Preservation of Lipid Molecular Fossils in the Sediments from an Oil-Gas Releasing Zone in the South China Sea
2018-12-20QIUHongZOULiZHANGMinshengZHUChaoqiandJIAYonggang
QIU Hong, ZOU Li, 2), *, ZHANG Minsheng, 2), ZHU Chaoqi, and JIA Yonggang, 2)
Preservation of Lipid Molecular Fossils in the Sediments from an Oil-Gas Releasing Zone in the South China Sea
QIU Hong1), ZOU Li1), 2), *, ZHANG Minsheng1), 2), ZHU Chaoqi1), and JIA Yonggang1), 2)
1),,266100,2),,266100,
In order to explore the standing stock and preservation of organic carbon, different forms of compound specific lipids were analyzed, based on a 1.8m sediment core collected in the northern continental shelf of South China Sea in May, 2016. In the core sediments, 21 species of fatty acids, 6 species of alcohols, 8 species of sterols and phytol were detected. Fatty acids were dominated components with the percent of 67%–91% in the total lipids, in which saturated fatty acid C16:0 accounted for about 50%. The contents of lipid species generally decreased from the sediments at surface to those deep, except alcohols waving in a certain range at the same time. Fatty acids primarily existed in free form, followed by base hydrolytic form, and then acid hydrolytic form. Free alcohols were obviously dominated, while base and acid hydrolytic forms contributed small comparably. Phytol gave a significant priority to base hydrolytic form, while acid hydrolytic form could be neglected. Similar to phytol, sterols mostly presented in base hydrolytic form, and then in free form. The compositions of lipids and their forms suggested that organic carbon basically originated from primary production in upper waters in studying area, and were well preserved in the sediments.
lipids; sediments; preservation; South China Sea
1 Introduction
The South China Sea (SCS) represents one of the largest marginal seas in the world, located in the western boundary of Pacific Ocean, with an area of 350×104km2. Influenced by the Pacific and Eurasian plates, the southeastern SCS had experienced complex geological processes, intense magmatism and frequent tectonic movements, and then developed into the typical passive continental margin (Zhang., 2017). Meanwhile, the content of organic carbon in the sediments ranged from 0.50% to 1.49% due to abundant input of terrestrial materials and a high deposition rate of 54.2cmkyr−1since Neogene (Chen., 2016), which provided abundant material sources for the formation of natural gas hydrates. Further than that, the extensive geological fracture system formed a series of sedimentary basins and helped to migrate and accumulate the deep petroleum and gas hydrate in the northern South China Sea. It was reported that over 100 billion m3of gas reservoirs existed in the continental slope of South China Sea (Zhu., 2009; Huang.,2016) and the South China Sea became an important occurrence area of gas and oil (Zhang., 2010).
Although little organic carbon (<10%) produced in the euphotic zone was able to reach to the surface sediment in continental shelf (Hedges., 2001; Armstrong., 2001), they were the basic sources for the formation of gas and oil in the ocean, if associated with suitable geological conditions, such as the basin type, tectonic background and depositional environment (Zhou., 2007; Mi., 2008; Hammer., 2011). Thus, the geochemical characteristics, such as the total organic carbon (TOC), hydrogen index (HI), Rock-Eval (RE) pyrolysis (Espitalie., 2006; Hanson., 2007; Wu., 2014), played a key role in determining the potential sources of oil and gas during geochemical sedimentary processes of organic matters (Tissot, 1987; Shalaby., 2011). Lipid biomarkers, although as a small part of sedimentary organic matter, were highly resistant to biodegradation and could be well preserved during sedimentation and dia- genesis. So it provided important information about the biochemical processes and depositional environments of the organism (Saliot, 1991; Killops and Killops, 2005; Duan, 2008). For example, lipid biomarkers could be used as the index of the type, quality and depositional conditions of organic matters and the assessment indicator of maturity level, biodegradation extent and lithification degree. Such features had made biomarkers potentially applicable to oil-to-oil correlations (Isaksen, 1995; Willsch and Radke, 1995; Kruge, 2000; Arfaoui, 2014). Lipid biomarkers and associated13C abundance indicated that the marine production contributed far more than the terrestrial inputs on organic matters in the sediments in subtropical Pearl River Estuary and adjacent shelf (Hu., 2006), and Shenhu area (Zhu., 2014), where the sediment core was collected in this study. Indicated by highly diagenetically- altered amino acids, low accumulation rate of organic carbon was determined in oxic estuarine sediments (Zhang., 2014). Meanwhile, lipid biomarkers and biogenic methane were well preserved in anoxic cold seep area (Ge., 2015). It would be meaningful to discover the lipid preservation in anoxic sediments, which would help to understand the relationship between the lipid digenesis and oil-gas formation.
A sediment core was collected and different forms of lipid molecules were analyzed in this study, in order to issue the topics of standing stock and preservation of organic carbon in a gas slow releasing area in the northern South China Sea, which may enrich the information on the formation processes of gas hydrates.
2 Materials and Methods
2.1 Study Area and Sample Collection
The study area is located in northeast continental slope of the South China Sea, with water depths between 1200– 1300m, shown in Fig.1. A short column (40cm) was collected at Site S8 with a box corer in May, 2016. A 1.8m sediment core was obtained by gravity sampler attached with cylindrical polyvinyl chloride pipes (100cm×10cm) at Site S8 at the same time. The sediment columns were pored and covered with liquid nitrogen for quick freezing in situ and then kept frozen until analysis.
Sediment columns were thawed at room temperature and sliced at 1 or 2cm intervals before further analysis in laboratory.
Fig.1 Location map of study area and sampling site in the South China Sea in May, 2016.
2.2 Analysis on Lipid Molecules
Three forms of lipid molecules (free, base hydrolytic and acid hydrolytic lipids) were extracted under a modified method (Zou., 2004; Peng, 2012). The 5g sediment subsample was ultrasonically extracted with organic solvents (methanol and methylene chloride) for free lipids. The residues were saponified first and then ultrasonically extracted with organic solvents for base hydrolytic lipids. The last residues were acidified with HF-HCl solution and further extracted for acid hydrolytic lipids. Both base hydrolytic and acid hydrolytic lipids stood for the bound organic matter to the particles, and represented a closer binding to the particles in turn. Extracted lipids were saponified and then separated into neutral and acid fractions by further extraction under different pH conditions. Neutral lipids were treated with BSTFA (N,O-bis(trime- thylsilyl)-trifluoroacetamide) in acetoneitrile to form tri- methylsilyl-ethers (N-TMS) and fatty acids were methylated with 5% BF3-MeOH to form fatty acid methyl esters (FAMEs).
FAMEs and N-TMS were identified and quantified with GC-MS (Agilent 7890A-5975C). Separations of lipids were achieved with a DB-5 capillary column (30m×0.25mm×0.25μm). The temperature of inlet and detector was 300℃. The temperature condition was programmed as 50–150℃ at 20℃min−1, then 150–300℃ at 4℃min−1intervals, and held at 300℃ for 20min. N2was used as the carrier gas, with air flow rate at 300mLmin−1and hydrogen flow rate at 30mLmin−1and tail blowing rate at 30mLmin−1. The internal standard (nonadecanoic acid methyl ester for FAMEs and α(H)-cholestane for N-TMS, Sigma-Algrich) were added into samples before analysis to aid in quantification. The abundance of lipid compounds was calculated based on the ratios of their gas chromatography peaks versus those of the internal standards and reported asμgg−1(dry weight).
3 Results and Discussion
3.1 Bulk Contents and Compositions of Lipids
21 species of fatty acids, 6 species of alcohols, 8 species of sterols and phytol were detected in the sediment samples. The total content of fatty acids (TFAs) were the sum of the content of every fatty acid molecule detected, so were the total contents of alcohols (TOHs) and sterols (TSTs). TFAs, TOHs, phytol and TSTs, and their relative percentages of free, base hydrolytic and acid hydrolytic forms were shown in Fig.2.
TFAs presented an obviously higher content in the surface sediment (104.66μgg−1), decreased rapidly from sur- face to subsurface (25.46μgg−1at 20cm) sediments, and then continuously kept at relative low contents in the sedi- ments below 20cm layer (11.18–17.39μgg−1). Free and base hydrolytic forms of fatty acids were comparable in contents and accounted for 33.61%–62.21% (ave. 41.64%) and 27.85%–56.62% (ave. 45.10%) respectively, while acid hydrolytic forms of fatty acids accounted for 6.15%– 22.97% (ave. 13.26%). The contents of TOHs waved among 3.30–6.67μgg−1from surface to 35cm layer, and held at 4.08–5.48μgg−1below 35cm layer. Free forms of alcohols dominated for the percent of 53.42%–83.60% (ave. 69.16%), while base and acid hydrolytic alcohols accounted for 13.70%–32.40% (ave. 20.64%) and 2.38%– 24.86% (ave. 10.19%) respectively. Contents of phytol and TSTs were comparable and followed the similar distribution pattern vertically, ranging at 0.33– 2.89μgg−1(ave. 1.35μgg−1) and 0.17–3.41μgg−1(ave. 1.29μgg−1), respectively, and decreasing from surface to deep layers. Base hydrolytic phytol was absolutely dominated species for the percent of 76.23%–90.14% (ave. 82.09%), free hydrolytic phytol contributed little by 9.86%–23.77% (ave. 17.91%), while acid hydrolytic phytol was undetect- able. Similar to the composition of phytol, base hydrolyticTSTs took 50.92%–100.0% (ave. 71.66%), while free TSTs held for the other part and acid hydrolytic TSTs could be neglected.
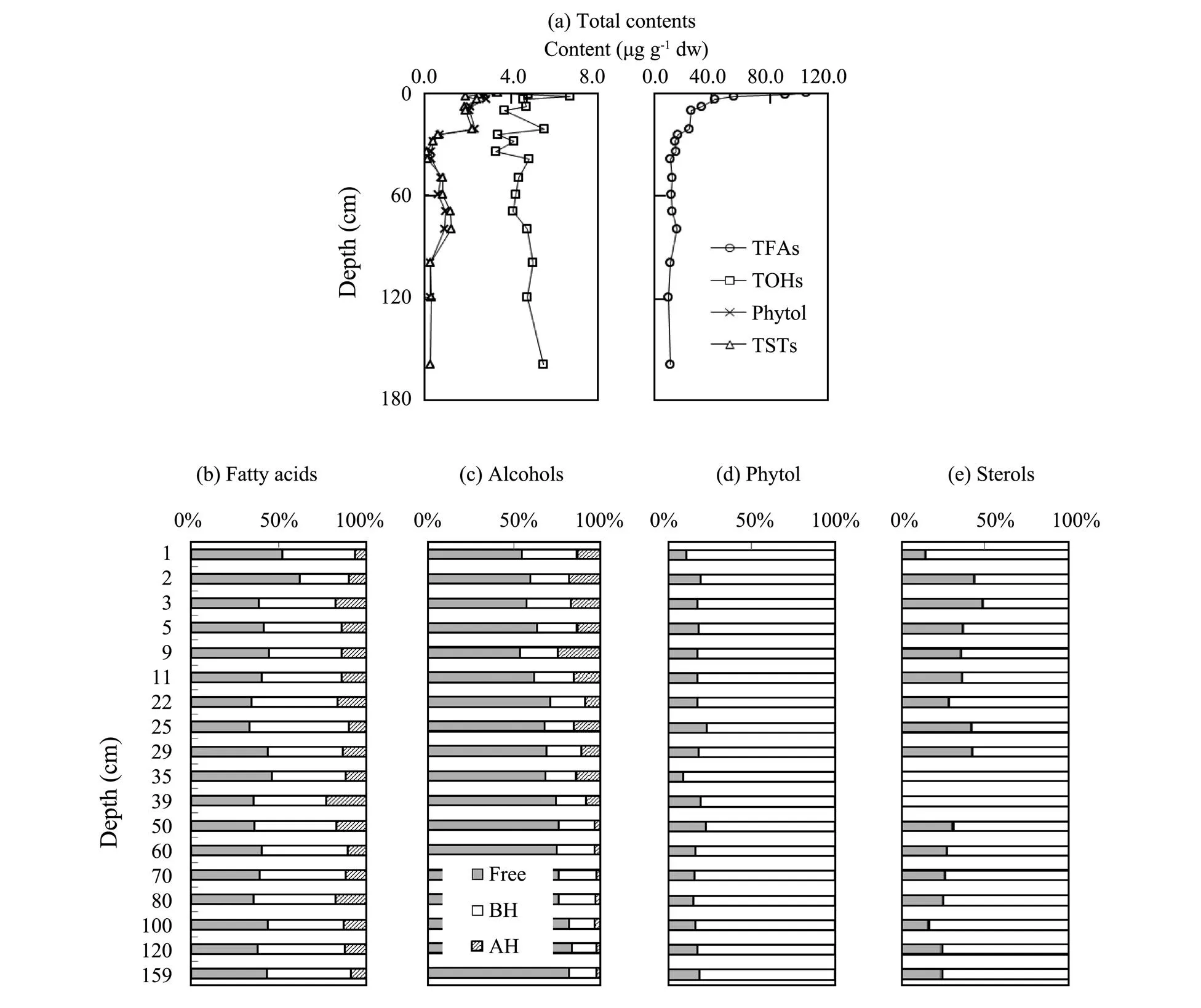
Fig.2 Total contents and compositions of fatty acids, alcohols, phytol and sterols in the core sediments (Free-free form; BH-based hydrolytic form; AH-acid hydrolytic form).
3.2 Vertical Distribution of Specific Lipid Molecule
Homologous compounds generally follow a similar pattern of distribution, and similar transportation and transformation history for their similar structure. Homologous compounds of lipids detected presented highly similarity in vertical distributions. Linear correlation analysis showedthat the homologous compounds detected significantly co-elated with each other. The vertical distribution of specific lipid molecules in different forms, based on the structural functional groups, was shown in Fig.3.
C16:0, a typical species of even saturated linear fatty acid, accounted for 50% of TFAs in surface sediment (0– 2cm) and 24%–37% (ave. 29%) in the sediments at 2cm below. C16:0 contents decreased rapidly from 53.69μgg−1in surface sediment to 10.54μgg−1 in the sediment at 10cm, and then kept at lower levels to the deep. C16:0 were dominated by free form (>40%) in sediments at 0–10cm layers, and the dominant of C16:0 changed from free form to base hydrolytic form (>40%) in sediments at 10cm below. Acid hydrolytic C16:0 contributed a little more than 10% in sediments at 10–50cm, while less than 10% at the other layers.
The vertical distribution patterns of other lipid molecules (except alcohol 18-OH) were quite similar with each other in both bulk content variations and contents of each form from surface to deep layers. Approximately similar to that of C16:0, the contents of these lipid molecules were higher in surface sediment, decreased significantly from surface to 10cm deep, and then changed little downward. The compound specific lipid molecules might indicate the sources and transformation processes directly (Volkman., 1980). Generally, sterols were more stable than fatty acids and phytol, while saturated fatty acids were more stable than unsaturated ones (Colombo., 1997). Moreover, the free fatty acids were easily trans- formed by bacteria, while base and acid hydrolytic components were relatively stable due to their combination with particles (Ding and Sun, 2005). Different from C16:0, free forms of C18:2(ω-6,9) and C18:1(ω-9) presented higher contents in the sediment at 10 cm, so do the base hydrolytic forms of Ci-15:0(ω-3), C15:0, phytol and sterols of cholesta-7,22E-dien-3β-ol (27Δ7,22), cholest-22-en- 3β-ol (27Δ22), and 5a-cholestan -3β-ol (27Δ0). Form compositions of C15:0(ω-3) and C15:0 were similar in that, free and base hydrolytic contents were generally comparable with the percentage of about 45% for each and acid hydrolytic contents contributed for <10%, which agreed to that of C16:0. Form compositions of C18:2(ω-6,9) and C18:1(ω-9) were similar in that, base hydrolytic contents prioritized to free ones by 10% on average and acid hydrolytic contents might contributed 15%–25% on average. Base hydrolytic forms absolutely dominated for C24:0, phytol and 3 species of sterols, which were 20%–60% more than free forms. Further than that, as an indicator of marine productivity and environment (Johns., 1980; Rontani and Volkman, 2003; Carreira., 2010), about 75% phytol stayed in base hydrolytic form, while the other in free form. It was suggested that the general extraction with organic solvents in pre-treatment method might largely undervalue the existence of phytol in marine sediments.
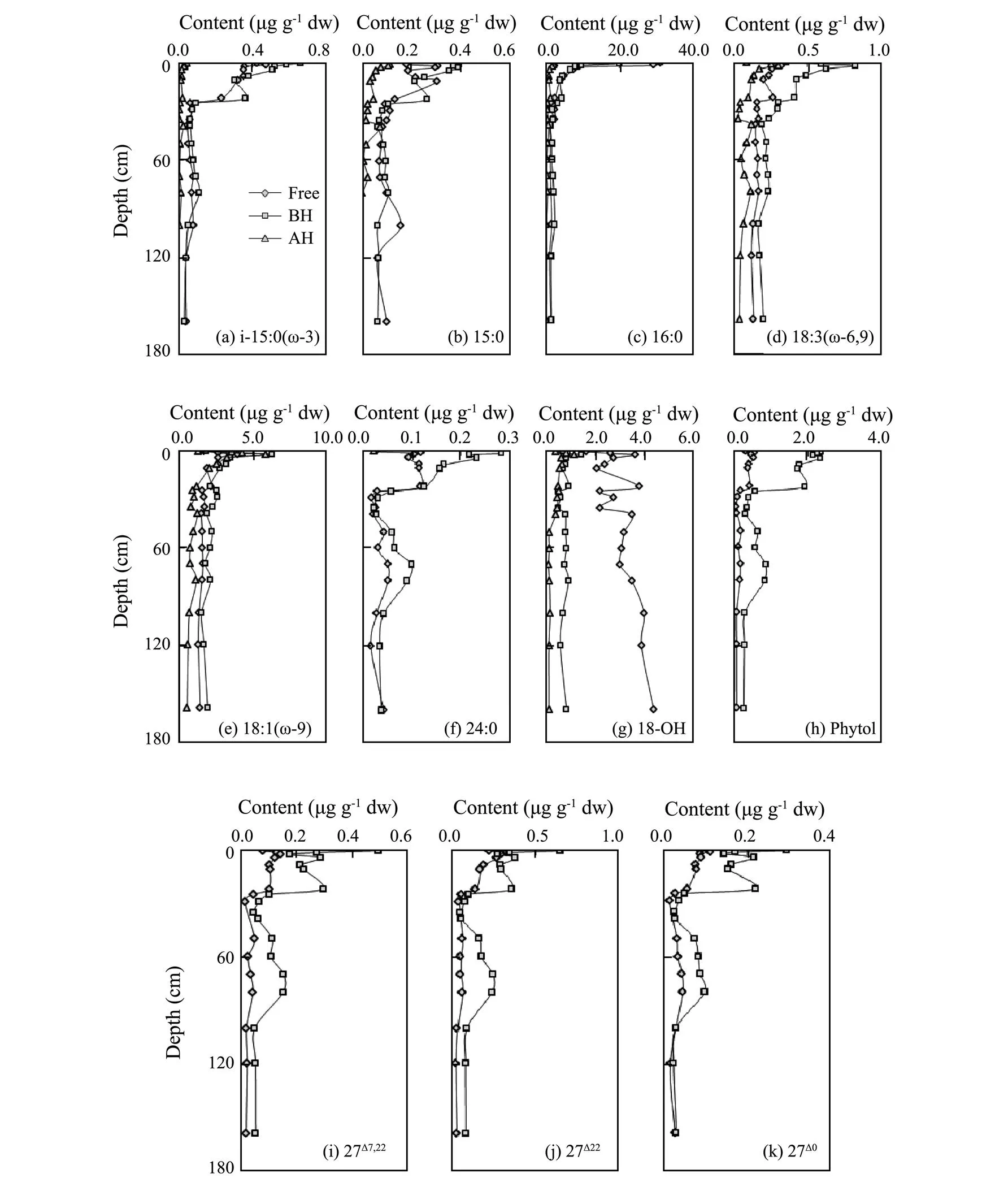
Fig.3 Contents of compound specific lipids in the sediment column (Free-free form; BH-based hydrolytic form; AH-acid hydrolytic form).
The vertical distribution of alcohol 18-OH differed from the other lipid molecules. Alcohol 18-OH contents kept in a certain range from surface to deep sediments in the whole column, but varied between 0 and 50cm (2.75– 6.24μgg−1), and relatively constant at 50cm below (3.89– 5.35μgg−1). Moreover, free 18-OH prevailed by 58%– 84% (ave. 71%), followed by base hydrolytic 18-OH (13%–25%, ave. 19%) and then by acid hydrolytic 18-OH (2%– 19%, ave. 10%).
Base on the distribution similarity of lipid molecules from surface to deep sediments, including odd linear sa- turated fatty acid (C15:0), branched saturated fatty acid (Ci-15:0(ω-3)), unsaturated fatty acids (C18:2(ω-6, 9) and C18:1(ω-9)), phytol, and sterols (27Δ7, 22, 27Δ22, 27Δ0), it was suggested that the lipids might primarily originated from similar sources. But based on the dissimilar distributions of form compositions, such as alcohols, phytol and sterols, it was suggested that the different structure of lipids might experience different transformation during sedimentary processes.
3.3 Vertical Distribution of Fatty Acids and the Species Grouped by Potential Functions
Fatty acids are major components in lipids, and they accounted for 67%–91% of total lipids in the studied core sediments. Most molecules of fatty acids are compound specific and available in tracing the source and transformation of organic carbon. Hereby, the fatty acids were grouped on structures which were produced by specific biological groups. Long chain fatty acids (LCFA) were originated from terrestrial input and derived from higher plants (Volkman., 1980; Meyers, 2003; Lv and Zhai, 2006), odd and branched saturated fatty acids (OBFA) were principally produced by marine bacteria (Volkman., 1980; Budge., 2001; Ishiwatari., 2006), unsaturated fatty acids (USFA) were derived from marine phytoplankton, and even saturated fatty acids (ESFA) came from multiple sources (Shi., 2001; Lebreton., 2011). The contents and compositions of fatty acids were shown in Fig.4.
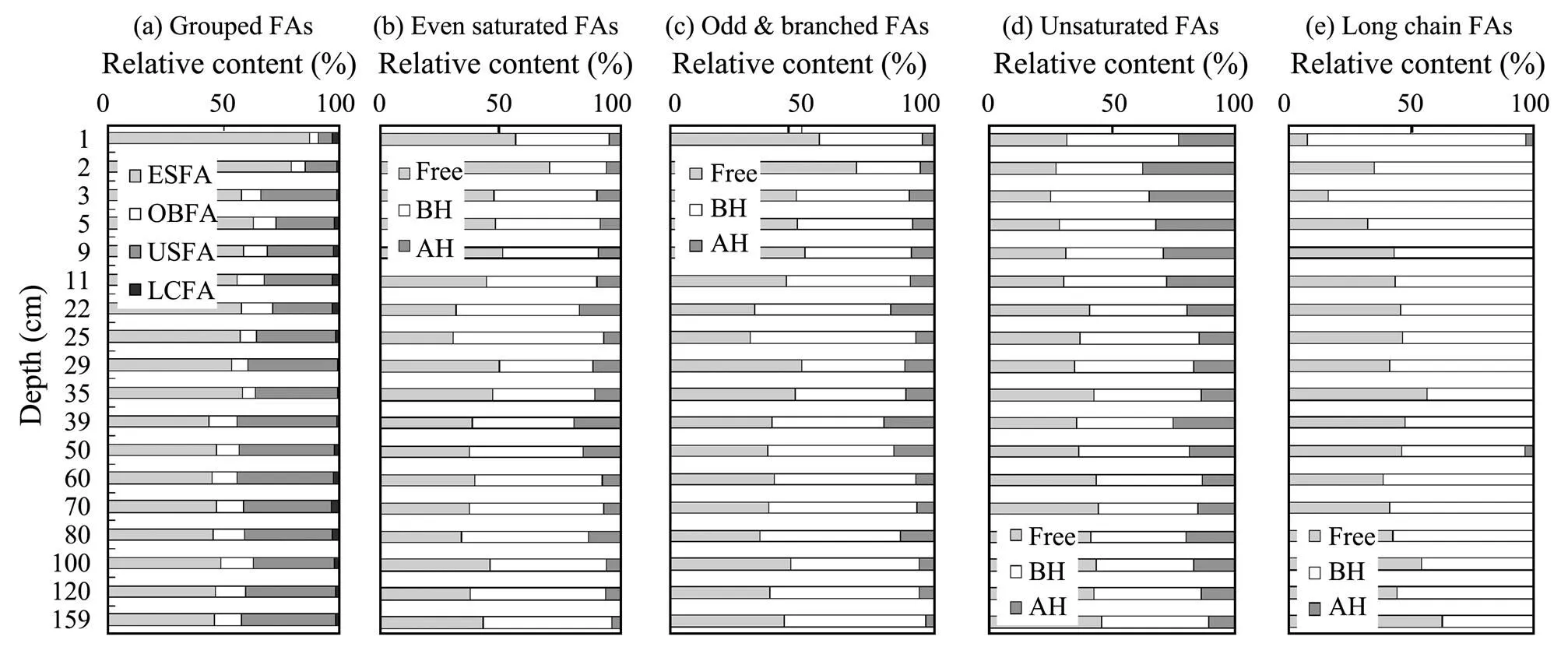
Fig.4 Relative contents and compositions of fatty acids in ptential functional groups in the core sediments (Free-free form; BH-based hydrolytic form; AH-acid hydrolytic form).
The multiple fatty acids (ESFA) dominated with the relative contents of more than 50% but tended to decrease from surface to deep. Relative contents of free and base hydrolytic ESFA varied with comparable percentage of 45% respectively through the column, while acid hydrolytic ESFA contributed <10%. Terrestrial inputting LCFA always donated minor percentages (<4%) through the column. LCFA were almost all composed by free and base hydrolytic ones, in which base hydrolytic LCFA were more than free LCFA, especially in surface sediments. Relative contents of USFA exceeded to those of OBFA by 0.5–3 times. These features were unusually differed from the results discovered in dissolved lipids and coastal sediments in Bohai and Yellow Seas (Gao., 2017). The higher relative contents of USFA suggested that more organic matters in primary productions had been well preserved in studying area, which might be in accordance with the sedimentary environment for oil and gas formation. Further than that, as shown in Fig.2, the high contents of phytol, an indicator of marine productivity and environment (Johns., 1980; Rontani and Volkman, 2003; Carreira., 2010), also confirmed the good pre- servation of primary organic carbon in studying area. About 75% phytol occurred as base hydrolytic form, while the other in free form. A similar high content of base hydrolytic phytol was also reported in the coastal sediments of East China Sea (Wang., 2011).
4 Conclusions
Lipids, relatively stable among the biological organic carbon, are not only the predecessor of natural gas and oil in the ocean, but also the good record to the sources, transportation and transformation processes of organic carbon. The northern continental shelf of South China Sea is one of the most important standing areas for natural gas and oil. Hereby, compound specific molecules of lipids in a 1.8m sediment core were analyzed in free, base hydrolytic and acid hydrolytic forms to determine the standing stock and preservation of organic carbon in an oil-gas releasing zone, and provide more detailed information on the formation of oil-gas reservoirs.
A total of 36 species compounds were detected, belonging to fatty acids (21), alcohols (8), phytol and sterols (6). The total amount of lipids (estimated by the content of every molecule) were higher in surface sediment (about 100μgg−1), then decreased rapidly to the 10cm layer (about 20μgg−1), and kept at lower contents in the deep sediments. Fatty acids were dominated with the percent of over 60% in total lipids, and C16:0 contributed >50% of total fatty acids. Vertical distribution of compound specific molecules in the core followed similar patterns with that of total lipids, except for alcohols.
Lipid molecules primarily presented in free and base hydrolytic forms, and existed little in acid hydrolytic form. Free fatty acids and alcohols were dominated, while phytol and sterols mainly occurred in base hydrolytic forms. Phytol and sterols in acid hydrolytic form could be neglected.
Sources could be speculated by compound specific molecular compositions. Results showed that more than 50% of fatty acids were contributed by marine phytoplankton, and specific fatty acids from marine phytoplankton were 1.5–3.8 times as much as that from bacteria. Moreover, terrestrial fatty acids accounted for <4%.
The contents and form compositions of lipids suggested that both source and structure influenced the standing state of lipid molecules in the sediments. The organic carbon in the sediments was primarily originated from the primary production in upper waters, and well preserved in the sediments, which might be important to the oil-gas formation in studying area.
Acknowledgements
This study is supported by the National Natural Science Foundation of China (No. 41176064) and China- ASEAN maritime cooperation fund: Comparative Study of Holocene Sedimentary Evolution of the Yangtze River Delta and the Red River Delta.
Arfaoui, A., 2014. The advantages of using n-alkanes, triterpane, and steranes to determine thecharacterization of sedimentary organic matter., 7 (1): 369- 383.
Armstrong, R. A., Lee, C., Hedges, J. I., Honjo, S., and Wakeham, S. G., 2001. A new, mechanistic model for organic carbon fluxes in the ocean based on the quantitative association of POC with ballast minerals., 49 (1): 219-236.
Budge, S. M., Parrish, C. C., and Mckenzie, C. H., 2001. Fatty acid composition of phytoplankton, settling particulate matter and sediments at a sheltered bivalve aquaculture site., 76 (4): 285-303.
Carreira, R. S., Araújo, M. P., Costa, T. L. F., Ansari, N. R., and Pires, L. C. M., 2010. Lipid biomarkers in deep sea sediments from the Campos Basin, SE Brazilian continental margin., 41 (9): 879-884.
Chen, F., Lu, H. F., Liu, J., Zhuang, C., Wu, C., Cao, J., Zhou, Y., and Liu, G. H., 2016. Sedimentary geochemical response to gas hydrate episodic release on the northeastern slope of the South China Sea., 41 (10): 1619-1629 (in Chinese with English abstract).
Colombo, J. C., Silverberg, N., and Gearing, J. N., 1997. Lipid biogeochemistry in the Laurentian Trough–II. Changes in composition of fatty acids, sterols and aliphatic hydrocarbons during early diagenesis., 26 (3): 257- 274.
Ding, H., and Sun, M. Y., 2005. Biochemical degradation of algal fatty acids in oxic and anoxic sediment-seawater interface systems: Effects of structural association and relative roles of aerobic and anaerobic bacteria., 93 (1): 1-19.
Duan, Y., 2008.. Science Press, Beijing, 34pp.
Espitalié, J., Laporte, J. L., Madec, M., Marquis, F., and Leplat, P., 2006. Méthode rapide de caractérisation des roches mètres, de leur potentiel pétrolier et de leur degré d'évolution., 32 (1): 23-42.
Gao, H. L., Zou, L., Wang, K., and Ye, X. W., 2017. Compositional distribution and transformation of terrestrial lipid organic matter in the sediments of the Yellow Sea and Bohai Sea., 39 (2): 53-61 (in Chinese with English abstract).
Ge, L., Jiang, S. Y., Blumenberg, M., and Reitner, J., 2015. Lipid biomarkers and their specific carbon isotopic compositions of cold seep carbonates from the South China Sea., 66: 501-510.
Hammes, U., Hamlin, H. S., and Ewing, T. E., 2011. Geologic analysis of the upper Jurassic Haynesville Shale in east Texas and west Louisiana., 95 (10): 1643-1666.
Hanson, A. D., Ritts, B. D., and Moldowan, J. M., 2007. Organic geochemistry of oil and source rock strata of the Ordos Basin, north-central China., 91 (9): 1273-1293.
Hedges, J. I., Baldock, J. A., Gélinas, Y., Lee, C., Peterson, M., and Wakeham, S. G., 2001. Evidence for non-selective preservation of organic matter in sinking marine particles., 409 (6822): 801.
Hu, J., Zhang, H., and Peng, P., 2006. Fatty acid composition of surface sediments in the subtropical Pearl River Estuary and adjacent shelf, southern China., 66 (1-2): 346-356.
Huang, B., Tian, H., Li, X., Wang, Z., and Xiao, X., 2016. Geochemistry, origin and accumulation of natural gases in the deepwater area of the Qiongdongnan Basin, South China Sea., 72: 254-267.
Isaksen, G. H., 1995. Organic geochemistry of paleodepositional environments with a predominance of terrigenous higher-plant organic matter. In:. Huc, A. Y., ed., The American Association of Petroleum, Tulsa Oklahoma, 81-104.
Ishiwatari, R., Yamamoto, S., and Shinoyama, S., 2006. Lignin and fatty acid records in Lake Baikal sediments over the last 130kyr: A comparison with pollen records., 37 (12): 1787-1802.
Johns, R. B., Gillan, F. T., and Volkman, J. K., 1980. Early diagenesis of phytyl esters in a contemporary temperate intertidal sediment., 44 (2): 183- 188.
Killops, S. D., and Killops, V. J., 2005.. Blackwell Publication, Malden, 1-393.
Kruge, M. A., 2000. Determination of thermal maturity and organic matter type by principal components analysis of the distributions of polycyclic aromatic compounds., 43 (1-4): 27-51.
Lebreton, B., Richard, P., Galois, R., Radenac, G., and Pfléger, C., 2011. Trophic importance of diatoms in an intertidalseagrass bed: Evidence from stable isotope and fatty acid analyses., 92 (1): 140-153.
Lv, X., and Zhai, S., 2006. Distributions and sources of organic biomarkers in surface sediments from the Changjiang (Yangtze River) Estuary, China., 26 (1): 1-14.
Meyers, P. A., 2003. Applications of organic geochemistry to paleolimnological reconstructions: A summary of examples from the Laurentian Great Lakes., 34 (2): 261-289.
Mi, L., Zhang, G., Shen, H., Liu, Z., Guo, R., and Zhong, K., 2008. Eocene–lower Oligocene sedimentation characteristics of Baiyun Sag in the deep water area of Pearl River Mouth Basin., 29 (1): 11-13.
Peng, R., 2012. Sedimentary of organic carbon in the sediments of North Yellow Sea: Indicated by lipid compounds. Master thesis.Ocean University of China.
Rontani, J. F., and Volkman, J. K., 2003. Phytol degradation products as biogeochemical tracers in aquatic environments., 34 (1): 1-35.
Saliot, A., Laureillard, J., Scribe, P., and Sicre, M. A., 1991. Evolutionary trends in the lipid biomarker approach for investigating the biogeochemistry of organic matter in the marine environment., 36 (1): 233-248.
Shalaby, M. R., Hakimi, M. H., and Wan, H. A., 2011. Geochemical characteristics and hydrocarbon generation modeling of the Jurassic source rocks in the Shoushan Basin, north Western Desert, Egypt., 28 (9): 1611-1624.
Shi, W., Sun, M. Y., Molina, M., and Hodson, R. E., 2001. Variability in the distribution of lipid biomarkers and their molecular isotopic composition in Altamaha estuarine sediments: Implications for the relative contribution of organic matter from various sources., 32 (4): 453-467.
Tissot, B. P., 1987. Thermal history of sedimentary basins, ma- turation indices, and kinetics of oil and gas generation., 71 (12): 1445-1466.
Volkman, J. K., Johns, R. B., Gillan, F. T., and Perry, G. J., 1980. Microbial lipids of an intertidal sediment–I. Fatty acids and hydrocarbons., 44 (8): 1133- 1143.
Wang, J. T., Yang, S., Tian, L. J., and Hu, J., 2011. Composition and form distribution of lipids biomarkers in a sediment core from southern coastal area of Zhejiang Province., 33 (5): 83-90 (in Chinese with English abstract).
Willsch, H., and Radke, M., 1995. Distribution of polycyclic aromatic compounds in coals of high rank., 7 (4): 231-251.
Wu, Y., Zhang, J., and Yu, Z., 2001. Distribution of lipids in the core sediments of the Bohai., 37 (2): 273-277 (in Chinese with English abstract).
Zhang, G. X., Chen, F., Sha, Z. B., Liang, J. Q., Su, X., and Lu, H. F., 2017. The geological evolution process of natural gas hydrate reservoirs in the northeastern South China Sea., 24 (4): 15-23 (in Chinese with English abstract).
Zhang, G., Zhu, W., and Mi, L., 2010. The theory of hydrocarbon generation controlled by source rock and heat from circle distribution of outside-oil fields and inside-gas fields in South China Sea., 28 (5): 987-1005 (in Chinese with English abstract).
Zhang, Y., Kaiser, K., Li, L., Zhang, D., Ran, Y., and Benner, R., 2014. Sources, distributions, and early diagenesis of sedimentary organic matter in the Pearl River region of the South China Sea., 158 (2): 39-48.
Zhou, D., Sun, Z., and Chen, H. Z., 2007. Tectonic features of world’s major deep-water oil/gas fields and their enlightenment to deep-water exploration in northern South China Sea., 22 (6): 561-572 (in Chinese with English abstract).
Zhu, W., Huang, B., Mi, L., Wilkins, R. W. T., Fu, N., and Xiao, X., 2009. Geochemistry, origin and deep-water exploration potential of natural gases in the Pearl River Mouth and Qiongdongnan Basins, South China Sea., 93 (6): 741- 761.
Zhu, X., Mao, S., Wu, N., Sun, Y., and Guan, H., 2014. Molecular and stable carbon isotopic compositions of saturated fatty acids within one sedimentary profile in the Shenhu, northern South China Sea: Source implications., 92 (5): 262-275.
Zou, L., Wang, X. C., Callahan, J., Culp, R. A., Chen, R. F., and Altabet, M. A., 2004. Bacterial roles in the formation of high- molecular-weight dissolved organic matter in estuarine and coastal waters: Evidence from lipids and the compound-spe- cific isotopic ratios., 49 (1): 297-302.
October 17, 2017;
March 9, 2018;
May 3, 2018
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2018
. Tel: 0086-532-66782260 E-mail: zouli@ouc.edu.cn
(Edited by Chen Wenwen)
杂志排行
Journal of Ocean University of China的其它文章
- Comparative Evaluation of Toleration to Heating and Hypoxia of Three Kinds of Salmonids
- Morphological Change in the Northern Red River Delta, Vietnam
- Internal Facies Architecture and Evolution History of Changxing Mouth-Bar Complex in the Changjiang (Yangtze) Delta, China
- The Holocene Environmental Evolution of the Inner Hangzhou Bay and Its Significance
- Provenance of Sediments Filling a Paleo-Channel that Formed on the Western Yellow Sea Continental Shelf During the Last Glacial Period
- Distribution and Characteristics of Hazardous Geological Features in the Marine Coastal and Offshore Areas of Zhejiang Province, East China Sea
