESHSathianeson
ESKANDER Rehab, AL-SOFYANI Abdulmohsin A., EL-SHERBINY Mohsen M. O., BA-AKDAH Mohammad Abdulaziz, and SATHEESH Sathianeson
Chemical Defense of Soft Coralfrom the Red Sea Against Marine Biofilm-Forming Bacteria
ESKANDER Rehab, AL-SOFYANI Abdulmohsin A., EL-SHERBINY Mohsen M. O., BA-AKDAH Mohammad Abdulaziz, and SATHEESH Sathianeson*
,,,21589,
Soft corals are an important group of organisms that live in reef ecosystems. In this study, the chemical defense of soft coralagainst biofilm-forming bacteria was assessed. The soft coral.was collected from the Ob- hur creek of the Red Sea and the bioactive compounds were extracted under different conditions using methanol and hexane as sol- vents. Results revealed that the bioactive compounds produced by the soft coral.were active against the growth, hy- drophobicity and extracellular polymeric substances production of the biofilm-forming bacteria. However, the damage or injury in soft coral tissue caused a decrease in the activity against biofilm-forming bacteria. GC-MS analysis revealed that sesquiterpenes were abundant in normal coral sample extract while cembranoids were abundant in damaged coral sample extracts. In general, the results indicated that the soft corals either under stress or with damage may not have a strong chemical defense against the colonizing ma- rine organisms and competitors.
chemical ecology; antifouling; antibiofilm activity; bioactive compound; coral tissue damage
1 Introduction
Coral reef ecosystems are considered as a generic li- brary for future generations and are characterized for the biological diversity with rich habitats in the world (Mo- berg and Folke, 1999). The Red Sea has a high reef diver- sity including scleractinian corals (reef-building) in the central and northern areas. Soft corals (Cnidaria: Antho- zoa) cover three families, Nephtheidae, Xeniidae and Al- cyoniidae. Alcyoniidae includes common genera such as,and(Aratake, 2012). Alcyonacea provides habitat and food for various organ- isms. Therefore, they are considered as an important com- ponent of coral reef assemblages (Evans., 2011).
Many chemical and physical defense strategies are present in sessile invertebrates to defend against predators. Soft corals and scleractinians produce allelochemical compounds that help them to extend or evade the com- petitors (Lages., 2010). Development of defense mechanisms in corals is caused by predation, which is considered as one of the factors affecting marine inverte- brate diversity (Xuan., 2014). The chemical com- pounds produced by corals could be exploited for phar- macological and other applications (Kamel and Slattery 2005; Khalesi., 2008). For instance, terpenes from the genusshow considerable biological activi-ties such as cytotoxic, anti-inflammatory, antiarthritic, HIV- inhibitory, antimicrobial, and ichthyotoxic activities (Se- lim., 2012). Soft coralalso produces cem- branoid diterpenes like sinulariolide and flexibilide, which can inhibit the development of Gram-positive bacteriaand(Kamel and Slattery, 2005).
Biofouling is a serious problem in the marine environ- ment, which is usually caused by the settlement of micro- organisms and macroorganisms on living and non-living surfaces (Satheesh., 2016). Many marine organisms have various strategies of preventing their surfaces from fouling, particularly by producing secondary metabolites (Qian., 2013; Satheesh., 2016). The organisms which can defend their surfaces effectively from fouling are considered as a potential naturalsource of antifouling compounds (Satheesh., 2016). After the world-wide ban on the use of Tributyl tin (TBT)-based antifouling paints for marine applications, many studies are con- ducted on non-toxic or less toxic antifouling compounds from marine sources (see reviews by Qian., 2013; Satheesh., 2016). Antifouling activities of soft corals were also reported in many previous studies (Mizobuchi., 1994, 1996; Dobretsov.,2015). However, the chemical defense and antifouling activities of Red Sea soft corals are not studied in detail. The aim of the present study was to analyze the chemical defense of soft coralagainst marine colonizing bacteria. Results obtained in this study will improve our under- standing the ecology of Red Sea soft corals and aid to searching natural antifouling compounds.
2 Materials and Methods
2.1 Collection of Soft Coral
Soft coral(Fig.1) was collected from the Jeddah Coast (Obhur Creek) of the central Red Sea. The samples were kept in polythene bags with sea- water and transferred to the laboratory (in living condi- tions for induction experiments). In the laboratory, coral samples were rinsed with filtered seawater to remove the debris and other associated organisms. Then the samples were used for extraction or chemical defense induction experiments.

Fig.1 Soft coral Sinulariapolydactyla collected from the Obhur Creek of the Red Sea.
2.2 Extraction Methods
Two methods were employed for the extraction of bio- active compounds from the soft coral samples using me- thanol and hexane as solvents.
a) To extract the compounds from the surface, the coral sample (about 15g) was dipped in 5mL of solvents (me- thanol and hexane) for 10s. The extract was filtered and concentrated by vacuum evaporation. The surface extracts were concentrated into 25% of the original volume and used for antimicrobial assays.
b) To extract the compounds from the whole tissue, the coral sample was macerated and kept in the solvents (methanol and hexane) for 24h. Then the extracts were centrifuged and filtered to remove the debris. Finally, the extracts were vacuum-evaporated and maintained at −20℃ for further assays.
In addition, extractions were also conducted from the samples subjected to various defense-induction experi- ments under laboratory conditions. The induction experi- ments are outlined below.
2.2.1 Local wounding
In this experiment, a wound (small cut) was made in the coral sample using sharp forceps and kept for 1h in a glass jar with filtered seawater (Millipore pore filtered, 0.47µm). Control coral sample was kept in filtered sea- water for 1h without any damage. Then the samples were frozen at −80℃ for 2h. The frozen samples were macer- ated in a pestle and mortar, and then were extracted using methanol for 3h. The extraction was centrifuged at 3000rmin−1and −4℃ for 15min and filtered. The extract was vacuum-evaporated and kept at −20℃ for further bioas- says.Every experiment was repeated as triplicates.
2.2.2 Neighboring effects on chemical defense induction (role of waterborne molecules)
The soft coral samples were divided into two groups, and the tips of one group were damaged with sharp for- ceps. Both of the damaged and undamaged samples were kept in a beaker (1L) with filtered (Millipore, 0.47µm) seawater for 3h at room temperature. After 3h, the un- damaged soft coral samples were collected and kept in the freezer at −80℃. The frozen samples were extracted us- ing methanol as described before. The extracts were cen- trifuged at 3000rmin−1to remove solid materials and then were filtered. The extract was vacuum-evaporated and kept at −20℃ for further bioassays.
2.2.3 Effects of epibiotic bacterial strain on chemical defense of soft corals
An epibiotic bacterial strain isolated from marine algasp. was inoculated (50mL) in jars with 1L seawater which contains soft coral samples. The jars were kept at room temperature and under normal light for 4h, and then the coral samples were frozen immediately at −80℃ and extracted using methanol. After they were centrifuged at 3000rmin−1and then filtered to remove the debris, the extracts were vacuum-evaporated and kept at −20℃ for further bioassays.
2.3 Antimicrobial Bioassays Against Marine Biofilm-Forming Bacteria
All the extracts obtained with different methods were used for assays against marine biofilm-forming bacterial strain(NCBI GenBank accession number: KY266820). This strain was isolated from the artificial substrate submerged in the Obhur Creek waters of the central Red Sea.
2.3.1 Bacterial growth inhibition assay (spectrophotometer assay)
The biofilm bacterial strain was inoculated in nutrient broth (Zobell marine broth) and cultured overnight before being used for bacterial growth inhibition assay. The OD of the culture was adjusted to 0.2 at 540nm to maintain the equal number of bacterial cells in each experiment. The coral tissue extracts prepared with different methods (except for surface extraction) were dissolved in methanol or hexane at the concentration of 1mgL−1. This extract concentration was used as a stock crude extract for further experiments. The bacterial culture (3mL) was taken in test tubes and 50µL soft coral extract (from the stock crude extract) was added. Control tubes were maintained without any extracts. The optical density of the bacterial broth was measured at 1h interval for a period of 4h at 670nm in a spectrophotometer. The experiment was con- ducted in triplicate (=3) and the mean±standard error (SE) values were calculated. The percentage of bacterial growth/inhibition was calculated using the following for- mula:
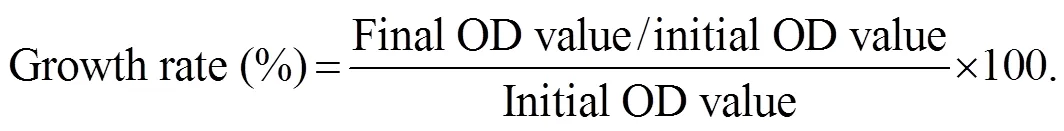
2.3.2 Bacterial growth inhibition assay (culture plate method)
The bacterial culture (3mL) was taken in test tubes and 50µL soft coral extract was added as mentioned above. The test tubes were incubated at room temperature for 24h. Then 50µL of bacterial culture was spread on Zobell marine agar plates and the plates were incubated in an incubator at 28℃ for 24–48h. The bacterial colonies de- veloped on each plate were counted with the mean colony count expressed as CFUmL−1.
2.3.3 Measurement of hydrophobicity of bacterial cells treated with soft coral extracts
The bacterial cell surface hydrophobicity was mea- sured by MATHS assay according to Rosenberg(1980). After the OD of the culture was adjusted to 0.2 at 540nm to maintain an equal number of bacterial cells,about 3mL of bacterial culturewas taken into a test tube and mixed with 100μL of coral extract. After 1h, the OD of the culture medium was measured at 400nm in a spectrophotometer. To this culture, 100μL of toluene was added and vortexed for 1min. The culture was then left for 15min to allow the separation of two phases. The aqueous phase of the culture was separated and used for OD measurement as above. The percentage of hydropho- bicity was calculated using the following formula:
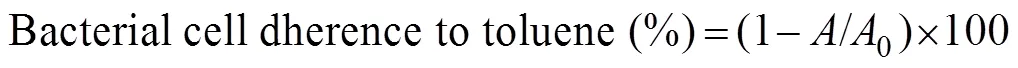
where0is the OD value of the aqueous phase of suspen- sion before adding toluene,andisthe OD value after adding the toluene.
2.3.4 Effects of soft coral extracts on extracellular polymeric substances production in biofilm-forming bacteria
Bacterial strain was inoculated into marine broth (25mL) and soft coral extract (50µLmL−1) was added to the broth. The culture without any soft coral extract treatment was considered as control. The cultures were incubated at 37℃ for 24h. After the incubation, the broth was centri- fuged at 5000rmin−1at ℃for 15min. The supernatant was collected, mixed with equal volume of ice-cold ethanol and kept for 24h at room temperature. After 24h, the precipitate was collected and stored at 4℃. The pre- cipitated EPS was filtered through a membrane filter and diluted with a known volume of distilled water. The car- bohydrate concentration of the EPS was measured using phenol-sulphuric acid method (Dubois, 1956) using glu- cose as standard.
2.4 Data Analysis
The results obtained from the biological assays were subjected to Student’stest to understand the difference between control and coral extract treatment.
2.5 GC-MS Analysis of Coral Extracts
The extracts obtained from the coral samples without any damage (control) and coral samples subjected to damages (local wounding) were analyzed using GC-MS (Shimadzu GC-MS QP 2010) to understand the difference in biochemical profile between two extracts. For GC-MS analysis, the coral extracts were partially purified using silica gel column chromatography. The compounds pre- sent in the extracts were analyzed with a capillary silica column (30m×0.25mm×0.25µm) using helium as the carrier gas. Electron impact (EI) mode at 70eV (scan range 40–700m/z) was the specification used for the mass spectrometer. The MS conditions for the analysis of the extracts were as the following: split ratio was 1:10; in- jected volume was 1µL; and the injector temperature was maintained at 250℃. The oven temperature was adjusted initially as 70℃ and increased to 250℃ at a speed of 14℃min−1. The peaks observed in the GC-MS spectrum were subjected to library search (NIST 11-Mass Spectral Library) for the identification of the compounds.
3 Results
3.1 Bacteria Growth Inhibiting Activities of S. polydactyla Through Spectrophotometer Assay
Results of the present study revealed that extracts from the soft coral inhibited the growth of the biofilm-forming bacteria. The bacterial culture without soft coral extract (control) showed a growth rate of 9.11% during the 4h experimental period. The bacterial culture treated with hexane extract and methanol surface extract of.showed a negative growth rate of −10.24% and −7.63%, respectively after 4h (Fig.2). Furthermore, the growth rate of bacteria treated with other extracts of.showed a slight decrease when compared to the control. The extracts are hexane surface extract (−2.96%), methanol extract of coral samples subjected to local wounding (−1.97%), extract of coral samples subjected to chemical signaling experiment (−1.76%), and the metha- nol extract of coralsamples (0.98%). Also, the methanol extract of.sample subjected to bacterial induction experiment did not show much inhibitory activ- ity (6.81%) against biofilm-forming bacteria.
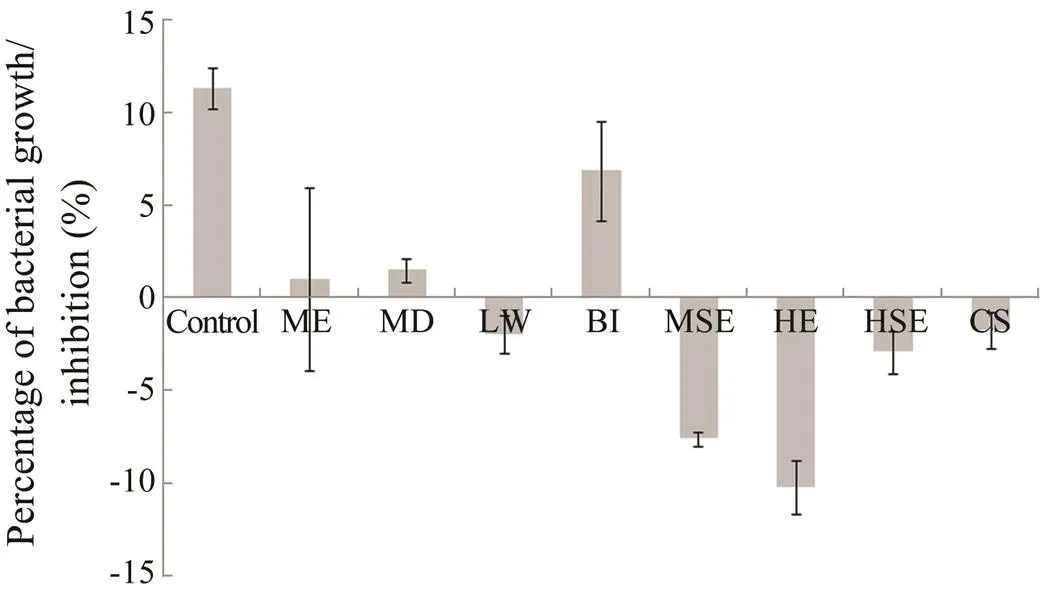
Fig.2 Bacterial growth inhibiting activities of S. polydac- tyla extracts. Bacterial growth inhibiting activity was ob- served by spectrophotometer assay for a period of 4h. Error bars indicate SE (n=3). Control,without extract; ME, methanol extract; HE, hexane extract; MSE, metha- nol surface extract; HSE, hexane surface extract; MD, mechanical damage; LW, local wounding; BI, bacterial induction assay; CS, chemical signal experiment.
Student’stesting revealed a significant difference among the growth of biofilm-forming bacteria treated with soft coral extracts. The bacterial culture treated with methanol extract of.subjected to a local wounding assay (=7.09529,=1,=0.009) showed a significant difference with control. Likewise, methanol surface extract of.(=12.85133,=1,=0.003) showed a significant variation in the bacterial growth. Other extracts which showed significant inhi- bitory activities were hexane extract (=9.40442,=1,=0.005), hexane surface extract of.(=7.28627,=1,=0.009) and methanol extract of coral samples subjected for waterborne molecules induction (=7.02782,=1,=0.009).
3.2 Bacterial Growth Inhibiting Activities of S. polydactyla Analyzed with Culture Plate Method
The extracts also showed bacterial growth inhibitory activities in culture plate method (Fig.3). The extract of.treated for bacterial induction experiment (190000CFUmL−1), extract of coral sample subjected to local wounding assay (655500CFUmL−1), methanol ex- tract of.(866400CFUmL−1) and extract of.subjected to waterborne molecules induc- tion (606100CFUmL−1) showed inhibitory activities against the bacterial culture. The hexane extract of.(1398400CFUmL−1) did not show much in- hibitory activity against bacterial culture. Furthermore, too many bacteria were found in the cultures treated with methanol surface extract and hexane surface extracts of.. Hence, the colonies were not counted for these extracts treated cultures.
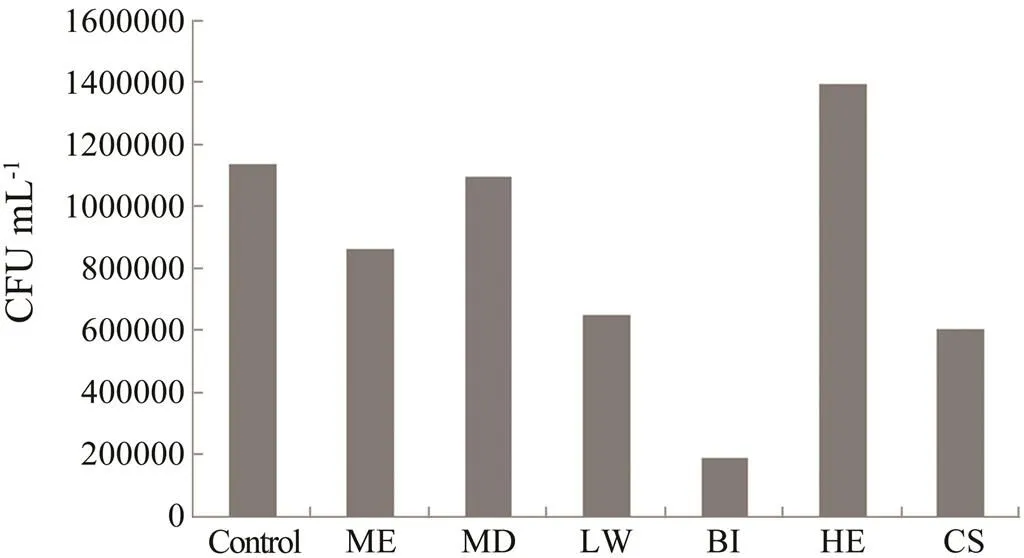
Fig.3 Bacterial growth inhibiting activities of S. polydac- tyla extracts. The growth inhibiting activity was observed by culture plate method. Control, without extract; ME, methanol extract; H, hexane extract; MD, mechanical damage; LW, local wounding; BI, bacterial induction as- say; CS, chemical signal experiment.
3.3 Effects of Soft Coral Extracts on the Hydropho- bicity of Biofilm-Forming Bacterial Cells
Results of the present study revealed that extracts from the soft coral showed a negative effect on the hydropho- bicity of biofilm-forming bacterial cells (Fig.4). Six ex- tracts exhibited strong activities against the hydrophobic- ity of the biofilm-forming bacterial strain. The extracts were hexane extract (2.68%), extract of.treated for bacterial induction assay (3.41%), hexane sur- face extract (3.53%), extract of.samples treated for waterborne molecules induction (6.89%), the methanol surface extract (6.42%) and the methanol ex- tract of.(without any treatment) (6.82%). However, the extract of.samples subjected to local wounding (20.40%) did not show obvious effects on bacterial cell surface hydrophobicity.
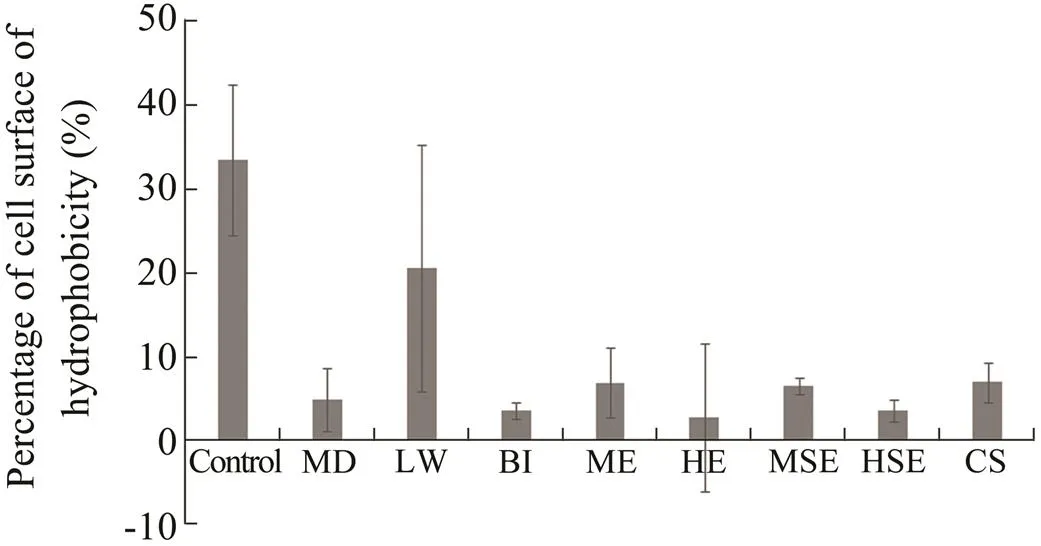
Fig.4 Effects of S.polydactyla extracts on cell surface hy- drophobicity of biofilm-forming bacteria. Error bars indi- cate SE (n=3). Control,without extract; ME,methanol extract; HE,hexane extract; MSE,methanol surface ex- tract; HSE,hexane surface extract; MD,mechanical da- mage; LW,local wounding; BI,bacterial induction assay; CS,chemical signal experiment.
3.4 Effects of Soft Coral Extracts on Extracellular Polymeric Substances Production in Biofilm-Forming Bacteria
The extracellular polymeric substances production (EPS) in biofilm-forming bacteria was affected by the soft coral extracts (Fig.5). Some extracts of.showed strong effects on EPS concentration of the biofilm bacte- ria. The extracts included methanol extract (2.04µgmL−1), hexane extract (2.30µgmL−1) and the extract of.samples subjected to local wounding experiment (2.65µgmL−1). However, some extracts such as methanol surface extract and hexane surface extract (4.45µgmL−1and 6.18 µgmL−1, respectively), extract of.samples subjected to bacterial induction assay (13.51µgmL−1) and extract of.subjected to water- borne molecules induction experiment (14.39µgmL−1) did not show much effects on EPS production of the biofilm- forming bacteria.
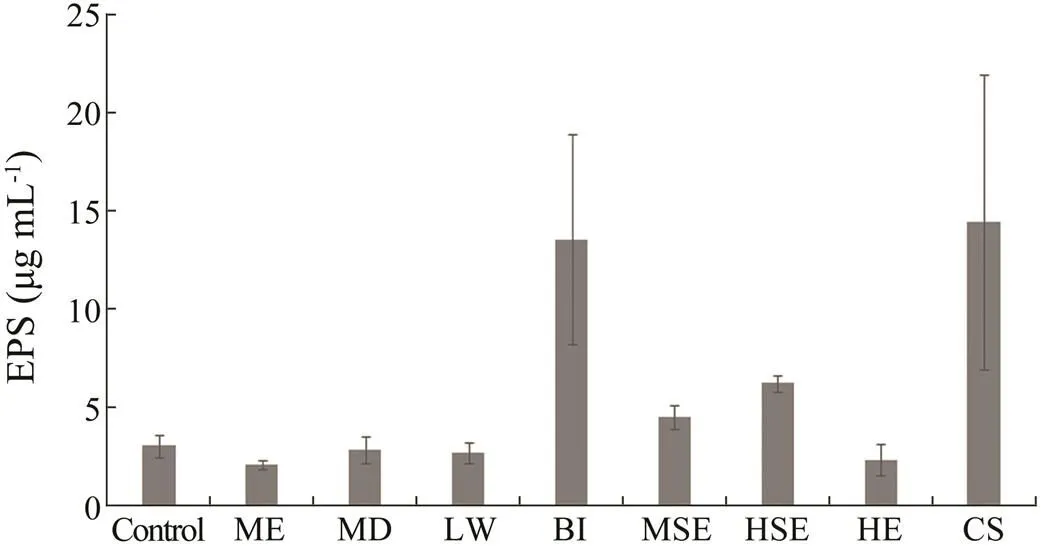
Fig.5 Effects of soft coral S.polydactyla extracts on EPS production in biofilm-forming bacteria. Error bars indi- cate SE (n=3). Control,without extract; ME,methanol extract; HE,hexane extract; MSE,methanol surface ex- tract; HSE,hexane surface extract; MD,mechanical da- mage; LW,local wounding; BI,bacterial induction assay; CS,chemical signal experiment.
3.5 GC-MS Analysis of Coral Extracts
The GC-MS spectrum of normal.extract revealed the presence of fatty acids,sesquiterpenes, pipe- ridine and imidazole derivatives (Fig.6). Among these compounds, sesquiterpenes were abundant in the extract of normal samples. The extract of coral samples damaged with local wounding revealed the abundance of cembra- noids (Fig.7). Specifically, the peaks related to the cem- branoid desulphosinigrinwas dominantly observed.
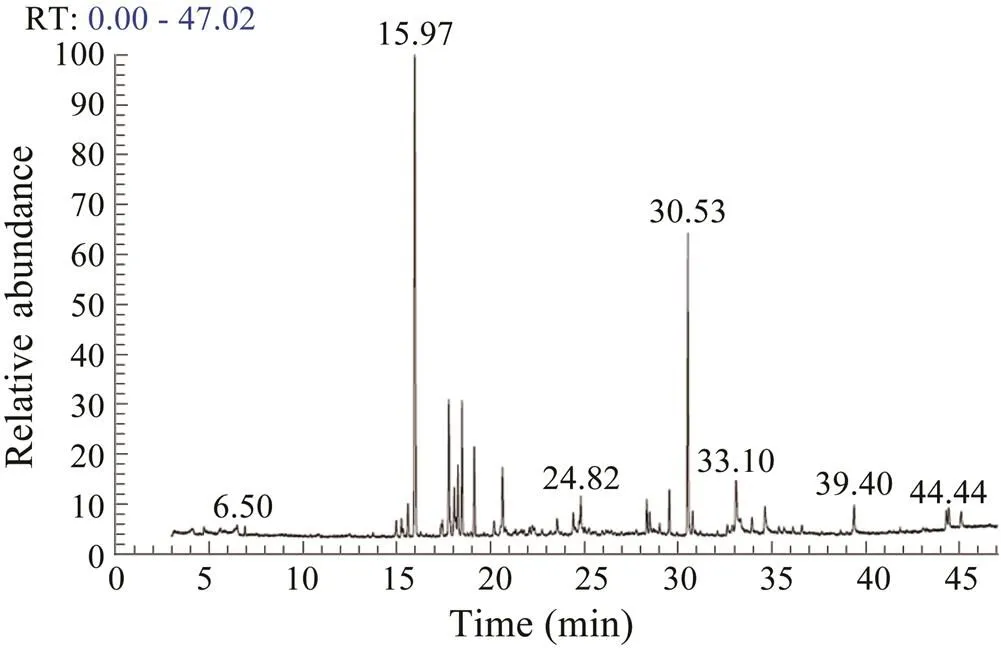
Fig.6 GC-MS spectrum of the extracts obtained from the normal S.polydactyla samples.
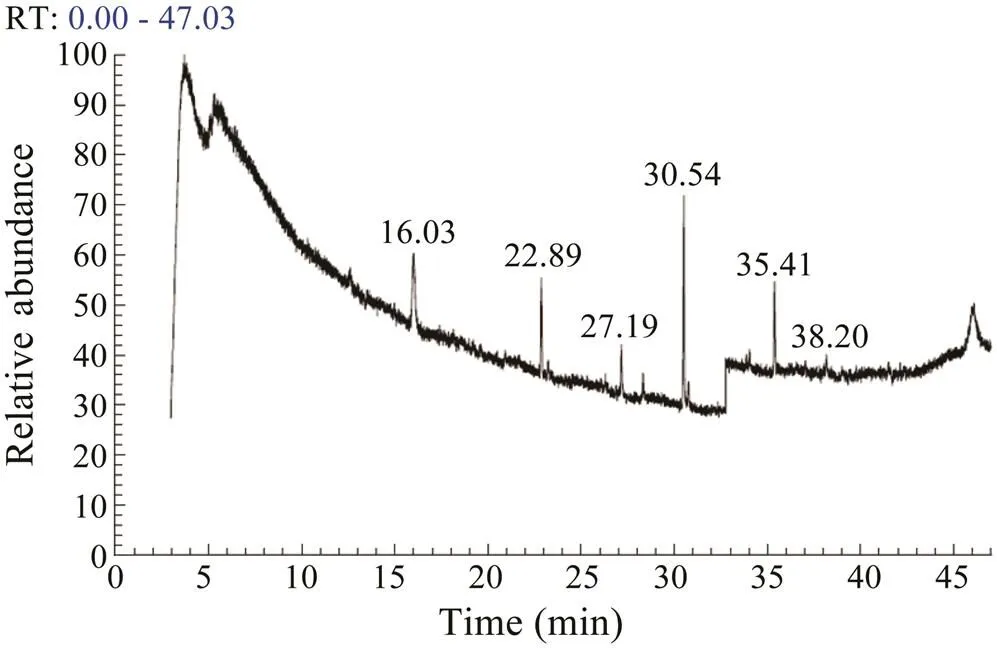
Fig.7 The GC-MS spectrum of the extracts of coral sam- ples subjected to local wounding.
4 Discussion
In this study, the chemical defenses of soft coral.collected from the Red Sea were assessed against a colonizing marine bacterial strain. The main objective of this work was to assess the ability of soft coral species for the production of secondary metabolites under different conditions. Generally, soft corals inhibit bacterial fouling by producing natural products (Dobret- sov., 2015). Hence, soft coral extracts were tested against some important properties of the bacteria such as EPS production, hydrophobicity and bacteria growth. Results of this study indicated that the extracts obtained from.exhibit a strong activity against the bacteria. Several previous studies also reported that sec- ondary metabolites of soft coralhave potent ecological functions including antifouling activities (Mizo- buchi., 1994; Mizobuchi., 1996; Limna Mol., 2010). Additionally, a high concentration of toxins were reported inspecies than other soft coral species, and because of the abundance of metabolites, the surface is free of fouling organisms (Fabricius, 1995; Wang., 2017).
The hexane extract of.showed a strong effect against biofilm-forming bacteria in the spectropho- tometer assay. This indicated that soft corals in its natural form in the environment without any stress or damage may produce compounds that protect them from predators and competitors. However, results of the present study showed that if any injury or stress from the environment existed, corals could not produce defense compounds in an effective way. The weak chemical defense was obvious from the results of low inhibitory activity against the biofilm-forming bacterial strain by the extracts of coral samples subjected to damage. In a natural environment, corals are subjected to injury or damage due to different factors. While the extents of damage and recovery rate have been studied by many researchers (Meszaros and Bigger, 1999; Work and Aeby, 2010), the defense func- tions of corals during tissue damage were not analyzed in detail in this research. Though the physiological mechanism for the low defense due to tissue damage is not clear, one possible reason may be that the corals spend much energy on healing the injury than producing secondary metabolites. It is well established in many organisms that production of secondary metabolites is an energy con- suming process (Coll, 1992; Van Alstyne., 2001). In general, the results indicated that injury of soft corals may decrease the defenses against microbes in the ecosystem, which may lead to more diseases.
Another important finding in this study was the higher bacterial growth inhibition by extracts obtained from the coral samples challenged with epibiotic bacterial strain. This higher growth inhibition was mainly observed in culture plate. This indicated that soft corals, when in- duced by bacteria, can produce chemical defense against invasive bacteria (Kelman., 2006). Normally, corals produce chemical compounds to prevent microbial and macrofouling (Slattery., 1995). Hence, whensoft corals are challenged with the colonizing bacteria under laboratory conditions, they might produce some metabo- lites to prevent the bacterial settlement. As they already produced some possible secondary metabolites against the bacteria, the extracts obtained from these samples inhibited the bacterial growth more strongly.
The soft coral extract also affected the hydrophobicity of the biofilm-forming bacterial strains. Hydrophobicity is essential for the attachment of bacteria on surfaces (Satheesh., 2012). The extracellular polymeric sub- stances production (EPS) in biofilm-forming bacteria was affected by the coral extracts. This was evidenced from the low carbohydrate concentration in the EPS produced by the bacterial strains treated with the extracts obtained from extracts of.. Some studies (Maida., 2006; Wang., 2008) showed that EPS is an adhesive material for the attachment of bacteria on hard surfaces. For successful prevention of bacterial colonization on surfaces, the host organisms should produce compounds which affect the attachment capability of the bacteria (Satheesh., 2012). Hence, results of the present study showed that the metabolites produced by the soft coral reduced the EPS production in biofilm-forming bacteria and thereby inhibited the attachment.
The soft coral genusconsists of around 100 species with a vast diversity of chemical compounds (Lakshmi and Kumar, 2009; Shi., 2012). Soft corals produce metabolites such as sesquiterpenes, norditerpenes, diterpenes, steroids and polyamine compounds (Coll, 1992; Khalesi., 2008; Shi., 2012). Some of these metabolites are reported to play key roles in chemi- cal defense of the soft corals against competitors and predators (Coll, 1992; Takaki., 2003). Antifouling and biofilm inhibiting activities of the soft coralhave been reported in previous researches (Mizo- buchi., 1994; Raveendran., 2011). Since the concentrations of secondary metabolites are very low in soft corals, more corals need to be collected for the ex- traction of the compounds. This study indicated that the chemical defense of the soft corals could be induced un- der laboratory conditions using epibiotic bacterial strains. Further research incorporating more chemical defense inducing factors may be helpful for getting secondary metabolites with strong activity against the target bio- fouling organisms.
GC-MS analysis of extracts obtained from normal and damaged coral samples revealed considerable differences. While peaks corresponding to sesquiterpenes were domi- nant in the extract of normal samples, the damaged sam- ples revealed the abundance of cembranoid desulphosini- grin. Both sesquiterpenes and cembranoids are reported to have myriad of biological activities (Kamel and Slattery, 2005; Qi and Ma, 2017). In soft corals, secondary me- tabolites are abundantly found as a repellent against predators (Coll, 1992; Paul and Ritson-Williams, 2008; Lai., 2013; Qi and Ma, 2017). Cembranoid diter- penes are the most dominant bioactive metabolites iso- lated from differentspecies (Chen., 2012; Blunt., 2013). Moreover, sesquiterpenes from marine organisms exhibited strong antifouling activities in labo- ratory and field studies (Hiroto., 1998; Tsoukatou., 2007; Raveendran., 2011). The observed higher chemical defense activity against the biofilm-forming bacterium by the extracts obtained from the normal coral samples may be due to the abundance of sesquiterpenes.
In conclusion, results of the present study showed that the compounds present in the soft corals could be used as antifouling agents for preventing biofouling on marine structures. The results also revealed that tissue damage or abiotic stress may down-regulate the chemical defense activities of the corals, which has strong implications in coral reef ecology (Lamp., 2015). The variations between antimicrofouling activities of two types of ex- tracts (normal and damaged coral samples) need to be investigated. Hence, additional studies on the active compounds present in the soft coral species from the Red Sea may provide more information on the chemical ecol- ogy of Red Sea soft corals.
Acknowledgement
We thank King Abdulaziz City for Science and Technology (KACST) for providing financial assistance to this study (No. PS-37-988).
Aratake, S., Tomura, T., Saitoh, S., Yokokura, R., Kawanishi, Y., Shinjo, R., Reimer, J. D., Tanaka, J., and Maekawa, H., 2012. Soft coral(Cnidaria: Anthozoa: Octocorallia) species diversity and chemotypes., 7: e30410.
Blunt, J. W., Copp, B. R., Keyzers, R. A., Munro, M. H., and Prinsep, M. R., 2013. Marine natural products., 30: 237-323.
Chen, W. T., Li, Y., and Guo, Y. W., 2012. Terpenoids ofsoft corals: Chemistry and bioactivity., 2: 227-237.
Coll, J. C., 1992. The chemistry and chemical ecology of octo- corals (Coelentrata, Anthozoa, Octocorallia)., 92: 613-631.
Dobretsov, S., Al-Wahaibi, A. S., Lai, D., Al-Sabahi, J., Claere- boudt, M., Proksch, P., and Soussi, B., 2015. Inhibition of bacterial fouling by soft coral natural products., 98: 53-58.
Dubois, M., 1956. Calorimetric method for determination of sugars and related substances., 28: 350- 356.
Evans, A. J., Steer, M. D., and Belle, E. M., 2011. The Alcyo- nacea (soft corals and sea fans) of Antsiranana Bay, northern Madagascar., 6: 29- 35.
Fabricius, K. E., 1995. Slow population turnover in the soft coral generaandon mid-and outer- shelf reefs of the Great Barrier Reef., 126: 145-152.
Hirota, H., Okino, T., Yoshimura, E., and Fusetani, N., 1998. Five new antifouling sesquiterpenes from two marine sponges of the genusand the nudibranch., 54: 13971-13980.
Kamel, H. N., and Slattery, M., 2005. Terpenoids of: Chemistry and biomedical applications., 43: 253-269.
Kelman, D., Kashman, Y., Rosenberg, E., Kushmaro, A., and Loya, Y., 2006. Antimicrobial activity of Red Sea corals., 149: 357-363.
Khalesi, M. K., Beeftink, H. H., and Wijffels, R. H., 2008. The soft coral: Potential for drug development. In:Lee- wis, R. J., and Janse, M., eds., Burgers’ Zoo, Arnhem, 47-60.
Lages, B. G., Fleury, B. G., Pinto, A. C., and Creed, J. C., 2010. Chemical defenses against generalist fish predators and foul- ing organisms in two invasive ahermatypic corals in the genus., 31: 473-482.
Lai, D., Geng, Z., Deng, Z., van Ofwegen, L., Proksch, P., and Lin, W., 2013. Cembranoids from the soft coralwith antifouling activities., 61: 4585-4592.
Lakshmi, V., and Kumar, R., 2009. Metabolites fromspecies., 23: 801-850.
Lamb, J. B., Williamson, D. H., Russ, G. R., and Willis, B. L., 2015. Protected areas mitigate diseases of reef‐building corals by reducing damage from fishing., 96: 2555-2567.
LimnaMol, V. P., Raveendran, T. V., Parameswaran, P. S., Kun- nath, R. J., and Sathyan, N., 2010. Antifouling sesquiterpene from the Indian soft coral,Alderslade & Prita., 39: 270-273.
Maida, M., Sammarco, P. W., and Coll, J. C., 2006. A diffusion chamber for assessing efficacy of natural anti-fouling de- fenses in marine organisms., 337: 59-64.
Meszaros, A., and Bigger, C., 1999. Qualitative and quantitative study of wound healing processes in the coelenterate,: Spatial, temporal, and environmental (light at- tenuation) influences., 73: 321-331.
Mizobuchi, S., Adachi, K., and Miki, W., 1996. Antifouling polyhydroxysterols isolated from a Palauan octocoral ofsp., 62: 98-100.
Mizobuchi, S., Kon-ya, K., Adachi, K., Sakai, M., and Miki, W., 1994. Antifouling substances from a Palauan octocoralsp., 60: 345-346.
Moberg, F., and Folke, C., 1999. Ecological goods and services of coral reef ecosystems., 29: 215-233.
Paul, V. J., and Ritson-Williams, R., 2008. Marine chemical ecol- ogy., 25: 662-695.
Qi, S. H., and Ma, X., 2017. Antifouling compounds from ma- rine invertebrates., 15: 263.
Qian, P. Y., Chen, L., and Xu, Y., 2013. Mini-review: Molecular mechanisms of antifouling compounds.,29: 318- 400.
Raveendran, T. V., Mol, V. L., and Parameswaran, P. S., 2011. Natural product antifoulants from the octocorals of Indian waters., 65: 265-268.
Rosenberg, M., Gutnick, D., and Rosenberg, E., 1980. Adher- ence of bacteria to hydrocarbons: A simple method for meas- uring cell-surface hydrophobicity., 9: 29-33.
Satheesh, S., Ba-akdah, M. A., and Al-Sofyani, A. A., 2016. Natural antifouling compound production by microbes asso- ciated with marine macroorganisms–A review., 21: 26-35.
Satheesh, S., Soniamby, A. R., Shankar, C. S., and Punitha, S. M. J., 2012. Antifouling activities of marine bacteria associated with sponge (sp.)., 11: 354-360.
Selim, N. M. A., 2012. Chemical and bioactivity studies of the red sea soft corals:(Ehrenberg) and(Marenzellar) (Fam. Alcyoniidae). PhD thesis, Cairo University.
Shi, H., Yu, S., Liu, D., van Ofwegen, L., Proksch, P., and Lin, W., 2012. Sinularones A–I, new cyclopentenone and buteno- lide derivatives from a marine soft coralsp. and their antifouling activity., 10: 1331-1344.
Slattery, M., McClintock, J. B., and Heine, J. N., 1995. Chemi- cal defenses in Antarctic soft corals: Evidence for antifouling compounds., 190: 61-77.
Takaki, H., Koganemaru, R., Iwakawa, Y., Higuchi, R., and Miyamoto, T., 2003. Inhibitory effect of norditerpenes on LPS-induced TNF-α production from the Okinawan soft coral,sp., 26: 380- 382.
Tsoukatou, M., Maréchal, J. P., Hellio, C., Novaković, I., Tu- fegdzic, S., Sladić, D., Gašić, M. J., Clare, A. S., Vagias, C., and Roussis, V., 2007. Evaluation of the activity of the sponge metabolites avarol and avarone and their synthetic deriva- tives against fouling micro- and macroorganisms., 12: 1022-1034.
Van Alstyne, K. L., Dethier, M. N., and Duggins, D. O., 2001. Spatial patterns in macroalgal chemical defenses. In:. McClintock, J. B., and Baker, B. J., eds., CRC Press, Boca Raton, 301-324.
Wang, C. Y., Liu, H. Y., Shao, C. L., Wang, Y. N., Li, L., and Guan, H. S., 2008. Chemical defensive substances of soft corals and gorgonians., 28: 2320-2328.
Wang, J., Su, P., Gu, Q., Li, W. D., Guo, J. L., Qiao, W., Feng, D. Q., and Tang, S. A., 2017. Antifouling activity against bryo- zoan and barnacle by cembrane diterpenes from the soft coral., 120: 97-103.
Work, T. M., and Aeby, G. S., 2010. Wound repair in., 105: 116-119.
Xuan, H. B., 2014. Soft coral (Octocorallia, Alcyonacea) diver- sity and distribution along a latitudinal environmental gradi- ent and the role of their chemical defenses against predatory fish in the Red Sea. PhD thesis. Christian-Albrechts-Univer- sität Kiel.
September 13, 2017;
December 27, 2017;
July 3, 2018
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2018
. E-mail: ssathianeson@kau.edu.sa
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Comparative Evaluation of Toleration to Heating and Hypoxia of Three Kinds of Salmonids
- Morphological Change in the Northern Red River Delta, Vietnam
- Internal Facies Architecture and Evolution History of Changxing Mouth-Bar Complex in the Changjiang (Yangtze) Delta, China
- The Holocene Environmental Evolution of the Inner Hangzhou Bay and Its Significance
- Provenance of Sediments Filling a Paleo-Channel that Formed on the Western Yellow Sea Continental Shelf During the Last Glacial Period
- Distribution and Characteristics of Hazardous Geological Features in the Marine Coastal and Offshore Areas of Zhejiang Province, East China Sea
