Assembly of a Heteronuclear POM{[K5Na6CuII5(CH3COO)20(CH3CN)]Cl}n via Two Kinds of Clusters as the Basic Bridge Unit①
2018-11-22DAIYuMeiXUJie
DAI Yu-Mei XU Jie
(Minnan Science and Technology Institute, Fujian Normal University, Quanzhou 362332, China)

A novel 3D hetero-nuclear framework, [K5Na6CuII5(CH3COO)20(CH3CN)]Cl}n(1),was obtained by hydrothermal reaction.Triple metal centers (including sodium/potassium or/and copper) were coordinated to acetate anions in such two heteronuclear clusters, respectively.Moreover, the nine kinds of binding modes for acetate anions were illustrated in polymer 1, with the first reported pentadentate coordination mode for the acetate ligand.
1 INTRODUCTION
Rational design for constructing polyoxometalates(POMs) has been attracting extensive interest in material chemistry in the past several decades, not only because of the unique topologies, but also of the wide range of potential applications as functional solid materials[1-3].In general, such strategies were mainly focusing on: i) using some precursors incorporate in situ formed transition-metal (TM)clusters binding with multidentate inorganic ligands,generating a rapidly growing class of transition-metal substituted polyoxometalates (TMSPs)[4], and affording the array of 1-, 2-, and 3-D networks via self-assembly.Additional, some of the 1-D and 2-D structures might be further extended to 3-D frameworks through hydrogen bonding or/and p-pstacking interactions.ii) the research on polymers that incorporate with alkali or alkali earth metal ions,which can act as both counter ions and structural units in the synthesis[5,6].The current trends towards“rational design” are mainly based on the accumulated knowledge of crystal chemistry, thermodynamics and reactivity, as well as the relationship between structures and properties.However, little attention has been paid to the research of new strategy for constructing multi-dimensional architecture hitherto, for instance, the selection of heteronuclear clusters as bridge units to substitute the organic ligands to form the skeleton of framework.To our knowledge, the chemistry of heteronuclear complexes especially those containing nuclear triple metal elements coordinating with simple carboxylate ligands have rarely been reported, and there are only two examples up to now[7,8].
On the other hand, the properties and supramolecular behaviors of copper-(II)/carboxylates have been studied extensively since the structure of copper(II) acetate monohydrate, Cu2(OCOCH3)4·2H2O,was first reported by Van Niekerk et al[9].The flexibility of the coordination sphere of Cu(II) in combination with steric and crystal packing forces leads to its tremendous structural diversity.Various Cu(II) carboxylate derivatives analogous to this complex which incorporated alkali metal ions, often sodium, have been investigated.The recent attempts to synthesize double salts consisting of alkali metal cations and Cu(II) acetate anions met with limited success.Such work helps us to understand the influence of alkali metal ions on the structures and properties of transition metal complexes[10-12].However, all such dinuclear copper(II) based systems both contained the well-characterized[Cu2(CH3COO)4] binuclear units.Such units were linked by acetate-bridged Na+ions to generate a 3-D network, in which the Cu(II) centers are either isolated or square planar with fairly simple motifs.And no occurrence of heteronuclear clusters could be found in structures incorporating acetate anions according to the search of CCDC.Herein, we report the hydrothermal synthesis and X-ray structure of{[K5Na6CuII5(CH3COO)20(CH3CN)]Cl}n(1).
2 EXPERIMENTAL
2.1 Synthesis of the title complex 1
A mixture of Cu(Ac)2(9.1 mg, 0.05 mmol), NaAc(5.0 mg, 0.06 mmol), KAc (4.1 mg, 0.04 mmol), KCl(1 mg, 0.01 mmol), and CH3CN (3 mL) in a Pyrex glass tube (15 cm in length, 7 mm in inner diameter).The tube was then sealed and heated in an oven at 423 K for 50 h to form dark blue prism crystals of1,which were collected by filtration, washed with acetonitrile, and dried in air (yield: 66% based on Cu).The elemental analyses for C, H and N were performed on a Carlo-Erba CHNO-S microanalyzer.Elemental analysis found: C, 26.41; H, 3.35; N,0.74%.C42H63O40NK5Na6Cu5Cl requires C, 26.43; H,3.33; N, 0.73%.IR spectra were recorded on a Varian 1000 FT-IR spectrometer as KBr disks (4000~400 cm-1).IR (KBr, cm-1): 3437(s), 3414(s), 3253(m),3221(m), 3195(m), 3030(w), 2912(w), 1686(m),1655(w), 1625(w), 1528(w), 1438(w), 1385(m),1244(w), 1227(w), 1038(w).
2.2 Structure determination
X-ray diffraction data were collected on a Rigaku Mercury CCD diffractometer using graphite-monochromated Mo-Kα (λ = 0.70073 Å).One blue single crystal with dimensions of 0.30 mm × 0.20 mm×0.05 mm was mounted on a glass fiber and cooled at 193 K in a liquid nitrogen stream.Crystal data for C42H63O40NK5Na6Cu5Cl: Mr= 1908.57, orthorhombic, space group Pccn, a = 19.000(4), b = 20.120(4),c = 20.192(4) Å, V = 7719(3) Å3, Z = 4, Dc= 1.642 g/cm3, m = 1.781 mm-1, 24365 measured reflections,7994 unique reflections (Rint= 0.0226), 8858 observed reflections (I > 2s(I)), 467 parameters, R =0.0492, wR = 0.1188, S = 1.052, max./min.residual electron density 0.724~0.677 e/Å3.The crystal structure was solved by direct methods and refined with full-matrix least-squares techniques using the SHELXS-97 and SHELXL-97 programs (Sheldrick,1997).All non-hydrogen atoms were refined anisotropically.Carbon-bound hydrogen atoms were generated geometrically, and were allowed to ride on their parent carbon atoms.All other hydrogen atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms.
3 RESULTS AND DISCUSSION
3.1 Structure analysis
The selected bond lengths and bond angles are listed in Table 1.The title compound exhibits an unprecedented 3-D POM using two kinds of heteronuclear metal-oxygen clusters as the basic bridge units and features a number of distinct acetate binding modes.To our knowledge, both heteronuclear metal-oxygen clusters and the binding modes of acetate are novel, not only in coordination chemistry, but also in POM chemistry[13].The whole structure of1was divided into the[K5Na6CuII5(CH3COO)20(CH3CN)]n+cationic host framework and Cl¯ as the inclusion.And such cationic framework was described as three sections:i) bridge unit, ii) extending way and iii) acetate binding mode, respectively.

Table 1.Selected Bond Lengths (Å) and Bond Angles (°)
Two kinds of heteronuclear clusters in such cationic framework were matryoshka-like K4Na4CuII6-O cluster unit (A) and flexure type KNa2-O cluster unit (B), respectively.In unit A, a K4Na4-O cluster was formed firstly through interleaving K4 and Na4 tetrahedra in opposite directions coordinating to acetate anions (Fig.1a).In K4Na4-O cluster, each K(1) atom was pentadentated-coordinated by five oxygen atoms from five acetate ligands: the oxygen atoms of the three acetate ligands adopt m2-O bridging K(1), Na(2)atoms, K(1), Cu(1) atoms and K(1), Cu(3) atoms with the K–O bond distances varying from 2.741(2)to 2.773(2) Å.And the oxygen atoms of the last two acetate ligands were a m3-O which coordinates to K(1), Na(1), Na(2) and K(1), K(2), Na(2) atoms,respectively (K–O = 2.773(2) and 2.881(2) Å).The bond distances between K–O are comparable to those in potassium compounds reported[14].As to K(2) atom, both K atoms were hexadentate-coordinated by six oxygen atoms from six acetate ligands while some difference is found compared to that of K(1).One of the acetate ligands uses an oxygen atom to monodentate-coordinate to the K(2)atom (K–O = 2.769(2) Å).The other two acetates use their m2-O to bridge K(2) and Na(1) atoms with the K–O bond distances of 2.841(2) and 2.812(2) Å,respectively.Moreover, the last acetate ligand tridentated-coordinates to K(2), Na(1) and Na(2)atoms via m3-O atom (K–O = 2.844(2) Å).The average distances of Na··Cl and K··Cl are 3.091 and 3.1286 Å, respectively.Thus, the Cl¯ anion might be treated as template reagent in forming such interleaving tetrahedron.Furthermore, three crystallographically independent Cu(II) ions in unit A and a Cu(II) ion of adjacent heteronuclear cluster A occupied the vertexes of six directions of K4Na4O,forming a cage of distorted octahedral geometry resulting in the tight trapping of Cl¯.Fig.1b shows six CuIIatoms coordinating to acetate anions to form a distorted octahedral geometry.Fig.1c shows a matryoshka-like K4Na4CuII6-O cluster unit (A)and Fig.1d depicts the view of Cl¯ which is tightly trapped in A and further acts as a counter anion to stabilize the architecture of complex.Each of two Cu(1) atoms was tetradentate-coordinated by four oxygen atoms from four acetate ligands.Two of such four acetates use their m2-O to bridge Cu(1)and Na(1), Cu(1) and K(1) atoms with the Cu–O bond distances being 1.986(2) and 1.980(2) Å,respectively.An oxygen atom of the third acetate ligand monodentate-coordinates to Cu(1) atom and the Cu–O distance was 1.942(2) Å, and the oxygen atom of the last acetate ligand bridges Cu(1) and Na(3) atoms with the Cu–O to be 1.973(2) Å.The coordination environment of Cu(2) was the same as that of Cu(1) while the situation of tetradentatedcoordinated Cu(3) atom was different from Cu(1)and Cu(2) atoms.There are two oxygen atoms from two acetate ligands bridging K(1) and Cu(3) atoms with the Cu–O bond distance of 1.959(2) Å, and them2-O atoms of the other two acetate ligands bridged K(2) and Cu(3) atoms (Cu–O = 1.965(2) Å), and all the Cu–O bond distances were classical in the range for Cu(II)–O.
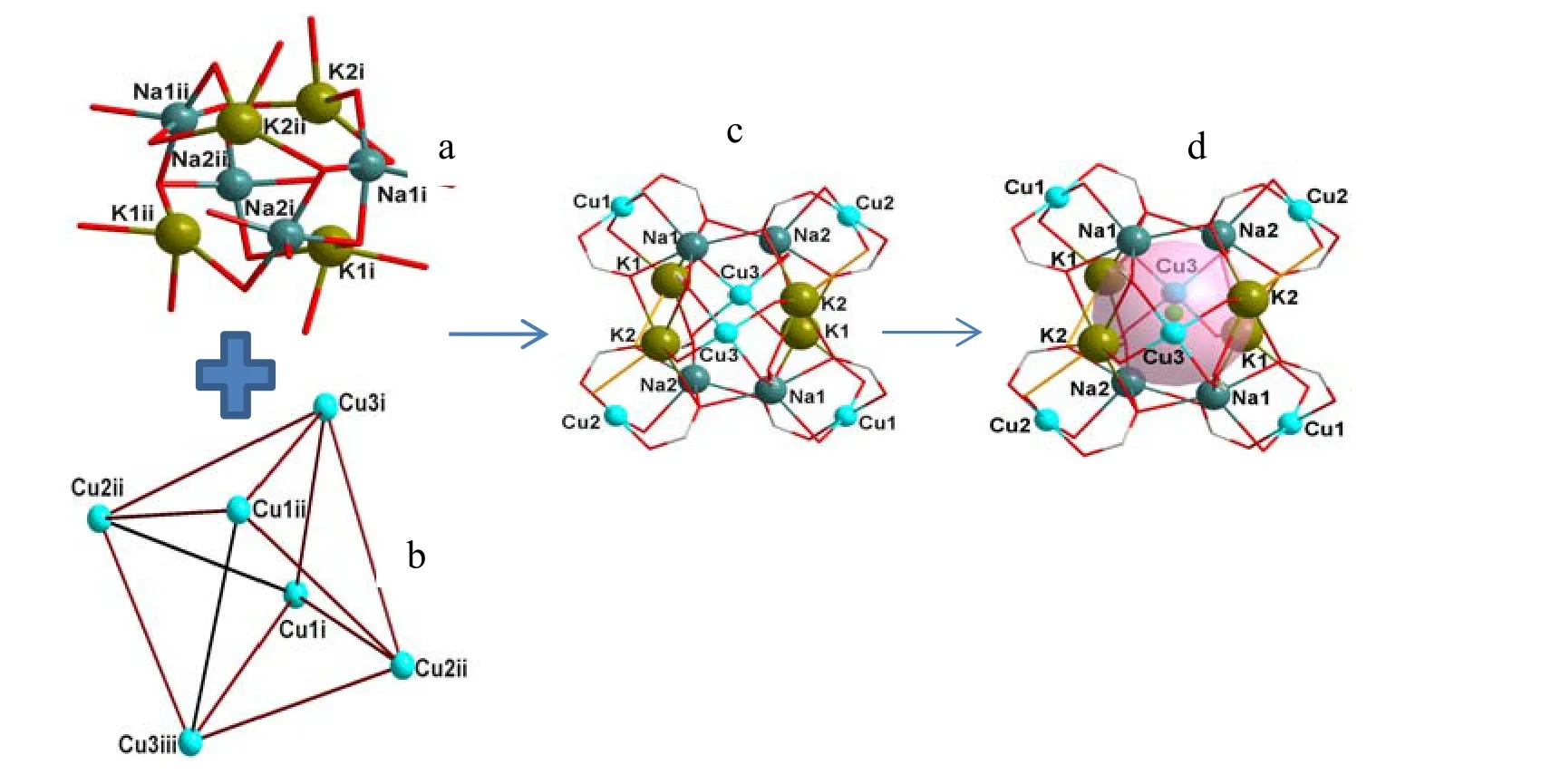
Fig.1.a) View of the K4Na4-O cluster formed through the interleaving of K4 and Na4 tetrahedra in opposite directions coordinating to acetate anions (Symmetry codes: i: x, 1+y, z; ii: 1/2–x, 1/2–y, z).b) Six Cu(II) atoms coordinating to acetate anions to form a distorted octahedral geometry(Symmetry codes: i: x, 1+y, z; ii: 1/2–x, 1/2–y, z; iii: 1/2–x, 1+y, 1/2+z).c) A matryoshka-like K4Na4CuII6-O cluster unit (A).d) View of Cl– which was tightly trapped in A and further acting as the counter anion to stabilizethe architecture of the complex
The unit B contents a flexure type KNa2-O cluster (Fig.2), and the K(3) atom was heptadentate-coordinated by six oxygen atoms from four acetate ligands and one N atom from CH3CN.Both acetate ligands use their one m2-O atom to bridge K(3) and Na(3) atoms, respectively.The K–O bond distance is 2.814(2)~2.950(2) Å.And as to the Na(3) atoms, both of them are hexadentate-coordinated by six oxygen atoms from six acetate ligands.For each Na(3) atom, two of the six acetate ligands use their oxygen atoms to monodentate-coordinate to the Na(3) atom (Na–O =2.329(3)~2.453(3) Å), two O atoms of the other two acetate ligands bridge K(3) and Na(3) atoms viam2-O atom (Na–O = 2.396(3)~2.373(2) Å), and two O atoms of the last two acetate ligands usem2-O atom to bridge the Na(3) (Na–O = 2.356(2) Å),Cu(1) atoms, and Na(3) (Na–O = 2.395(2) Å) and Cu(2) atoms, respectively .
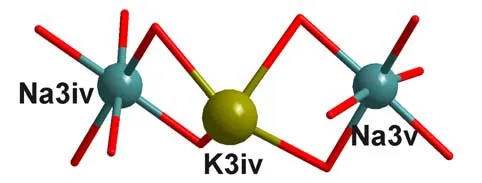
Fig.2.View of the flexure type KNa2-O cluster unit (B)Symmetry codes: iv: 1/2–x, y, –1/2+z; v: x, 1/2–y, –1/2+z
There are four units B connect with A to form a windmill fashion AB4(Fig.3a).On the other hand,four A connect with unit B in an A4B X-style through two Na(3)–O–Cu(1) and two Na(3)–O–Cu(2) bridges binding the acetate anions, respectively (Fig.3b).Furthermore, the Cu(3) and Cu(3a)atoms in unit A occupied the vertexes of K4Na4-O nuclear cluster perpendicular to such windmill architecture, and link the neighboring two units A with the rotation of 90º to form a (4,6)-connected framework finely.Fig.3c shows the 3D architecture of1.If A and B are treated as nodes respectively,such cationic skeleton of1was rationalized to be a topological net with the Schläfli symbol(4462)(446108) (Fig.3d).Such strategy for the construction of 3-D architecture is quite different from the examples which have been reported before.In those polymers, sodium/potassium atoms are coordinating to oxygen atoms, forming the isolate unit or extending the framework through H-bonding between oxygen atoms.The acetate ligands act as counterions and connect the symmetry-related constructs.
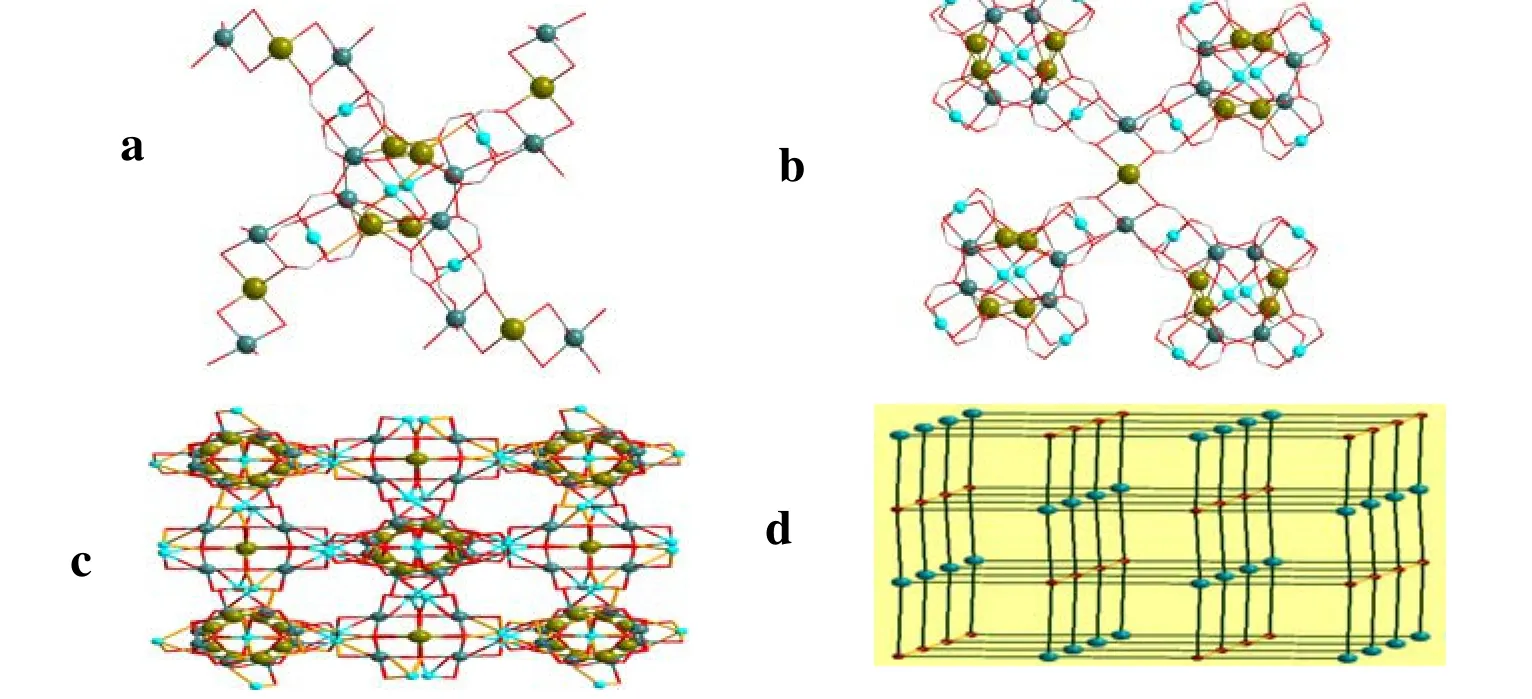
Fig.3.a) X fashion AB4.b) View of the windmill fashion of A4B formed by four unit A connecting with Cu(1) and Cu(2) atoms binding the acetate anions.c) 3D architecture of complex.d) Topological view of the complex with Schläfli symbol (4462)(446108), and no means for the lines
As to the two carboxylate oxygen atoms of each acetate coordinating to metal center, the number of coordination was 1+1, 2+2, 1+3, 2+1 for A (Fig.4)and 2+1, 2+2, 2+3 for B (Fig.5), respectively.Thus,it leads to four coordination types with eighteen modes for unit A while three coordination types with five kinds of binding modes for B, respectively according to all of the binding acetate ions.Based on such consideration, the acetate ligands act finally as di-, tri- and tetradentate ligands in A and tri-,tetra- and pentadentate ligands correlated to B,respectively.To our surprise, the unexpected pentadentate coordination mode for acetate ligand was firstly reported with the inclusion of carboxylates being part of the ligand assembly, except the reported structure of CuII(CH3COO)(CH3O)[15]which exhibits a tetradentate ligand for acetate.And no occurrence of such nine binding modes could be found in structures incorporating acetate anions according to the search of CCDC.

Fig.4.Coordination modes (I-IV) for acetate anions in the heteronuclear cluster unit A
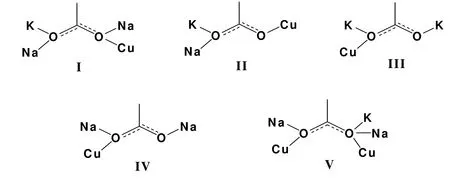
Fig.5.Coordination modes (I-V) for acetate anions in heteronuclear cluster unit B
3.2 Thermogravimetric analysis
The thermogravimetric analysis (TGA) of complex1reveals that four stages of weight loss(30~90, 135~150, 240~320 and 450~510 ºC,respectively) in the temperature range of 30~750ºC (Fig.6).To understand the process of weight loss,the thermogravimetric analyses of free KAc, NaAc and Cu(Ac)2were carried out, respectively.The TGA of complex1is completely different from that offree KAc, NaAc and Cu(Ac)2, which further proves the complex coordination of1.The result also means that1is more stable in such construction, and the covalent bonds between these carboxylic groups and the triple metal centers are strong enough to withstand the stability of the complex.
3.3 Ultraviolet absorption spectrum analysis
The ultraviolet absorption spectrum of complex1is shown in Fig.7.It can be observed that the maximum absorption wavelength occurs at 226 nm for complex1(DMSO as solvent, 0.05 mg/mL).To understand the nature of the ultraviolet absorption spectrum of1, we analyzed the ultraviolet absorption spectrum of the reactants and found that the maximum absorption wavelength occurs at 280 nm for the reactants.The maximum absorption wavelength of1, compared to the reactants, exhibits blue shift due to the steric effect[16].Complex1is a 3D framework which can destroy the conjugate structure.The ultraviolet absorption spectrum indicates that1can be a candidate for anti-ultraviolet material.

Fig.6.TGA plot of complex 1
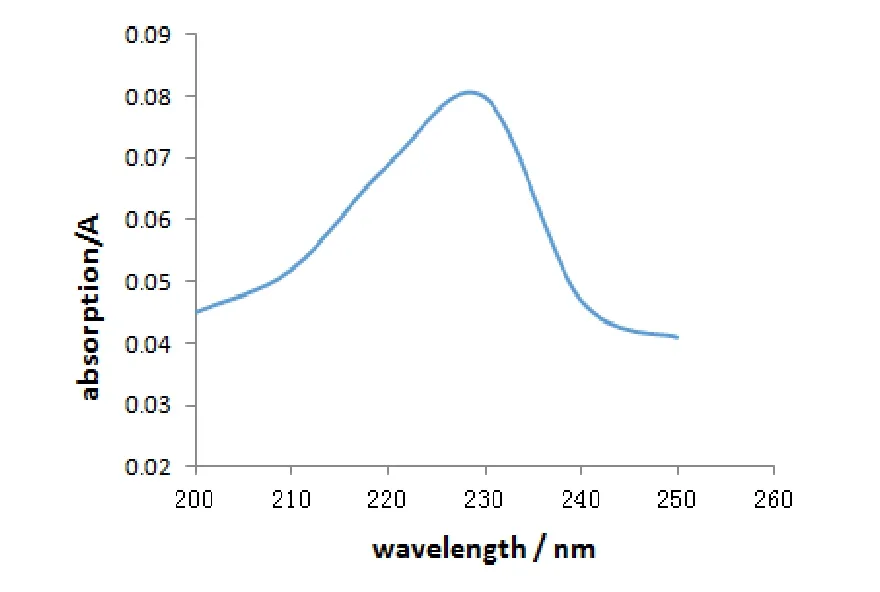
Fig.7.Ultraviolet absorption spectrum of complex 1
In summary, we report here the hydrothermal method to produce a 3D polymer using heteronuclear clusters as the basic bridge unit.The nine kinds of binding modes for acetate anions were illustrated with the first reported pentadentate coordination mode for acetate ligand.
REFERENCES
(1) Ma, L.; David, J.M.; Lin, W.Highly porous and robust 4,8-connected metal-organic frameworks for hydrogen storage.J.Am.Chem.Soc.2009, 131, 4610–4612.
(2) Zhao, J.W.; Wang, C.M.; Zhang, J.; Zheng, S.T.; Yang, G.Y.Combination of lacunary polyoxometalates and high-nuclear transition metal clusters under hydrothermal conditions: IX.A series of novel polyoxotungsta-tes Sandwiched by octa-copper clusters.Chem.Eur.J.2008, 14, 9223–9239.
(3) Tang, E.; Dai, Y.M.; Zhang, J.; Li, Z.J.; Yao, Y.G.; Zhang, J.; Huang, X.D.Two cobalt(II) 5-aminoisophthalate complexes and their stable supramolecular microporous frameworks.Inorg.Chem.2006, 45, 6276–6281.
(4) Nsouli, N.H.; Bassil, B.S.; Dickman, M.H.; Kortz, U.; Keita, B.; Nadjo, L.Synthesis and structure of dilacunary decatungstogermanate,[V-GeW10O36]8-.Inorg.Chem.2006, 45, 3858–3860.
(5) Guillaume, V.; Sax, A.M.; Paul, D.P.; Peter, C.J.; Jonathan, W.S.Intramolecular versus intermolecular hydrogen bonding of coordinated acetate to organic acids:? a neutron, X-ray, and database study.Cryst.Growth Des.2003, 3, 699–704.
(6) Kennedy, A.R.; Kirkhouse, J.B.A.; Whyte, L.Supramolecuar motifs in s-block metal-bound sulfonated monoazo dyes:? the case of orange G.Inorg.Chem.2006, 45, 2965–2971.
(7) Richard, D.M.; Tetsuya, O.; Quintus, F.Crystal structure of copper(I) acetate.Inorg.Chem.1974, 13, 802–805.
(8) Zhou,Y.X.; Shen, X.Q.; Liu, H.L.; Zhang, H.Y.; Wu, Q.A.; Niu, C.Y.Studies on synthesis and crystal structures of heteronuclear complexes of tartarate with Na(I), K(I) and Cu(II).Synth.React.Inorg.Met.Org.Nano-Met.Chem.2006, 36, 563–568.
(9) Niekerk, J.N.V.; Schoening, F.R.L.A new type of copper complex as found in the crystal structure of cupric acetate Cu2(CH3COO)4·2H2O.Acta Crystallogr.1953, 6, 227–232.
(10) Liu, H.L.; Mao, H.Y.; Zhang, H.Y.; Xu, C.; Wu, Q.A.; Li, G.; Zhu, Y.; Hou, H.W.Two novel heterometallacrowns: syntheses and crystal structures of two copper(II) complexes with malonate and o-pthalate.Polyhedron2004, 23, 943–948.
(11) Laborda, S.; Clerac R.; Anson C.E.; Powell, A.K.Intra- and intermolecular magnetic interactions in a series of dinuclear Cu(II)/hxta complexes {H5hxta =N,N΄-(2-hydroxy-1,3-xylylene)-bis-(N-carboxymethylglycine)}:?correlation of magnetic properties with geometry.Inorg.Chem.2004, 43, 5931–5943.
(12) Proust, A.; Gouzerh, P.; Robert, F.Molybdenum oxo nitrosyl complexes 1.Defect Lindqvist compounds of the type [Mo5O13(OR)4(NO)]3-(R =CH3, C2H5).Solid-state interactions with alkali-metal cations.Inorg.Chem.1993, 32, 5291–5298.
(13) Warden, A.C.; Hearn, M.T.W.; Spiccia L.Novel acetate binding modes in [Na2Cu(CH3COO)4(H2O)].Inorg.Chem.2003, 42, 7037–7040.
(14) Dai, Y.M.; Tang, E.; Wang, X.Q.; Huang, J.F.; Wang, L, H.; Huang, X.D.A three-dimensional neodymium(III)-potassium(I) coordination polymer.Chin.J.Struct.Chem.2008, 9, 1031–1034.
(15) Koval, I.A.; Gamez, P.; Roubeau, O.; Driessen, W.L.; Lutz, M.; Spek, A.L.; Reedjik, J.Century-known copper salt Cu(OAc)(OMe) proven to be a unique magnetic lattice composed of tetranuclear copper(II) species with a rare binding mode of the acetate anion.Inorg.Chem.2003, 42, 868–872.
(16) Hu, H.W.Organic Chemistry.Higher Education Press2013.
杂志排行
结构化学的其它文章
- Ionothermal Synthesis, Structure and Luminescent Properties of a New 2-D Bismuth(III) Coordination Polymer with (6,5)-Connected Topological Sheet①
- A Doubly Interpenetrated Co(II) Framework: Synthesis,Crystal Structure and Selective Adsorption of CO2①
- Iodoplumbate(II)-based Hybrid Templated by 1,4-Diazabicyclo[2.2.2]octane Derivative: Structure,Photocurrent Response Behavior and Photocatalytic Activity for the Degradation of Organic Dye①
- Synthesis, Crystal Structure and Photoluminescence of a Dinulear Copper Complex①
- Synthesis, Crystal Structure and Properties of a 1D Heteronuclear Cobalt-sodium Polymer with Bridging Ligand 2-(2-Hydroxy-3-methoxybenzylidene)Hydrazinecarbothioamide①
- Structural, Electronic, Optical and Thermodynamic Properties of Nanolaminated Boride Cr4AlB6①
