The progress in the chemical constituents of the genus Picrasma during 2007-2017
2018-11-01JieZhangJianYangChuanXiWangHaoGaoXinShengYao
Jie Zhang,Jian Yang,Chuan-Xi Wang,Hao Gao,*,Xin-Sheng Yao,
1College of Traditional Chinese Materia Medica,Shenyang Pharmaceutical University,Shenyang,China.2Institute of Traditional Chinese Medicine&Natural Products,College of Pharmacy,Jinan University,Guangzhou,China
Background
The plants of the genus Picrasma,comprised of nine species,are mainly distributed in tropical and subtropical regions of America and Asia[1].The bark,roots,stems,branches,or leaves of some species are used as herbal medicine resources for the treatment of some diseases,such as anemopyretic cold,sore throat,dysentery,eczema,nausea,loss of appetite,diabetes mellitus,hypertension,and so on [2-5].In fact,the investigations on phytochemistry and pharmacology of the plants of the genusPicrasma aremainly focused on Picrasma quassioides(D.Don)Benn and Picrasma javanica Blume.
For the chemical constituents and bioactivities of the plants of the genus Picrasma,Jiao WH et al.made a review in 2007[6].Twenty-three β-carboline alkaloids,nine canthinone alkaloids,eleven bis β-carboline alkaloids,ninety-four quassinoids,eight triterpenoids,and twelve other compounds were reviewed in that report.In addition,the biological activities of those compounds and extracts were also reviewed,including anti-bacteria,anti-malaria, anti-hypertension, anti-tumor,anti-phosphodiesterase 4(PDE 4),protect-cardiovascular,and protect-gastrointestinal mucous membrane[6].From then on,some significant progresses on the plants of the genus Picrasma have been achieved over the last decade,and 101 compounds with various biological activities were reported.These compoundsare assigned to alkaloids,quassinoids,triterpenoids,and others,which are described herein.
Alkaloids
Alkaloids are the principal active components of the plants of the genus Picrasma.They can be divided into β-carbolinealkaloids,canthinonealkaloids,and bisb-carboline alkaloids.
β-Carboline alkaloids
In 2007, four β-carboline alkaloids,1-methoxy-9H-pyrido[3,4-b]indole (1),4-methoxy-1-methyl-9H-pyrido[3,4-b]indole (2),1-ethyl-4-methoxy-β-carboline (3),9H-pyrido[3,4-b]indole-1-carboxylic acid (4), were isolated from the stems of P.quassioides by Chen M et al.[7].At the same time,their inhibitory activities on the production of nitric oxide (NO) in mouse monocyte-macrophage RAW264.7 cells stimulated by lipopolysaccharide (LPS) were evaluated. Only compounds 1 and 2 showed weak inhibitory activities with IC50values of 100.0 mM and 57.3 mM,respectively[7].In 2010,twelve β-carboline alkaloids,picrasidine Y(5), 6-hydroxy-3-methoxycarbonyl-β-carboline (6),6,12-dimethoxy-3-(2-hydroxyethyl)-β-carboline (7),3,10-dihydroxy-β-carboline (8),6,12-dimethoxy-3-(1-hydroxyethyl)-β-carboline (9),6,12-dimethoxy-3-(1,2-dihydroxyethyl)-β-carboline(10),6-methoxy-3-(2-hydroxyl-1-ethoxyethyl)-β-carboline(11),6-methoxy-12-hydroxy-3-methoxycarbonyl-β-carboline(12), 3-hydroxy-β-carboline (13),3-(2-hydroxyethyl)-β-carboline (14),6-methoxy-3-(1,2-dihydroxyethyl)-β-carboline(15),and kumujancine(16),were obtained from the stems of P.quassioides by Jiao WH et al.[8].But the absolute configuration of 5 was not be determined at that time.In 2015,its absolute configuration was firstly identified by Koike K et al.on basis of the comparisons of1H and13C nuclear magnetic resonance(NMR)spectral data,specific opticalrotation values,High Performance Liquid Chromatography(HPLC)analysis using chiral columns,and circular dichroism(CD)spectra with the chemically synthesized stereoisomersof5 [9].In 2011,two β-carboline alkaloids,1-(dimethoxymethyl)-9H-pyrido[3,4-b]indole (17),4,8-dimethoxy-9H-pyrido[3,4-b]indole-1-carboxaldehyde(18),were obtained from the stems of P.quassioides by Jiao WH et al.[10].Both of them exhibited potent inhibitory activities on the production of NO,tumor necrosis factora(TNF-a),and interleukin 6(IL-6)in mouse RAW264.7 cells stimulated by LPS[10].In 2014,a β-carboline alkaloid,6-hydroxy-9H-pyrido[3,4-b]indole-1-carboxylic acid(19),was isolated from the stems of P.quassioides by Lai ZQ et al.,which showed no cytotoxic activities against four human cancer(K-562,SGC-7901,Hep G2,and A-549)and one mouse cancer(CT26.WT)cell lines[11].In 2016,two new β-carboline alkaloids,1-hydroxymethyl-8-hydroxy-β-carboline (20),6-hydroxy-9H-pyrido[3,4-b]indole-1-carboxylic acid ethyl ester(21),along with 6 and 13,were isolated from the stems of P.quassioides by Gong G et al.[12].Meanwhile,they exhibited different potency of the anti-angiogenic activities on zebrafish in vivo with antiangiogenic index(AI)>1[12].The structures and related information of compounds 1-21 are shown in the Figure 1 and Table 1.
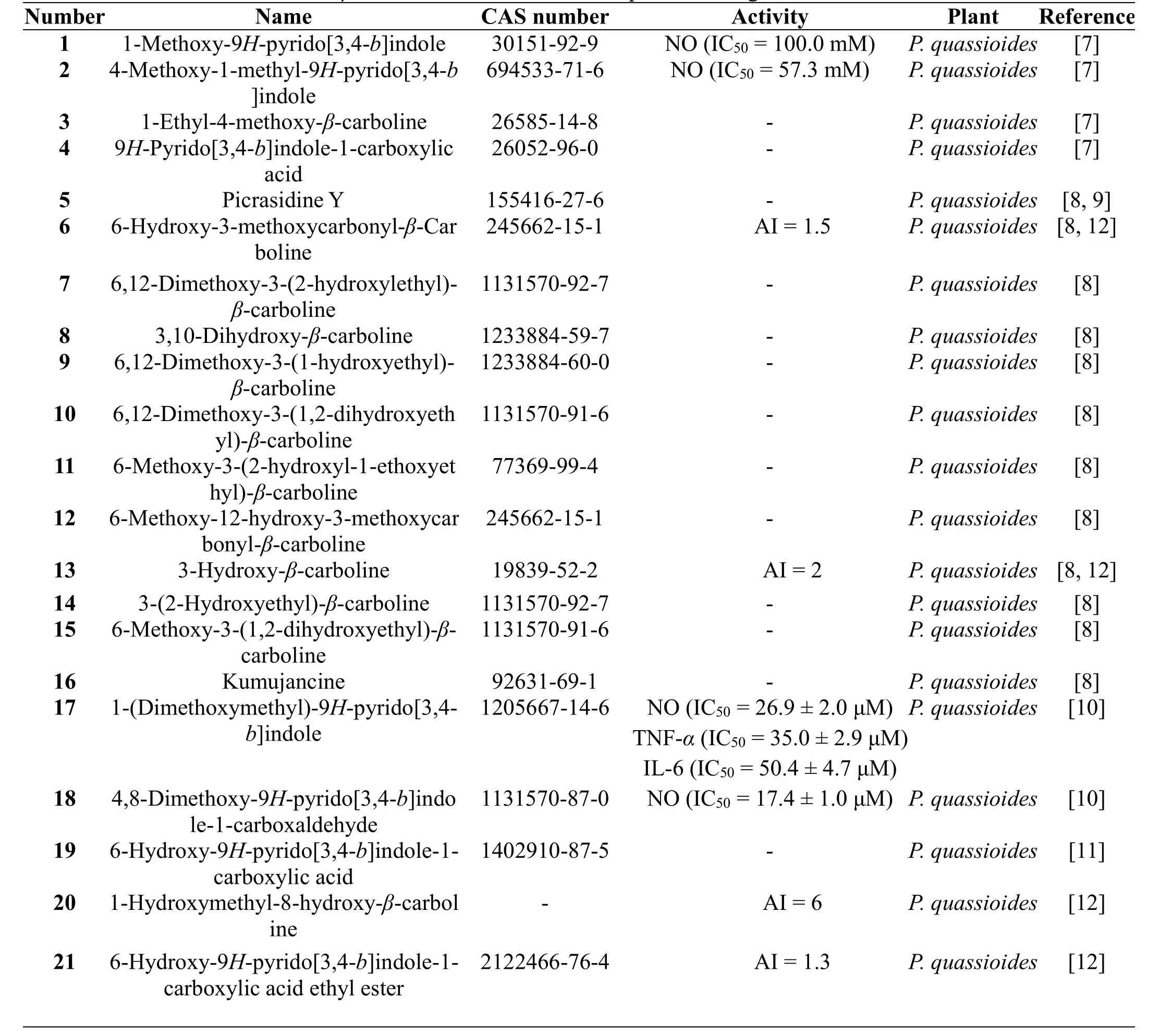
Table 1 β-Carboline alkaloids from the plants of the genus Picrasma
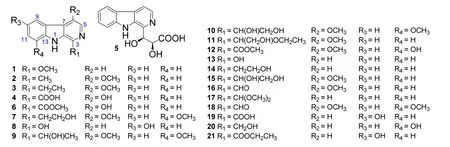
Figure 1 Chemical structures of 1-21
Canthinone alkaloids
In 2007,a canthinone alkaloid,11-hydroxycanthin-6-one(22),was isolated from the stems of P.quassioides by Chen M et al.[7].It showed inhibitory activity on the NO production of mouse RAW264.7 cells stimulated by LPS with IC50value of 46.3 mM[7].In 2008,three canthinone alkaloids, 8-hydroxycanthin-6-one (23),4,5-dimethoxy-10-hydroxycanthin-6-one (24), and 3-methyl-5,6-dioxo-4H-indole[3,2,1-de] [1,5]naphthyridinium(25),were obtained from the stems of P.quassioides by Jiang MX et al.[13].Their cytotoxic activities were evaluated against human nasopharyngeal carcinoma(CNE2)and human liver cancer(Bel-7402)cell lines with the result that only compounds 23 and 24 exhibited significant cytotoxic activities against CNE2 cell line [13]. In 2011,a canthinone alkaloid,6-oxo-6H-indole[3,2,1-de][1,5]naphthyridine-4-butanoic acid(26),was isolated from the stems of P.quassioides by Jiao WH et al.[10].Meanwhile,its inhibitory activities on the production of NO,TNF-a,or IL-6 in mouse RAW264.7 cells stimulated by LPS was evaluated.But it didn’t display obvious inhibitory activities[10].In 2016, two canthinone alkaloids,5-methoxy-11-hydroxycanthin-6-one (27) and furancanthin(28),were isolated from the stems of P.quassioides by Gong G et al.[12].Their biological results showed no anti-angiogenic activities on zebrafish in vivo[12]. The structures and related information of compounds 22-28 are shown in the Figure 2 and Table 2.
Bis β-carboline alkaloids
Eight bis β-carboline alkaloids,quassidines A-H(29-36)[10,14],along with two pairs of bis-β-carboline alkaloid enantiomers, (±)-quassidines I (37-38) and(±)-quassidines J(39-40)[15],were isolated from the stems of P.quassioides by Jiao WH et al.At the same time,their biological activities showed that compounds 29,33,34 and 35 displayed potent inhibitory activities on the production of NO,TNF-a,and IL-6 in mouse RAW 264.7 cells stimulated by LPS.Whereas,compounds 30,31,32 and 36 showed potent cytotoxicities on mouse RAW 264.7 cells at the concentration of 100 mg/ml[10,14].In addition,37-40 displayed potent cytotoxicities against human cervical HeLa and gastric MKN-28 cancer cell lines[15].The structures and related information of compounds 29-40 are shown in the Figure 3 and Table 3.
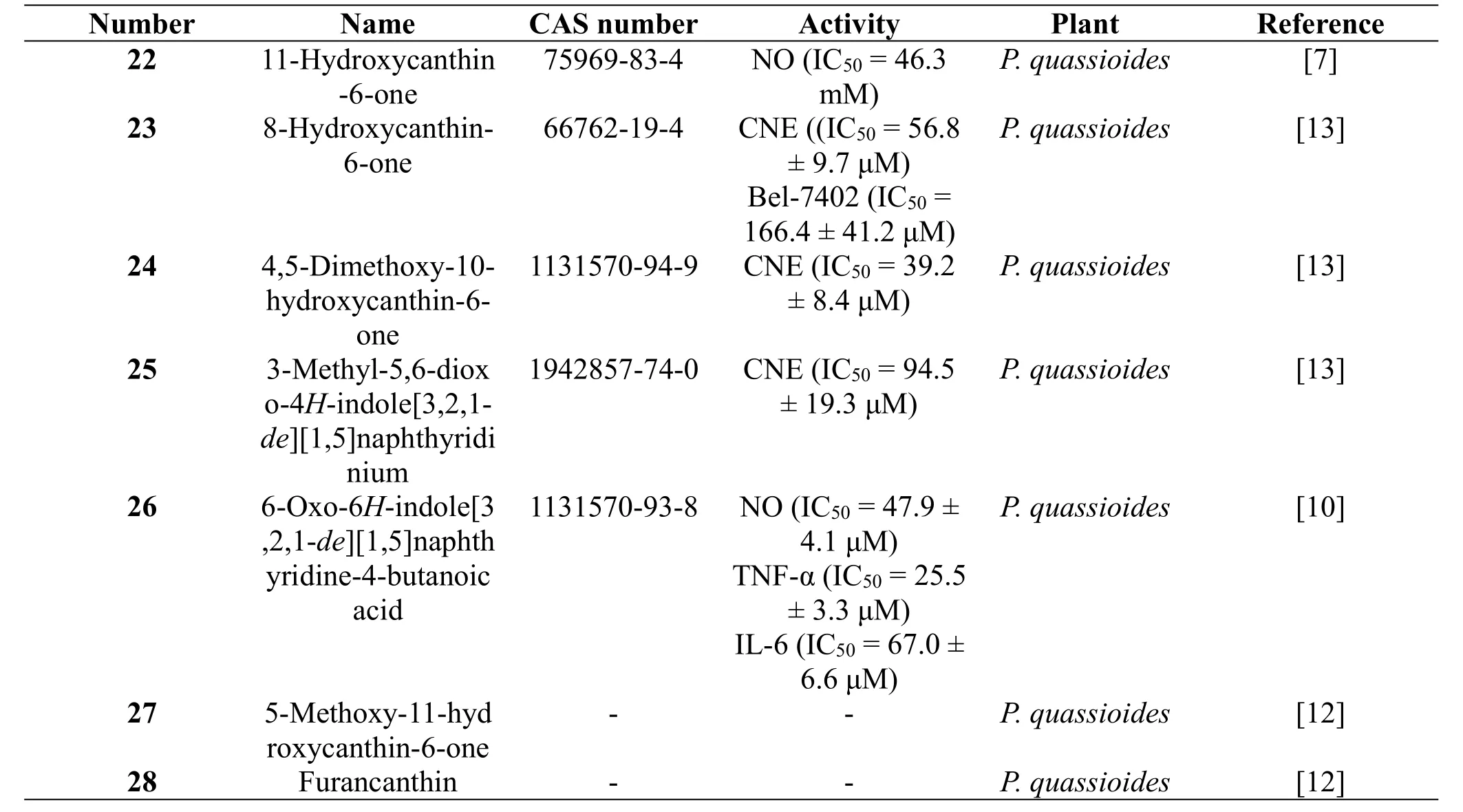
Table 2 Canthinone alkaloids from the plants of the genus Picrasma
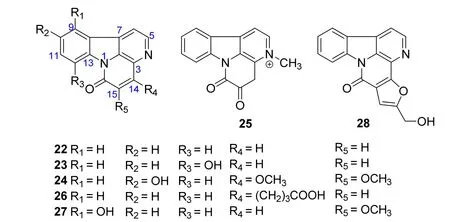
Figure 2 Chemical structures of 22-28.
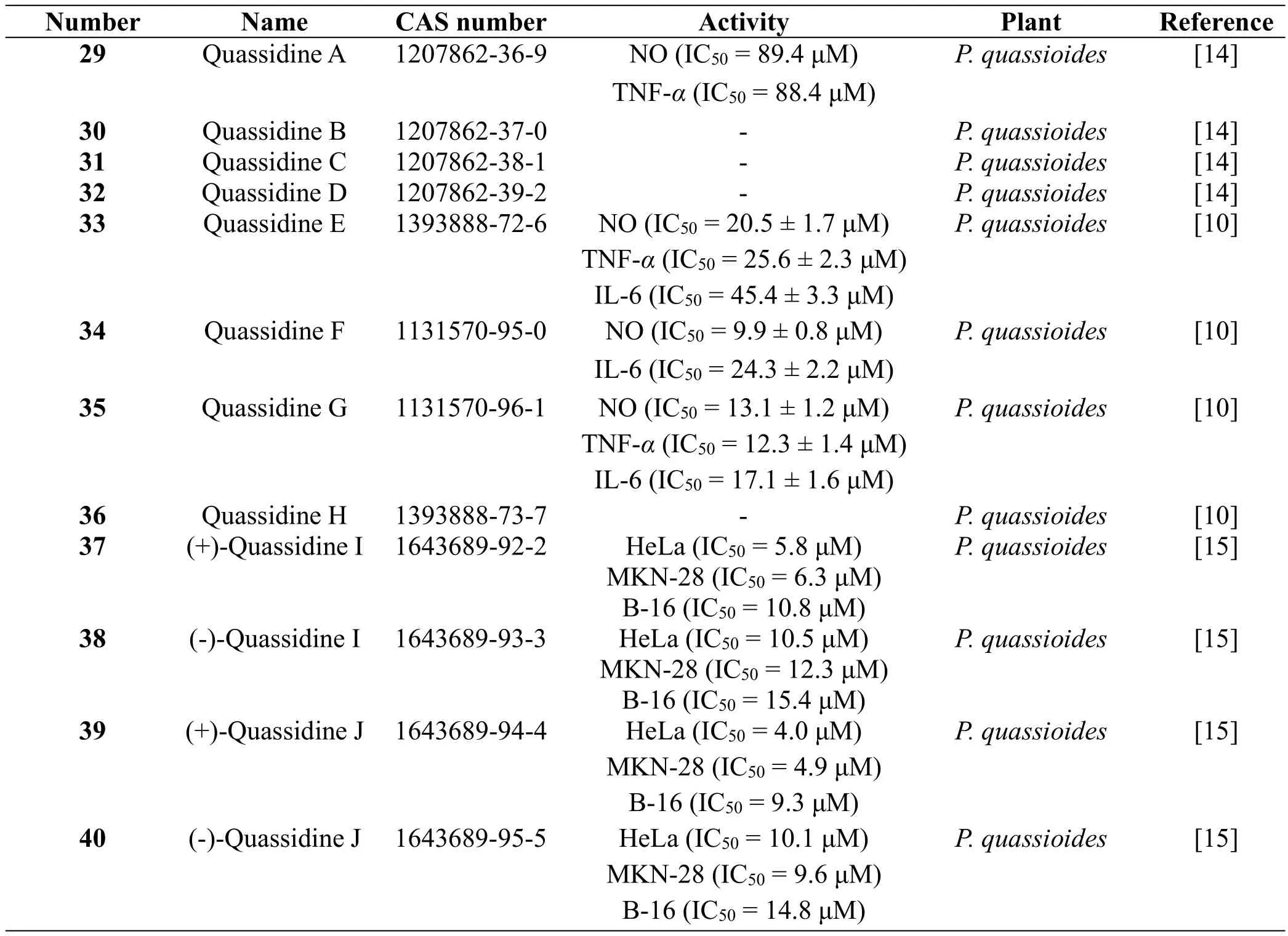
Table 3 Bis β-carboline alkaloids from the plants of the genus Picrasma
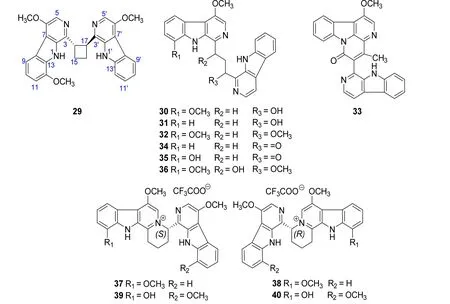
Figure 3 Chemical structures of 29-40
Quassinoids
In 2011,one quassinoid,2'-isopicrasin A(41),was isolated from the stems of P.quassioides without inhibitory activities on the production of NO,TNF-a,and IL-6 in mouse RAW 264.7 cells stimulated by LPS[16].In 2015 and 2016, thirteen new quassinoids,picrajavanicins A−M (42−54), together with the compound 41,were obtained from the bark of P.javanica collected in Myanmar by Nwet Nwet Win et al.[3,17].Meanwhile,41-54 were tested forantiproliferative activities against five human cancer cell lines of A549,HeLa,PANC-1,PSN-1,and MDA-MB-231.The results showed that 41 and 49-54 displayed potent antiproliferative activities against the human pancreatic cancer PANC-1 cell line[17].And compounds 41 and 49 exhibited antiproliferative activities against the human cervical cancer HeLa cell line[17].After that,an assay of anti-viral protein R(Vpr)activity for picrajavanicinsA–K(42–52)and M(54)was tested by Nwet Nwet Win et al.in 2016.The results revealed that 42–52 and 54 exhibited anti-VpractivitiesagainstTREx-HeLa-Vprcellsat concentrations of 1.25,2.5,and 5mM with damnacanthal as a positive control[18].In 2016,three quassinoids,nigakilactone P (55), picraqualide F (56) and nigakilactone Q(57),were identified from the stems of P.quassioides by Xu J et al.[19].They were tested for the cytotoxicities and inhibitory activity of NO production with the result of no cytotoxic activities against three human cancer cell lines of MCF-7,A-549 and HepG-2,and no inhibitory activity on the production of NO[19].The structures and related information of compounds 41-57 are shown in the Figure 4 and Table 4.
Triterpenoids
Thirty tirucallane-type triterpenoids,picraquassins A-D(58-61),6β-hydroxypicraquassin C(62),picraquassins E-J (63-68), 21β-ethoxybourjotinolone A (69),6-oxo-21β-ethoxybourjotinolone A (70),9,11-dehydrotoonaciliatin K (71),5,6,9,11-dehydrotoonaciliatin K (72),21β-ethoxy-20a-hydroxymelianodiol(73),picraquassin K (74), xanthocerasic acid methyl ester (75),11-oxobrumollisol A (76), picraquassin L (77),melianodiol (78), 6β-hydroxypicraquassin C (76),(13α,14β,17α,20S,21R,23R,24S)-21,23-epoxy-21-ethoxy-24,25-dihydroxylanost-7-en-3-one(79),toonaciliatin K(80),21-methoxy-21,23-epoxytirucalla-7,24-dien-3a-ol(81), bourjotinolone A (82), sapelin B (83),3β,29-dihydroxytirucalla-7,24-dien-21-oic acid (84),piscidinol A (85), brumollisol B (86), and 24S,25-dihydroxytirucall-7-en-3-one(87)were identified from the stems of P.quassioides by Xu J et al.in 2016[20].Cytotoxicities of the isolated compounds were evaluated using three human cancer cell lines of MKN-28,A-549,and MCF-7 with cis-platinum as a positive control.Among them,compounds 59,63,64,67,70,71,73,74,75,82,84,85 and 87 exhibited inhibitory activities against MKN-28 cells;compounds 59,67,82,and 85 showed inhibitory activities against A-549 cells;compounds 59,82,85 exhibited inhibitory activities against MCF-7 cells[20].The structures and related information of compounds 58-87 are shown in the Figure 5 and Table 5.

Table 4 Quassinoids from the plants of the genus Picrasma
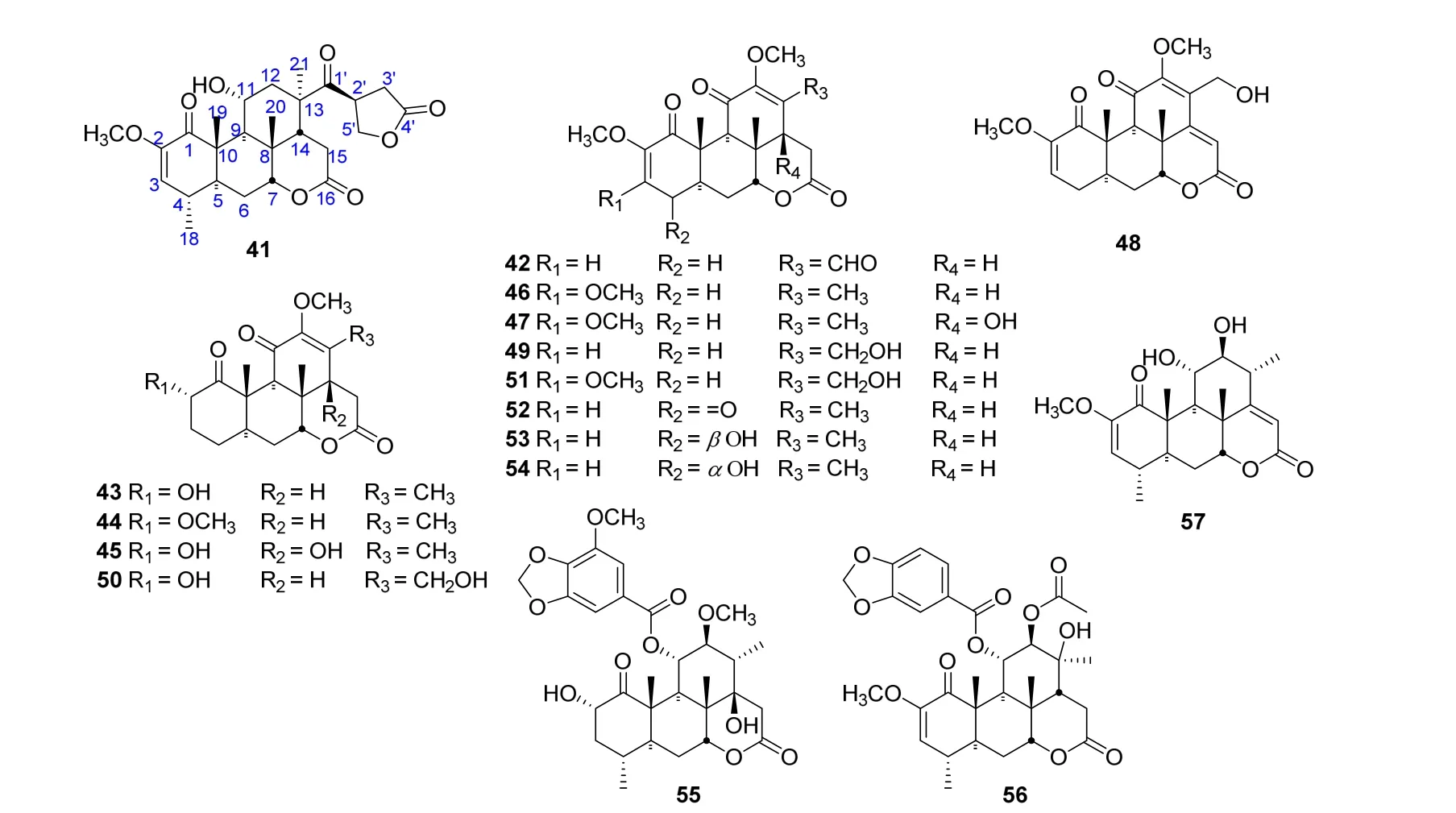
Figure 4 Chemical structures of 41-57.
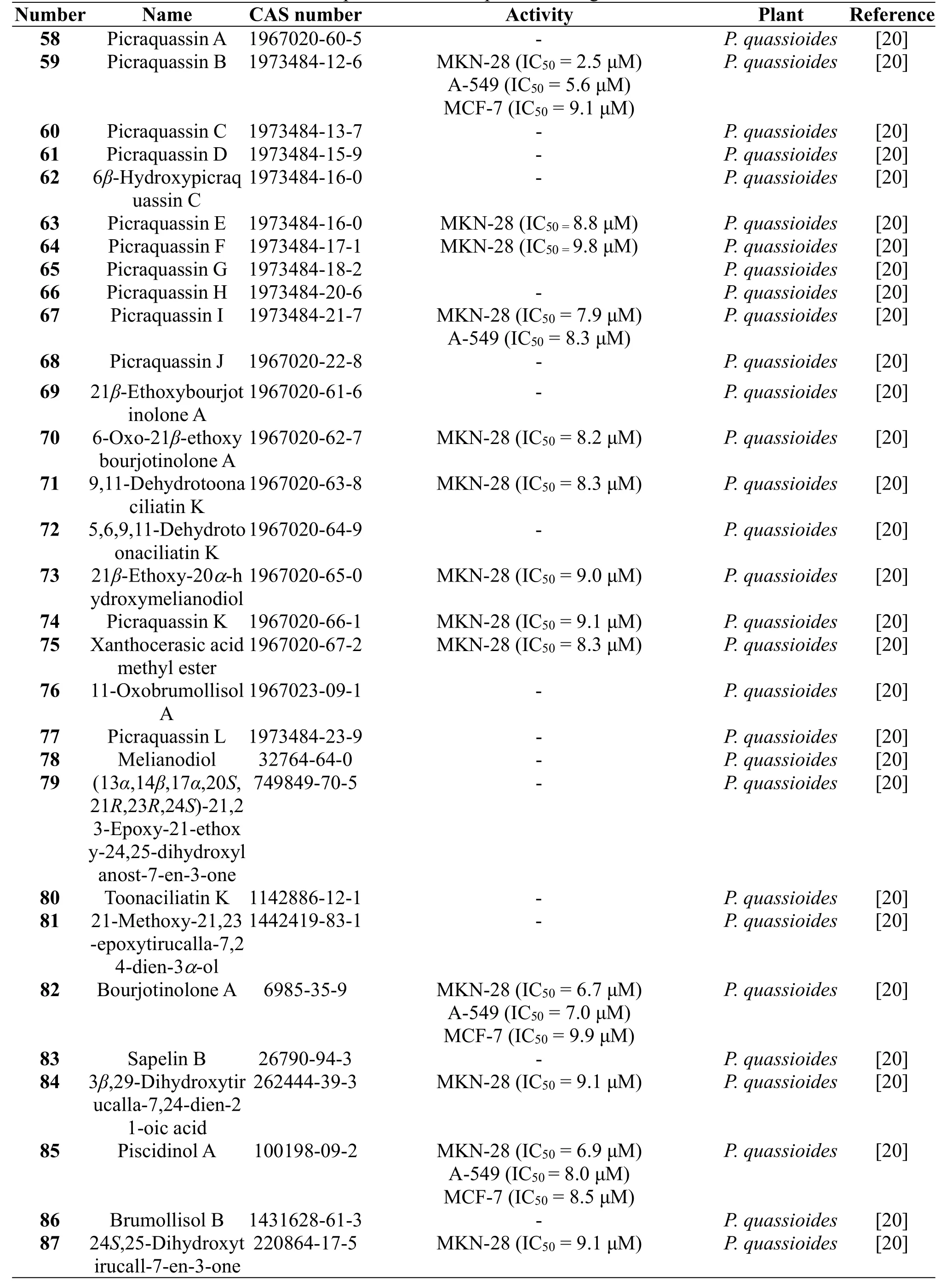
Table 5 Triterpenoids from the plants of the genus Picrasma
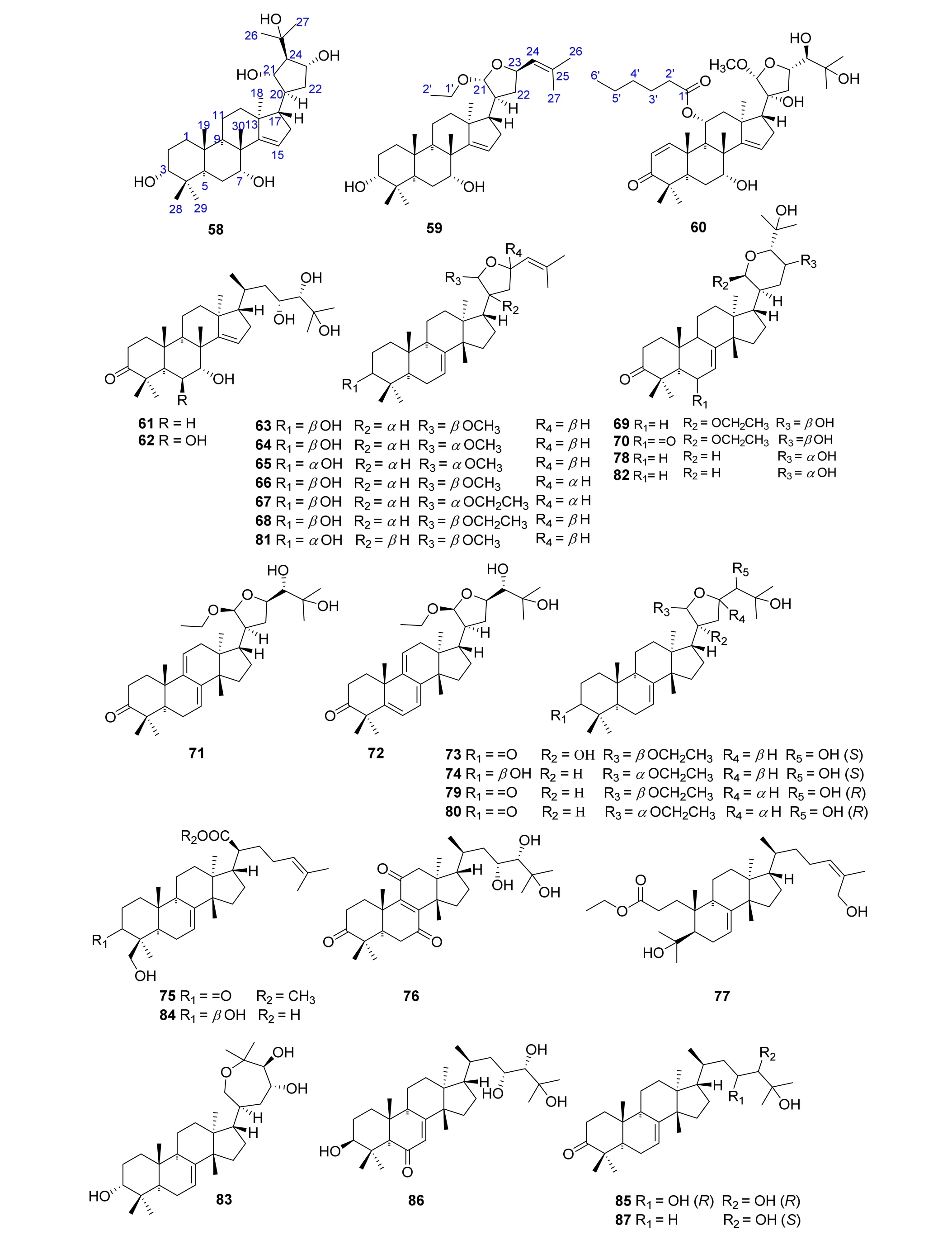
Figure 5 Chemical structures of 58-87.
Others
In 2011,ten compounds,calycosin(88),ononin(89),formononetin 7-O-β-D-apiofuranosyl-(1→6)-β-D-glucopyranoside(90),kushenol O (91),umbelliferone(92),emodin(93),maackiain (94), trifolirhizin (95),stigmasterol-3-O-β-glucopyranoside(96),and mannitol(97)were obtained from branches and leaves of P.quassioides by Deng GH et al.[21]Then in 2011,three neolignans,picrasmalignan A(98),buddlenol A(99),buddlenol C(100),together with a flavonol,fisetin(101),were isolated from the stems of P.quassioides and they showed inhibitory activities on the production of NO,TNF-a,and IL-6 in mouse RAW 264.7 cells stimulated by LPS[16].The structures and related information of compounds 88-101 are shown in the Figure 6 and Table 6.
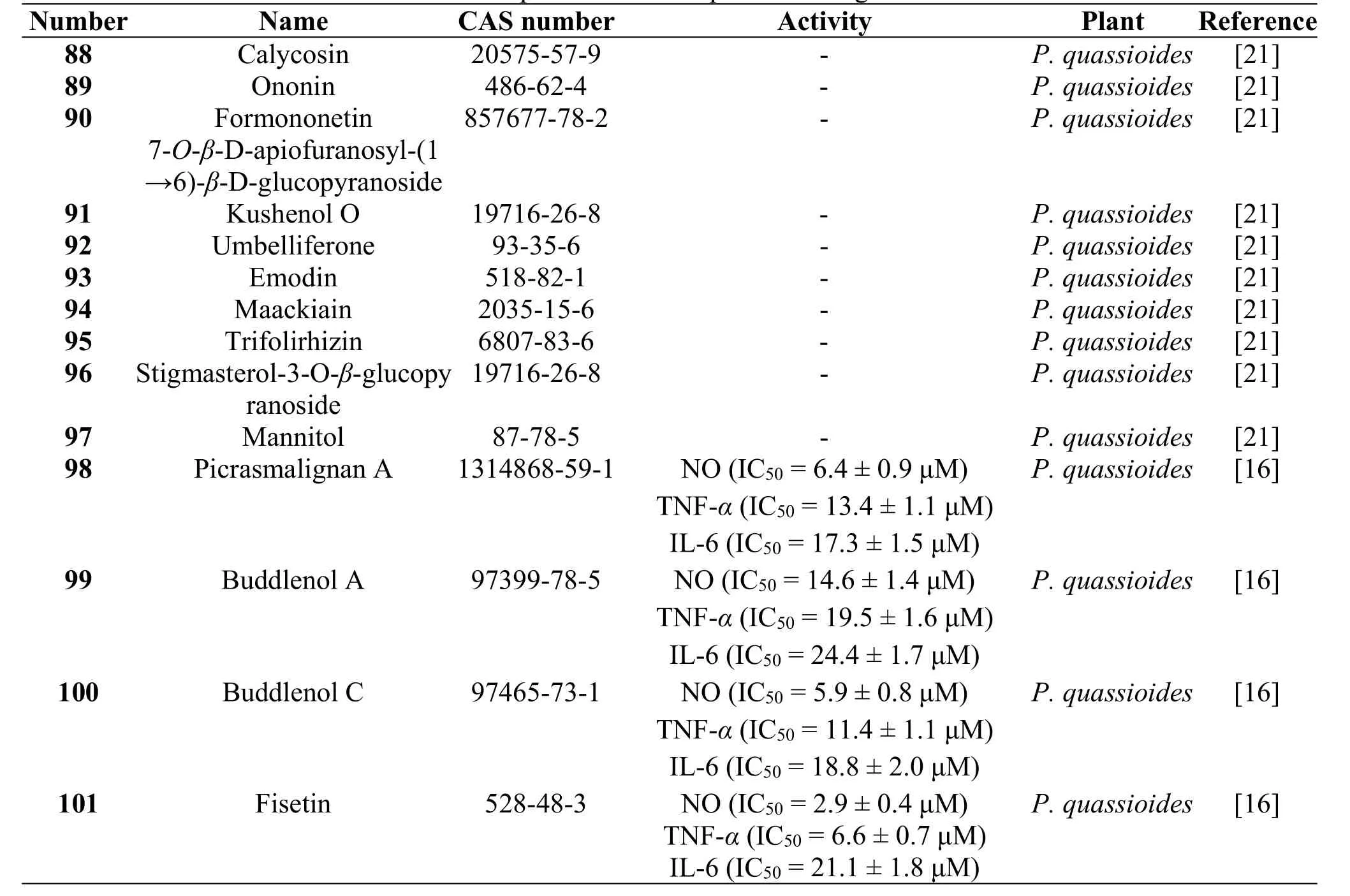
Table 6 Other compounds from the plants of the genus Picrasma

Figure 6 Chemical structures of 88-101.
Discussion and conclusion
The plants of the genus Picrasma consist of nine species.In fact,chemical investigation on the plants of the genus Picrasma only focus on three species(P.quassioides,P.javanica and P.excelsa)during the last decade,which is the same as the results of a decade ago[22-28].Among them,the moststudies on chemicalconstituents concentrate on the plants of P.quassioides,followed by the plants of P.javanica.The branches,leaves,stems or bark of the plants of P.quassioides are usually used as the traditional medicine for the treatment of infectious and inflammatory diseases in China,Japan and Korea[22-23].The bark of the plants of P.javanica is used in folk medicine as antimalarial drugs in Myanmar,Indonesia and Thailand[29-35].Little researches have been done on the plants of P.excelsa,and the extract of which is a natural bittering agent used as a food additive in Japan,Europe andAmerica[26,36].
Until now,a total of 258 compounds are identified from the plants of the genus Picrasma,of which 195 compounds are obtained from the plants of P.quassioides,71 compounds are identified from the plants of P.javanica,7 compounds are isolated from the plants of P.crenata.And the plants of P.quassioides and P.javanica shared 15 compounds.Among the 195 components of the plants of P.quassioides,there are 82 alkaloids,51 quassinoids,36 triterpenoids,and 26 other ingredients.Seventy-one constituents from the plants of P.javanica include 15 alkaloids,54 quassinoids and 2 triterpenoids.Seven compounds from the plants of P.crenata are all quassinoids.From the currentresultsofchemical constituents,there are large differences in the chemical constituents between the species of the genus Picrasma.
The biological activities of the chemical constituents from the plants of the genus Picrasma are mainly focused on anti-microbial,anti-inflammatory,anti-virus activities and cytotoxicities.It is consistent with the traditional applications,such as curing anemopyretic cold,sore throat,dysentery,eczema,and so on.Alkaloids in the plant of P.quassioides have been proved to be the main components with anti-inflammatory activities[37-38].Although quassinoids are the major chemical constituents in the plant of P.javanica.,alkaloids from the stembark of the plants of P.javanica are proved to be the main anti-malarial active ingredients[35,39-40].
1. Qian CS,Chen.HY.Flora of China.Beijing:Science Press 1997,43:7.
2. Chinese Pharmacopoeia Commission.Pharmacopoeia of the People's Republic of China(partI).Beijing:ChinaMedicalScienceand Technology Press 2015,200.
3. Win NN,Ito T,Kodama T,et al.Picrajavanicins A–G,Quassinoids from Picrasma javanica Collected in Myanmar.J Nat Prod 2015,78:3024-3030.
4. Woo GH,Shibutani M,Inoue K,et al.Promoting potential of a Jamaica quassia extract in a rat medium-term hepatocarcinogenesis bioassay.Food Chem Toxicol 2007,45(7):1160-1164.
5. Cardoso MLC,Kamei MS,Nunes RF,et al.Development and validation of an HPLC method for analysis of Picrasma crenata.J Liq Chromatogr Relat Technol 2009,32:72-79.
6. Jiao WH,Li CY,Gao H,et al.Advances in studies on chemical constituents and bioactivities for plants of Picrasma Bl.Chin Tradit Herbal Drugs 2007,38:1419-1424.
7. Chen M.Alkaloids from Picrasma quassioides(D.Dom)Benn.and theiranti-inflammatory and antibacterial activities.Yantai University,2007.
8. Jiao WH,Gao H,Li CY,et al.β-Carboline alkaloids from the stems of Picrasma quassioides.Magn Reson Chem 2010,48:490-495.
9. Koike K,Yoshino H,Li HY,et al.Determination of the absolute configuration of picrasidine Y,a naturally occurring β-carboline alkaloid.Tetahedron Lett 2015,56:5306-5308.
10.Jiao WH,Gao H,Zhao F,et al.Anti-inflammatory alkaloids from the stems of Picrasma quassioides Bennet.Chem Pharm Bull 2011,59:359-364.
11.Lai ZQ,Liu WH,Ip SP,et al.Seven alkaloids from Picrasma quassioides and their cytotoxic activities.Chem Nat Compd 2014,50:884-888.
12.Gong G,Li QH,Xu J,et al.In vivo SAR and STR analyses of alkaloids from Picrasma quassioides identify 1-hydroxymethyl-8-hydroxy-β-carboline as a novel natural angiogenesis inhibitor.RSC Adv 2016,6:9484-9494.
13.Jiang MX,Zhou YJ.Canthin-6-one alkaloids from Picrasma quassioides and their cytotoxic activity.J Asian Nat Prod Res 2008,10:1009-1012.
14.Jiao WH,Gao H,Li CY,et al.Quassidines A-D,bis-β-carboline alkaloids from the stems of Picrasma quassioides.J Nat Prod 2010,73:167-171.
15.Jiao WH,Chen GD,Gao H,et al.(±)-Quassidines I and J,two pairs of cytotoxic bis-β-carboline alkaloid enantiomers from Picrasma quassioides.J Nat Prod 2015,78:125-130.
16.Jiao WH,Gao H,Zhao F,et al.A new neolignan and a new sesterterpenoid from the stems of Picrasma quassioides Bennet.Chem Biodive 2011,8:1163-1169.
17.Win NN,Ito T,Kodama T,et al.Picrajavanicins H–M,new quassinoids from Picrasma javanica collected in Myanmar and their antiproliferative activities.Tetrahedron 2016,72:746-752.
18.Win NN,Ito T,Win YY,et al.Quassinoids:Viral protein R inhibitors from Picrasma javanica bark collected in Myanmar for HIV infection.Bioorg Med Chem Lett 2016,26:4620-4624.
19.Xu J,Xiao D,Song WW,et al.Quassinoids from the stems of Picrasma quassioides and their cytotoxic and NO production-inhibitory activities.Fitoterapia 2016,110:13-19.
20.Xu J,Xiao D,Lin QH,et al.Cytotoxic tirucallane and apotirucallane triterpenoids from the stems of Picrasma quassioides.J NatProd 2016,79:1899-1910.
21.Deng GH,Study on chemical constituens and quality evaluation ofRamulus etFolium Picrasmae.Guangzhou University of Chinese Medicine,2011.
22.Ohmoto T,Koike K.Studies on the constituents of Picrasma quassioides Bennet.II.On the alkaloidal constituents. Chem Pharm Bull 1983, 31:3198-3204.
23.Sung YI,Koike K,Nikaido T,et al.Inhibitors of cyclic AMP phosphodiesterase in medicinal plants.V.Inhibitors of cyclic AMP phosphodiesterase in Picrasma quassioides Bennet, and inhibitory activities of related β-carboline alkaloids.Chem Pharm Bull 1984,32:1872-1877.
24.Arbain D,Sargent MV.The alkaloids of Picrasma javanica.Aust J Chem 1987,40:1527-1536.
25.Koike K,Ohmoto T,Uchida A,et al.Javacarboline,a new β-carboline alkaloid from the stem of Picrasma javanica in Java.Heterocycles 1994,38:1413-1420.
26.Novello CR,FerreirA,MarquesLC,etal.Quassinoids from Picrasma crenata.Nat Prod Res 2003,17:145-148.
27.Tada A,Sugimoto N,Sato K,et al.Examination of original plant of Jamaica quassia extract,a natural bittering agent,based on composition ofthe constituents.Food Hyg Safe Sci 2009,50:16-21.
28.Yang SP,Yue JM.Five new quassinoids from the bark of Picrasma quassioides.Helv Chim Acta 2004,87:1591-1600.
29.Ohmoto T,Koike K,Kagei K.Alkaloids from Picrasma javanica growing in Indonesia.J Pharm Soc Jpn 1987,41:338-340.
30.Ishii K,Koike K,Ohmoto T.Javanicinosides D-H,quassinoid glucosides from Picrasma javanica.Phytochemistry 1991,30:4099-4103.
31.Koike K,Ishii K,Mitsunaga K,et al.Constituents from Picrasma javanica.Part 6.Quassinoids from Picrasma javanica. Phytochemistry 1991, 30:933-936.
32.Koike K,Ohmoto T.New quassinoid glucosides,javanicinosides I,J,K,and L,from Picrasma javanica.J Nat Prod 1992,55:482-486.
33.Yoshikawa M,Harada E,Aoki S,et al.Indonesian medicinal plants.VI.On the chemical constituents of the bark of Picrasma javanica BL.(Simaroubaceae)from Flores Island.Absolute stereostructures of picrajavanins A and B.Chem Pharm Bull 1993,41:2101-2105.
34.Koike K,Yokoh M,Furukawa M,et al.Picrasane quassinoids from Picrasma javanica.Phytochemistry 1995,40:233-238.
35.Saiin C,Sirithunyalug B.Review of the chemical structuresand antimalarialactivitiesofindole alkaloids isolated from Picrasma javanica Bl.Adv Med Plant Res 2017,5:29-36.
36.KrebsHC,SchillingPJ,Wartchow R,etal.Quassinoids and other constituents from Picrasma crenata.Z Naturforsch B Chem Sci 2001,56:315-318.
37.Jiao WH,Studies on Anti-inflammatory Constituents of Picrasma quassioides Bennt, Shenyang Pharmaceutical University,2010.
38.Song YS,Lee Y,Kwon TR,et al.Picrasma quassioidesinhibitsLPS-and IFN-γ-stimulated nitric oxide production and inflammatory response in RAW264.7 macrophage cells. Biotechnol Bioprocess Eng 2014,19:404-410.
39.Pavanand K,Yongvanitchit K,Webster HK,et al.In vitro antimalarial activity of a Thai medicinal plant Picrasma javanica Bl.Phytother Res 1988,2:33-36.
40.Saiin C,Sirithunyalug B,Rattanajak R,et al.Isolation and in vitro antimalarialactivity of chloroform extract from Thai Picrasma javanica Bl stembark.Adv Med Plant Res 2016,4:94-98.
杂志排行
TMR Modern Herbal Medicine的其它文章
- Effect of TongFengNing Decoction on UricAcid Levels and Xanthine Oxidase Activity in Hyperuricemia Rats
- Clinical experience in treating 78 cases of upper limb edema after breast cancer operation by WenYang HuoXue Washing Prescription
- Clinical observation of Furongtongmai capsule on the lower extremity Atherosclerotic Occlusive Disease after Intervention Operation
- Application of LC-MS based glutathione-trapped reactive metabolites in the discovery of toxicity of traditional Chinese medicine
- Survey of dose-effect relationship in Chinese materia medica
- Analysis of pharmacological action and clinical application of DaXueTeng based on its anti-inflammatory action
