Determination of airborne formaldehyde and ten other carbonyl pollutants using programmed temperature vaporization-large volume injection-gas chromatography
2018-12-06StefanoDUGHERINicolaMUCCIIleniaPOMPILIOGiovanniCAPPELLICostanzaBOSSIAlessandroBONARIGiulioARCANGELI
Stefano DUGHERI, Nicola MUCCI, Ilenia POMPILIO, Giovanni CAPPELLI,Costanza BOSSI, Alessandro BONARI, Giulio ARCANGELI
(Department of Experimental and Clinical Medicine, University of Florence, I-50141 Florence, Italy)
Abstract: Long-term indoor-air limit for formaldehyde stipulated by the European Commission is 1 μg/m3, while the World Health Organization has set a threshold of 100 μg/m3 that should not be exceeded for more than 30 min. To date, however, only a few analytical techniques have been developed that can be used to detect formaldehyde at these very restrictive limits. Thus, there is a need to develop for comprehensive methods for analyzing airborne formaldehyde and other carbonyl pollutants in the ambient environment. The aim of this study is to develop a highly sensitive online automated preconcentration gas chromatographic method using large-volume injection with a programmed temperature vaporization injector for the analysis of airborne formaldehyde and ten other carbonyl compounds. The influence of several parameters, such as the maximum volume injected, programmed temperature vaporization transfer time and temperature, carrier gas flow rate, and type of packing material was investigated. After optimization, highly satisfactory results in terms of the absolute and methodological detection limits were achieved, i. e. as low as the μg/m3 level for all the carbonyl pollutants studied. A commercially available sampler, originally designed for active sampling, was evaluated as a passive sampling device; this optimized technique was applied to monitor the concentrations of carbonyl pollutants in the indoor air of ten public buildings in Florence. The strength of this methodology lies both in the low detection limits reached in the simultaneous analysis of a wide group of 2,4-dinitrophenylhydrazine derivatives, and the potential adaptability of this method to other gas chromatographic applications to achieve lower sensitivity.
Key words: large volume injection (LVI); gas chromatography (GC); formaldehyde; passive sampler; airborne carbonyl compounds; programmed temperature vaporization injector
Formaldehyde (FA) is an important chemical in the global economy, and more than 65% of all FA is used to synthesize resins, including urea-, phenol-, and melamine-FA. These resins are often found in construction materials, and thus they, contribute directly to indoor FA pollution [1-3]. China is the single-largest market for FA, accounting for 47% of world consumption in 2017 [4]. To monitoring airborne FA levels, the Chinese Government has issued and updated a series of national regulatory standards. In 2012, the China State Council implemented a guideline for Ambient Air Quality Standards (AAQS, GB 3095-2012) with the dual aim of improving the ambient environment and protecting the human health. The ambient air quality standards implemented in 2016 throughout China [5,6] are comparable to the interim targets set by the World Health Organization (WHO).
FA has been classified as a human carcinogen that causes nasopharyngeal cancer and possibly leukemia [7]. Nielsen et al. [8] reviewed the WHO assessment thoroughly and concluded that the air quality FA guideline threshold of 100 μg/m3(80 ppb)--not to be exceeded for 30 min--is adequate for preventing all types of cancer. They further agreed that the WHO guideline is also protective against both acute and chronic sensory and airway irritation in the general population. The European Commission established a more stringent indoor air limit for FA of 1 μg/m3[9]; this level was based on the threshold for nose and throat irritation of 100 μg/m3(the lowest-observed-adverse-effect level, LOAEL), the no-observed-adverse-effect level (NOAEL) of 30 μg/m3, and an assessment factor of 30 [10].
These stricter standards for airborne FA and other carbonyl compounds have warranted the development of accurate, sensitive, and automated sampling and analysis tools. Moreover, improved monitoring campaigns have also provided extensive data about environmental contamination. One of the most significant developments in air sampling methodology in recent years is that of passive samplers, which have been on the rise. This technology was first introduced into the health and safety profession in 1973 by researchers Palmes and Gunnison [11]. As the applications of this environmental monitoring technique have grown and changed over the years [12-15], the number and types of passive samplers commercially available for carbonyl compounds have escalated [16-20]. Although liquid chromatography (LC) has been used for such analyses [21], most techniques use gas chromatography (GC) given the excellent separation efficiency of the latter when coupled to different detectors such as flame ionization detectors (FID) [22-24], nitrogen-phosphorus detectors (NPD) [20,25-28], electron capture detectors (ECD) [29], photoionization detectors (PID) [30], and mass spectrometry (MS) [24,31,32]. The current validated methods for detecting gaseous carbonyl pollutants based on either active or passive sampling use 2,4-dinitrophenylhydrazine (DNPH) as the reagent to form the corresponding hydrazones [33-35]. Confirmed findings [20,36,37], however, show poor results for the DNPH-LC/ultraviolet method at low or sub-μg/m3levels. Moreover, this technique is limited in terms of co-elution and specificity, given that the detection and quantification limits exceed the indoor air quality (IAQ) legal requirements during short sampling times. GC has been coupled to MS to solve this sensitivity issue; however, an additional preconcentration step is required to improve the detection/quantification capacity. Among the various options available for preconcentration, large volume injection (LVI) is very convenient for GC analyses as it can be automated and carried out on-line. In miniaturized techniques, such as 96-microwell plates [38], microextraction by packed sorbent (MEPS) [39-41], stir bar sorptive extraction (SBSE)/twister solvent back extraction (TBE) [42], monolithic material sorptive extraction/monotrap solvent extraction [43], and dispersive liquid-liquid microextraction (DLLME) [44], as well as in trace and ultra-trace component analyses, LVI coupled with GC helps to fulfill these goals. The currently available LVI methods include various injection modes, namely, programmed-temperature vaporizing (PTV) solvent split [45-48], on-column injection (OCI) [49,50], direct sample introduction (DSI)/difficult matrix injection (DMI) [51,52], splitless overflow/concurrent solvent recondensation (CSR) [53], AT-column [54], through oven transfer adsorption desorption (TOTAD) [55], and stomach-shaped insert liner [56]. The PTV injector has proven to be the most suitable device for LVI in capillary GC [57-61]; recent improvements in the injector design and the availability of inert packing materials have simplified the optimization procedures and extended the application of this injection mode to more labile and adsorptive analytes. The use of a packed liner is also attractive in LVI given the larger solvent capacity that can be achieved, and because the liner eliminates the need for liquid nitrogen or carbon dioxide for cooling, as required with regular liners. Moreover, the packing not only retains the analytes, but also prevents the transfer of high-boiling matrix constituents to the analytical column. However, the choice of the packing material is critical as the packing should: i) retain the analytes, but also allow their transfer to the column without degradation; ii) be thermally and chemically stable in the presence of the solvent, and iii) not increase the blank levels.
To the best of our knowledge, an on-line preconcentration method using a PTV inlet with a packed liner has never been optimized for the simultaneous determination of 2,4-dinitrophenylhydrazones. Therefore, it is the aim of this study to demonstrate the suitability of such a method for DNPH-derivatives and to optimize the PTV parameters influencing solvent elimination, hydrazone transfer to the GC, and subsequent separation and detection of these species. Sep-Pak XpoSure Aldehyde Plus Short DNPH-coated cartridges are used as a passive sampling device for FA and ten other carbonyl pollutants; the experimental and theoretical sampling rates (SR) demonstrate the applicability of this optimized PTV-LVI technique.
1 Experimental
1.1 Sampling procedures

Fig. 1 Diagram of the Sep-Pak XpoSure Aldehyde Plus Short DNPH-coated sampler
Area sampling was performed with Sep-Pak XpoSure Aldehyde Plus Short DNPH-coated cartridges on a silica sorbent (Fig. 1) (Cat. No. WAT047205, Waters, Milford, USA) attached to a 6-position Gascheck Basic/Air Cube Gas Pro Automatic Collector GSM Box (AMS Analitica, Pesaro, Italy) or an Automatic Cartridge Wi-Fi sampler (Chromline, Prato, Italy) for active and passive air sampling, respectively. The two samplers were remotely controlled by the Data Storing System (Chromline, Italy) as much as possible to avoid operator variability or mistakes. The sampling data and the analytical results were then integrated into a laboratory information management system (Bika Lab System, Western Cape, South Africa), which generates data reports.
The experimental SR of the Sep-Pak sampler used as the passive sampler was obtained by applying the equation:
(1)
The uptake is determined from the slope of the line obtained by correlating the mass of the carbonyl compound adsorbed on the passive sampler with the sampling time at calibrated air concentrations (Ccarbonyl air). The theoretical SR of the passive sampler was determined as:
(2)
whereZis the distance between the outlet tube opening of the Sep-Pak sampler and the DNPH- coated silica gel (0.82 cm),Ais the surface area of the outlet tube opening (0.125 6 cm2), andDgis the analyte diffusion coefficient in air (cm2/s).
1.2 Sample desorption and analysis
1.2.1Onlinexyzautosampler
A new, fully-automated Flexxyzautosampler (EST Analytical, Fairfield, USA) was coupled with a PTV-GC/Saturn 2200 MS/nitrogen phosphorus thermionic specific detector (TSD) (Varian Analytical Instruments) for online analysis of the airborne carbonyl compounds. The robotic sampling platform provides fully-automated functions, including desorption of the analyte from the Sep-Pak sampler cartridge and injection into the GC system using thexyzMulti Tool eXchange (MTX). The MTX fills a 5.0 mL syringe equipped with a polytetrafluoroethylene (PTFE) plunger and b-needle tip (Cat. No. 2600040, ILS Innovative Labor Systeme GmbH, Stützerbach, Germany) with 2 mL of ethyl acetate (EtAc) and first injects the sample into the Sep-Pak cartridge to elute the carbonyl-2,4-dinitrophenylhydrazone. It then injects the eluted solution into an Oasis MCX Plus cation exchange cartridge (Cat. No. 186003516, Waters, Milford, USA) to remove the excess derivatization agent [32]. A 1 mL syringe (Cat. No. 8131, SGE Analytical Science, Trajan, Ringwood, Australia) was used for GC LVI injection.
1.2.2PTV-GC-MS/TSD instrument
The calibration standards and eluate were injected into a Varian CP-3800 GC with a 1078/1079 Capillary Injector CO2Coolant system (Scion Instruments, Livingston, United Kingdom) equipped with Merlin Microseal Septa (Merlin Instrument Co., Newark, USA). The PTV injector was used in temperature ramp mode to vent the solvent vapor and inject the analytes into a 30 m×0.25 mm×0.25 μm DB-35MS UI column (Cat. No. 122-3832UI, Agilent J&W GC Column, Santa Clara, USA). The solvent vent exit solenoid valve was set to open when removing the solvent vapor, closed to inject the sample components into the separation column, and to open again to remove the remaining traces of solvent vapor from the liner. A time delay of 6 min was implemented before allowing the sample to enter the MS filament and electron multiplier. The peak threshold was set to three counts. For all the other compounds, the system operated in electron ionization (EI) full-scan mode, using the base peak from the 70 eV spectra as the quantitation ion. The transfer line, manifold, and trap temperatures were 250, 80, and 180 ℃, respectively. Helium, at respective flow rates of 1.2 and 2.0 mL/min, was used as the carrier gas for MS and TSD; the latter was maintained at 310 ℃ with the bead current set to 3.4 A.
1.3 Calibration and method validation
The model atmospheres containing carbonyl compounds were generated by using a Harvard Plus 11 syringe-pump, set to 2 μL/min, connected to an Adsorbent Tube Injector System (Supelco, Bellefonte, USA). The carbonyl mass concentration in the air (CCA-air, μg/L) was calculated according to the following formula:
(3)
where,CSolis the mass concentration of the solution (g/L),Fsyringeis the syringe-pump flow rate (μL/min), andFairis the air flow rate (L/min).
Five-point calibration curves were constructed by plotting the peak-area ratio for the base peak of the aldehyde/ketone DNPH mix (Cat. No. CRM4M7285, Sigma-Aldrich, Saint Louis, USA) versus the base peak of diphenylamine as the internal standard (IS). A linear regression plot was generated, and the instrumental limit of detection (LOD) is reported as [(YB+3SB)/m], whereYBis the intercept,SBis the standard deviation of the intercept, andmis the slope of the plot. The limit of quantification (LOQ) was then estimated in the same way by using 10SB, which corresponds to 3.3 LOD. The detection limit (based on the air mass and sample volume) depends on the total air volume sampled.
2 Results and discussion
In most previous studies employing passive samplers for area sampling of carbonyl compounds derivatized with DNPH, LC systems were utilized. These procedures, however, require a large number of manual operations and have a relatively high overall cost. Fortunately, in the last ten years, the proliferation ofxyzautosamplers, especially coupled to GC, has increased the use of hyphenated techniques in analytical chemistry, with resultant reduction of the solvent and sample quantities, faster sample preparation, traceability, and easier automation. Although most chromatography laboratories already use autosamplers as their standard form of sample injection, this modern instrumentation permits automation beyond injection only.
Accordingly, we developed a method where the Sep-Pak sampler DNPH cartridge, both as an active and passive sampler, was integrated with automated quantitative determination using GC-MS/TSD. Three fundamental aspects were optimized: i) the performance of the diffusive sampler, ii) the PTV-LVI process, and iii) automation of analysis.

Table 1 Theoretical SR vs. experimental SR determined with Sep-Pak XpoSure passive sampler
2.1 Performance of the Sep-Pak XpoSure Aldehyde Plus Short Sampler

Fig. 2 Weight of carbonyl compounds formed at 60 min exposure time as a function of various mass concentrations of carbonyl compounds in air with passive sampling
Fig. 2 shows plots of the amount of carbonyl compounds adsorbed as a function of the exposure time. The data were used to determine the SR, where the slope of the curve represents the uptake of the respective carbonyl compounds on the Sep-Pak sampler, as indicated in Equation 1. The experimental average SR values agree with the theoretical SR values calculated by applying Equation 2 (Table 1). The Fuller, Schettler, and Giddings (FSG) method was used to estimate the diffusion coefficient of the respective carbonyl compounds in air [62]. The correlation between active and passive sampling was experimentally demonstrated by the linear relationship with the Pearson’s product moment correlation coefficient, in line with the results reported by Shinohara and coworkers [63]; a correlation of 0.901-0.932 was observed for all the carbonyl compounds. The Sep-Pak cartridge, used as a passive sampler, allowed the detection of airborne FA at concentrations equal to 0.4 μg/m3and 100 μg/m3for sample times of 7 d and 30 min, respectively.
2.2 Optimization of the PTV injector parameters for LVI
To inject large amounts of EtAc containing the analytes into the 1078/1079 PTV inlet, most of the solvent had to be eliminated instantly while retaining all the target analytes in the liner. To achieve this feat during injection, the split vent valve must remain open while a large stream of carrier gas removes the solvent constantly. After complete removal of the solvent, the split vent valve is immediately closed and the inlet temperature swiftly increased. The analytes inside the liner will then rapidly vaporize and be transferred into the column. For injection volumes >3 μL, fine tuning of the PTV conditions is necessary to minimize the loss of analytes without sacrificing the chromatographic performance. Table 2 summarizes the physicochemical constants for the eleven 2,4-DNPH derivatives, obtained by using Performs Automated Reasoning in Chemistry (ARChem, Danielsville, USA), a physicochemical calculator that uses computational algorithms based on the basic chemical structures to predict a wide variety of reactivity parameters as a means of envisaging the injection condition trends.
To demonstrate the efficiency of analyte transfer during LVI, 1 μL of the calibration standard solution containing the target carbonyl-2,4-DNPH-derivatives was injected using normal splitless injection, and the reference area responses were obtained. By diluting these solutions and subsequently injecting more into the inlet to keep the amount of analyte constant, the inlet temperature, split flow rate, injection phase time, and injection speed were varied to obtain the final injection volume with optimal performance, recoveries, and reproducibility. High inlet temperatures may reduce the size of the solvent film, thus causing analyte loss due to solvent vaporization. Conversely, if the inlet temperature is too low, solvent flooding becomes an issue. Initially, as is common, the temperature was set to 30 ℃ below the boiling point of the solvent. Deviating from this temperature was found to cause significant analyte loss. Hence, the original temperature was retained. The injection phase time must be sufficient to allow the solvent to vaporize and then leave through the split vent during injection. Lastly, the injection speed is strongly related to the nature of the solvent and the initial inlet conditions; this can be estimated by using the following equation [64]:
Vinj=Vel=(M×Ps/ρ×R×T)×(P/Pinlet×Vgf)
(4)
where,Vinj(μL/min) is the maximum injection speed for sample introduction,Vel(μL/min) is the solvent elimination rate,M(kg/mol) is the molecular weight of the solvent,Ps(Pa) is the vapor pressure of the solvent at the initial inlet temperature,ρ(kg/m3) is the density of the solvent,R(J/(mol5K)) is the gas constant,T(K)is the outlet temperature,P(Pa) is the outlet pressure,Pinlet(Pa) is the inlet pressure, andVgf(μL/min) is the total gas flow under the outlet conditions.

Equation 4 indicates that the solvent evaporation rate is proportional to the gas flow rate in the liner, given by:P/Pinlet×Vgf. Therefore, reducing the pressure in the liner and/or increasing the total gas flow rate increases the solvent evaporation rate. For EtAc, a temperature increase of 10 ℃ increases the evaporation rate by a factor of 1.7, albeit with proportional loss of the less volatile analytes. This permits calculation of the elimination rate, and hence, the maximum acceptable speed of sample introduction as a function of the liner temperature and the purge gas flow rate for liners without packing materials. The magnitude of the cooling effect depends not only on the speed of introduction of the sample, but also on: i) the rate of solvent evaporation and ii) the saturated vapor volume (Vg). The heat of evaporation of EtAc is 79 cal/mL, and the solvent cools more quickly than other solvents [64].Vgcan be calculated for a defined liquid volume (V1) at a given temperature (T) according to the following equation:
Vg=(V1×ρ×R×T)×(M×Ps)
(5)
For EtAc,Vgis equal to 2.6 mL, which is much larger than the value for non-polar solvents. Because unpacked inlet liners (Cat. No. 2637101, Supelco, Bellefonte, USA, Cat. No. 20901-214.5, Restek Corporation, Bellefonte, USA and Cat. No. 092245, Trajan-SGE Analytical Science, Ringwood, Australia) only permit low solvent volumes (5 μL per injection), and considering also the low injector temperatures for cryo-focusing, we opted for packed liners.
2.2.1Optimization of retention and transfer of 2,4-DNPH-hydrazone to the column
Preliminary experiments were carried out to optimize the following parameters: i) the injector temperature program, ii) split vent, iii) transfer time, and iv) the packing materials of the liner. The initial temperature of the PTV was set to 40 ℃ (the boiling point of ethyl acetate is 77 ℃) to maximize retention of the volatile 2,4-DNPH-hydrazones, whereas the injection duration was set from 0.40-4.00 min (split) and the split rate was set from 50∶1 to 1 000∶1 during the venting phase to obtain optimal elimination of EtAc. To achieve transfer of the least volatile species, the temperature of the PTV inlet was maintained at 200 ℃ for 5 min (200 ℃/min). Compared to normal splitless injection (GC conditions; without PTV) two changes were made: i) the initial oven temperature was lowered to 40 ℃ (from 70 ℃) to better refocus the analytes at the head of the column, ii) the initial oven temperature was held for 2.0 min instead of 1.0 min, in order to accommodate the longer time for transfer of the species from the inlet. Given these modifications, the duration for each analysis increased to 34 min (where the temperature was held at 40 ℃ for 2 min, then increased to 310 ℃ at 10 ℃/min and held for 5 min) compared to 30 min without PTV.
2.2.2Multiple injection mode
Four packing materials were evaluated. Three Siltek deactivated liners (3.4 mm×5.0 mm×54 mm) were purchased from Restek Corporation (Bellefonte, USA), i. e., glass frit (Cat. No. 21709-214.5), deactivated wool (Cat. No. 20901-213.5), and carbo frit (Cat. No. 20901-216.5); 10% OV-101 on 80/100 Chromosorb W HP (Cat. No. 2637301) was obtained from Supelco (Bellefonte, USA). With Chromosorb W HP and carbo frit, the 2,4-DNPH derivatives were irreversibly retained on the packing, whereas with deactivated wool, poor reproducibility was observed. Thus, all the investigations were carried out using glass frit. The maximum volume that can be injected through multiple 25 μL injections was then evaluated. In this case, the time interval between injections is critical and should be optimized to allow the solvent to evaporate while avoiding species loss. In the present case, the time between two injections, required by the autosampler (60 s), was found to be enough to vent the solvent. Increasing the solvent injection rate from 5 to 200 μL/s did not lead to increased signal intensity. All carbonyls showed a linear signal increase up to the fifth injection (R2≥0.998), except for benzaldehyde-2,4-dinitrophenylhydrazone, the least volatile carbonyl, for which the signal deviated slightly for injection volumes between 75 and 125 μL (15% lower than the linear trend).
2.2.3Maximum volume injectable at once
After these first optimization steps, the maximum volume that can be injected at once was determined. The species could be clearly grouped into two pools where: i) the more volatile 2,4-dinitrophenylhydrazones showed a continuous signal rise over the full range of injection volumes; and ii) the least volatile 2,4-dinitrophenylhydrazones for which the signal intensity plateaued or decreased slightly at high injection volumes. In order to increase the volume injected, the effect of increasing the gas flow rate or lowering the solvent delivery rate to the liner during the injection period was evaluated. Increasing the split rate to 1 000∶1 during the injection period caused a significant loss of analytes. Given these results, 100 μL was chosen as the optimal volume for at-once injection, combined with a split rate of 250∶1, with 5 μL/s injection for a duration of 2.00 min.
2.2.4PTV-LVI performance
Attempts were made to simultaneously determine eleven 2,4-dinitrophenylhydrazones using PTV-LVI-GC-MS/TSD; the performance of this method was subsequently evaluated.
The injection precision for all analytes, in particular the late eluting compounds, was reasonable in five consecutive runs of the analytes, indicating that the LVI method was reproducible and robust. Similarly, the chromatograph peak shapes for all analytes were comparable to that achieved with the normal splitless injection method. Furthermore, efficient LVI was achieved with good recoveries (95%-98%) for all the analytes. To prevent evaporative loss of the low-boiling analytes, however, it was confirmed that the difference between the boiling point of the solvent and the analyte should be at least 150 ℃. The Siltek deactivated liner with the glass frit appeared to be effective for refocusing the analytes, thus increases the rate of solvent evaporation and, hence, the analysis time is reduced.
2.3 Automation of analysis
The final objective was to achieve high-throughput automated analysis, which was accomplished by exploiting the flexibility of thexyzrobotic system. All sampling management processes were available through the open-source Laboratory Information Management System (LIMS); thus, the automated processing steps could be easily customized. This enabled “just in time” sample preparation. Further, this technology allows the analysis of thirty-six samples in 24 h. Such specific automated robotic systems facilitate the use of GC devices, hence reducing the costs of the monitoring campaigns. Compared to the competitor, the 1078/1079 injector integrated with thexyzautosampler offers versatile GC system control, septum-less injection, and choice of packing liners (Table 3).
In light of these considerations, the final results are summarized in Tables 4 and 5. Fig. 3 presents the chromatogram of the aldehyde/ketone DNPH mix under the optimized conditions with PTV. The calibration curves were linear in the investigated range for all the hydrazones considered, with correlation coefficients >0.998. The precision of the assay (reported as the relative standard deviation, RSD), estimated both as within-session and as inter-session repeatability, was 2%-9% and 4%-13%, respectively. The accuracy was within 9% of the theoretical concentration, in line with the requirement of the US Food and Drug Administration for analytical method validation. To demonstrate the applicability of the method to air sampling (by comparing passive vs. active sampling), the concentration of FA and ten other carbonyl pollutants in indoor air was monitored in ten public buildings in Florence in 2018 (Table 6).

Table 3 List of companies with related commercially available LVI-mode instruments.
PTV: programmed temperature vaporizing; OCI: on-column injection; DMI: difficult matrix injection; CSR: concurrent solvent recondensation; TOTAD: through oven transfer adsorption desorption.

Table 4 Nominal mass concentrations of calibration curve standards ng/mL
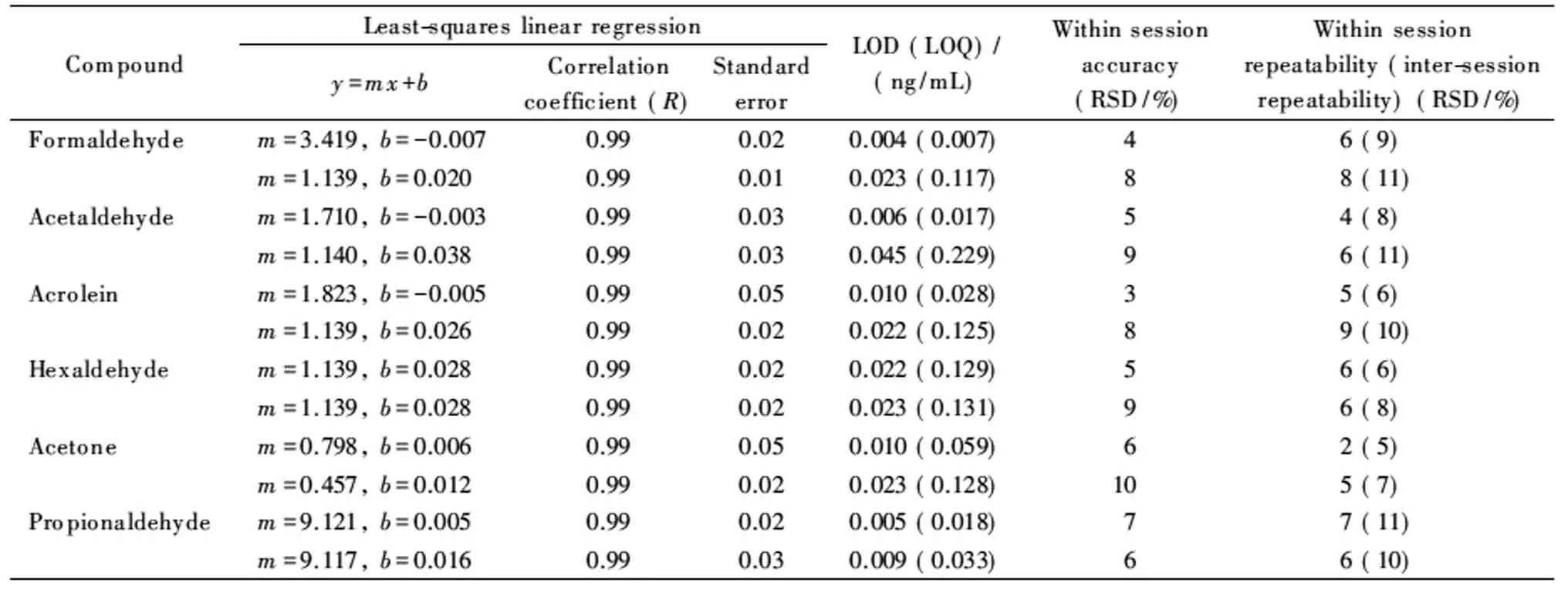
Table 5 Performance of PTV-LVI-GC-TSD (upper line)/MS(lower line) system

Table 5 (Continued)

Fig. 3 Chromatogram of DNPH-derivatives of aldehydes/ketones and their relative syn and anti conformation DNPH mix under the optimized conditions with PTV

Table 6 Comparison between passive and active sampling for determination of concentrations of eleven carbonyl pollutants in indoor air for ten public buildings in Florence
3 Conclusions
The PTV-LVI-GC-MS/TSD method was optimized to improve the detection of FA and other airborne carbonyl pollutants. The new online automated technology requires no manual intervention steps, thus providing several advantages over conventional chromatographic methods. Firstly, both Sep-Pak desorption and LVI were automated. Secondly, significant cost-reduction was achieved: i) the price of the Sep-Pak cartridge is the lowest relative to other commercially available diffusive samplers, ii) remote control of the active and passive sampling enables cost-savings for sample transportation and storage, iii) the use of the non-mass detector TSD affords a relatively lower purchase cost and maintenance expense, and this detector is much easier to use than the LC/UV and GC-MS systems. In addition, the injection consistency for different molecular weight compounds was optimized. Furthermore, the surface adsorption losses of high molecular weight carbonyl compounds were minimized.
