Conocarpus erectus L., a plant with a high content of structural sugars, ions and phenolic compounds, shows antioxidant and antimicrobial properties promoted by different organic fractions
2018-10-12DayaneKellyDiasdoNascimentoSantosWeslleyHenriquedeOliveiraMeloAnastssiaMariNunesdeOliveiraLimaIranildoJosdaCruzFilhoGluciaManoelladeSouzaLimalioDiegodaSilvaMaiaraCelinedeMourarciaSilvadoNascimentoAnaMariaSoutoMa
Dayane Kelly Dias do Nascimento Santos, Weslley Henrique de Oliveira Melo, Anastássia Mariáh Nunes de Oliveira Lima, Iranildo José da Cruz Filho, Gláucia Manoella de Souza Lima, Túlio Diego da Silva, Maiara Celine de Moura, Márcia Silva do Nascimento, Ana Maria Souto Maior, Thiago Henrique Napoleão, Cristiane Moutinho Lagos de Melo✉
1Department of Biochemistry, Biosciences Centre, Federal University of Pernambuco, Recife, Pernambuco, Brazil
2Department of Antibiotics, Biosciences Centre, Federal University of Pernambuco, Recife, Pernambuco, Brazil
3Center of Strategic Technologies of the Northeast, Recife, Pernambuco, Brazil
Keywords:Combretaceae Phenolic compounds Antimicrobial activity Antioxidant
ABSTRACT Objective: To evaluate the structural and chemical composition of plant and the antioxidant and antimicrobial activities promoted by hexanic, ethanolic and ethyl acetate fractions obtained from leaves of Conocarpus erectus. Methods: Organic fractions were characterized through UPLC-MS and GC-MS. Antioxidant potential was performed through DPPH and molybdenum phosphate techniques. Antibacterial and antifungal assays were performed in accordance with Clinical and Laboratory Standards Institute protocols. Results: The obtained biomass of Conocarpus erectus leaves showed the high presence of glucose (0.45 g/L), cellulose (28.69%),Na (55.126 μg/L) and K (31.163 μg/L). We identified seven compounds in the hexanic and ethyl acetate fractions, and eight compounds in ethanolic fraction. Moreover, phenolic compounds are prevalent in all organic fractions with values of (10.04 ± 0.24), (221.26 ±1.84), (340.53 ± 0.84) mg/g GAE to hexanic, ethyl acetate and ethanolic fraction, respectively.Antioxidant results showed a high potential in ethyl acetate fraction (71.82 ± 6.87)% and(10.89 ± 0.05)% in DPPH and molybdenum phosphate techniques, respectively. The ethanolic fraction showed moderate bacteriostatic and bactericidal activity against Staphylococcus aureus and presented a high fungistatic potential for all Candida species tested. Conclusions: Organic fractions obtained from leaves of Conocarpus erectus present antimicrobial and antioxidant properties, and these findings contribute to scientific information for the effectiveness on use of this plant in the development of a phytotherapic compound.
1. Introduction
The plants represent an important source of natural products,besides therapeutic agents. Moreover, they also can be a potential source from a variety of chemical constituents which are biologically active[1]. For millennia, plants with medicinal objectives have been used, to improve health and to treat some diseases like cardiovascular disorders and infections[2].
Products from medicinal plants are belonging to both, traditional and alternative medicine[3]. Currently, about 70% of people in the world rely on plants as therapeutic agents, either as botanical extracts (because plants are easily found, usually cheap and easy to prepare) or like isolated molecules, obtained in pharmaceutic industries[4].
However, the investigation of chemical composition and bioactivity of these medicinal plants are essential requirements for their safe and effective use[5]. Phytochemical potential, mechanisms of action and synergistic effects of natural products have been evaluated, in different areas of knowledge, with the objective of validating the benefits of the use of these products[1,6].
Conocarpus erectus (C. erectus) L. (Combretaceae), is widely distributed on shorelines in tropical and subtropical regions of the earth, and is popularly known as button mangrove[7]. In the folk medicine, the leaves are eaten and decoctions from bark, leaves and fruits are used against many diseases as catarrh, conjunctivitis,gonorrhea, diarrhea, fever, orchitis and syphilis[7-9].
Phenolic compounds are the major secondary metabolites of this species[10]. These molecules detected in different C. erectus extracts have been described to exhibit antioxidant, antibacterial, antifungal and antiviral activities, as well as act in the activation of the immune system[9,11,12]. Here, we investigated the structural and chemical composition of leaves of C. erectus, as well as, the phytochemical constituents, antioxidant and antimicrobial properties promoted by three organic fractions obtained from leaves of this plant.
2. Materials and methods
2.1. Plant material
Adult leaves of C. erectus L. were collected in April 2018 in the mangrove of the city of Ilha de Itamaracá, Pernambuco, Brazil(7°40’ S, 34°50’ W). It was selected health leaves with green and showy aspects, visually intact, free of bugs, diseases or altered color. A voucher specimen was identified by biologist and PhD.Marlene Barbosa and was deposited in the Herbarium Geraldo Mariz of the Universidade Federal de Pernambuco under the number 75,457. Plant collect was authorized by the Agência Estadual de Meio Ambiente, Recife, Pernambuco, under number CA DFRB N.120/2014.
2.2. Chemical composition from leaves of C. erectus
The chemical composition of the leaves of C. erectus in terms of cellulose, hemicellulose, lignin, pectin, extractives and ash was obtained through the adaptation of a technique performed by Gouveia et al.[13] for analysis of sugarcane biomass. The adaptation of the method was done in relation to the extractive solvents used, to allow the extraction and fractionation of the pectic material, based on the methodology of extraction of pectic polysaccharides from Habibi et al.[14].
2.3. Determination of carbohydrates, organic acids, and degradation products from leaves of C. erectus
Values of sugar and degradation products were obtained by hydrolysis with 72% H2SO4(v/v) in a high-performance liquid chromatograph (Agilent, series 1100), Aminex HPX87H column(Bio-Rad), temperature of 60 ℃, mobile phase: 5 mM H2SO4, flow of 0.6 mL/min and refractive index (IR) detector for identification and quantification of components (acetic and formic acids,arabinose, glucose, cellobiose and xylose sugars).
Concentrations of furfural and hydroxymethylfurfural were determined using a reverse phase (C-18) column (Agilent Technologies),with a mobile phase consisting of a 1:8:1 acetonitrile:water:acetic acid solution using a UV/Vis detector (274 nm) at 25 ℃. Samples were filtered on a 0.22 μm membrane for the analytical procedure.A correction was used to determine the final amount to 0.95 of polysaccharides (cellulose and hemicellulose), 0.90 of Cellobiose-C,0.88 of Glucose-C, 0.88 of Xylose-H, 0.88 of Amino Acid-H, 0.72 of Acetic Acid-H, 3.52 of Formic Acid-C, 1.37 of Furfural-H and 1.29 of hydroxymethylfurfural-C[15].
2.4. Determination of metal ions in leaves from C. erectus L.
Metals (Na, Mn, Zn, Cd, Cu, Cr, Pb, Fe, Ni and K) were detected and quantified using the Atomic Absorption Spectrophotometer,series: AA-6300, brand of Shimadzu. For the sodium (Na) and potassium (K), assays were performed in the Flame Photometer,series: DM-61 and Digimed brand.
2.5. Preparation of organic fractions of C. erectus leaves
Dried leaves of C. erectus (5 g) were passed by fractional extraction in Soxhlet with hexanic, ethyl acetate and ethanolic, respectively for 8 h for each solvent. The fractions obtained were dried in rotary evaporator at 40 ℃ at 70 rpm.
2.6. Investigation of total phenolic content
Folin-Ciocalteu method was used to measure total phenols content in accordance with Li et al.[16] with some changes. Folin solution (2 mL; 1:10 v/v) was added to 0.2 mL of the fractions diluted in water(1 mg/mL). After 4 min, the solution was added 1.6 mL of sodium carbonate (7.5%) with incubation for 120 min in the dark at room temperature. Absorbances of the samples were measured at 765 nm against a blank (reagent added to the sample solvent). A calibration curve was prepared by plotting the absorbance as a function of the gallic acid concentration (0-500 μg/mL) and then finding the linear equation (y = 0.004 8x + 0.001 6; R²= 0.999 9). The assay was performed in five replicates and the phenols contents are expressed in gallic acid equivalent (mg/g GAE).
2.7. Investigation of flavonoid content
For determination of total flavonoids, 1.5 mL of the AlCl3in 2% of alcohol solution was added to 1.5 mL of the sample (0.5 mg/mL). After 1 h at room temperature, the absorbance was measured at 420 nm. A standard quercetin curve (0-0.5 mg/mL) was performed to obtain the equation (y = 0.023x + 0.150 9; R²= 0.995 6). The assay was performed in five replicates and the flavonoid content is expressed as quercetin equivalent (mg/g QE).
2.8. Gas chromatography coupled to mass spectrometry (GCMS)
GC-MS was utilized to identify nonpolar compounds in the hexanic fracion. The oven temperature was programmed at 70 ℃ with an increase of 4 ℃/min until 280 ℃, and maintained for 15 min. The carrier gas was helium, with a constant flow of 1.4 mL/min. The temperature of the ionization source was maintained at 280 ℃, the ionization energy at 70 eV, and the ionization current at 0.7 kV.Mass spectra were recorded from 300 m/z to 450 m/z. Individual components were identified by matching their 70 eV mass spectra with those of the spectrometer data base using the Wiley L-Built library, and by comparing their retention indices with those of NIST computer MS library and with the fragmentation patterns of the mass spectra with those reported in the literature.
2.9. Ultra-performance liquid chromatography coupled to mass spectrometry (UPLC-MS)
UPLC-MS was utilized to identify polar compounds in the ethyl acetate and ethanolic fractions. Chromatography was performed with an Acquity H-Class (Waters) ultra-performance liquid chromatograph(UPLC). A BEH 2.1 mm × 100 mm column and a 1.7 μm particle size were employed. The mobile phases used consisted of aqueous solution containing 2 % MeOH, 5 mM ammonium formate and 0.1% formic acid (eluent A) and methanolic solution containing 0.1% formic acid (eluent B), which were pumped at a flow rate of 0.3 mL/min. Elution was performed in gradient mode and the initial condition (98% A / 2% B) was maintained for 0.25 min. The B ratio increased linearly to 99% in 8.5 min, remaining at 99% B for 1 min,followed by immediate decrease to 2% B, where it was maintained for up to 11 min. Ten microliters of sample were injected. The column temperature was maintained at 40 ℃ and the auto injector at 10 ℃. The UPLC system was coupled to a single quadrupole mass spectrometer SQ Detector 2 (Waters). The capillary voltage was 3.5 Kv, the voltage of the 30 V cone, the desolvation temperature was 450 ℃, with gas flow from the source of 650 L/h. The data acquisition was done in fullscan mode, searching for masses between 100 and 1 000 Da, in negative ionization. Chromatograms and mass spectra were acquired through MassLynx™ software (Waters).Interpretation of mass spectrum was conducted using the database of Centro de Tecnologias Estratégicas do Nordeste (CETENE), Wiley L-Built library and NIST computer MS library.
2.10. Study of antioxidant actions promoted by organic extract in vitro
2.10.1. Free radical sequestration: DPPH method
The antioxidant activity of the extracts was evaluated by the stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH·)[17]. A total of 2.5 mL of the DPPH· solution [1 mM and OD 517 = (0.650 ± 0.50) nm] was mixed in 0.2 mL of extracts in different concentrations (500, 250,125, 62.5, 31.2, 15.6, 7.8 and 3.9 μg/mL) to find the IC50of each extract. Acid ascorbic and butylated hydroxytoluene (BHT) were used as standard. After 25 min, the absorbance was read at 517 nm.The control was DPPH added to 0.2 mL of water. The percentage of radical scavenging radicals was measured by the formula: radical scavenging radicals (DPPH·) (%) = [(Sa - Ca)/Ca] × 100.
Where: Sa = Sample absorbance and Ca = Control absorbance.The concentration of the sample required to inhibit 50% (IC50) of DPPH was calculated by calibration curve prepared by plotting the absorbance as a function of DPPH concentration (0-500 μg/mL)and then finding the linear equation (y = 0.000 07x + 0.010 6; R²=0.996 4). The y value was substituted by 50 to obtain IC50. The assay was performed in five replicates.
2.10.2. Total antioxidant activity
The total antioxidant activity[18] was determined as a function of ascorbic acid, compound considered with 100% activity. A total of 300 μL of each sample and ascorbic acid (1 mg/mL) was added to 3 mL of the phosphomolybdenum solution (600 mM sulfuric acid,28 mM sodium phosphate and 4 mM ammonium molybdate) and incubated in water at 95 ℃ for 90 min. After returning to room temperature, the absorbances were measured at 695 nm against a blank (1 mL of phosphomolybdenum solution and 0.1 mL water).The total antioxidant activity (TAA) is calculated by the formula TAA(%) = [(Sa - Ca)/(Aaa - Ca)] ×100, where: Ca = Control absorbance,Sa = Sample absorbance and Aaa = Ascorbic acid absorbance. A calibration curve of ascorbic acid (0-0.5 mg/mL) was performed to obtain the equation (y = 0.019x + 0.072 3, R2= 0.993 70. The assay was performed in five replicates.
2.11. Antifungal assays
2.11.1. Fungal strains, culture conditions and sample preparation
The fungal strains used were: Candida krusei (C. krusei), Candida tropicalis (C. tropicalis), Candida albicans (C. albicans) and Candida glabrata (C. glabrata) from the Collection of Cultures of the Departamento de Micologia of Universidade Federal de Pernambuco(UFPE). Stock cultures were maintained with sterile powdered skim milk containing 10% (v/v) glycerol or Sabourand-dextrose broth medium with 30% (v/v) glycerol (-20 ℃). To perform the experiments, the yeasts were cultured on Sabourand Dextrose overnight agar medium at 36 ℃ and, subsequently, the colonies were resuspended in sterile saline solution (0.15 M NaCl) and adjusted turbidimetrically at a wavelength of 600 nm (OD600) to obtain suspension equivalent to 106colony forming units (CFU) per mL.For the assay, the organic extracts were filtered through a 13 mm ×0.22 μm sterile PVDF syringe filter.
2.11.2. Determination of minimum inhibitory concentration(MIC) and minimum fungicidal concentration (MFC)
The MIC of the samples was evaluated by the microtiter test proposed by the Clinical and Laboratory Standards Institute. In 96-well microtiter plates, the sample was added (80 μL) into the fourth well from which it was serially diluted in sterile Milli-Q water to the twelfth well of the same row. Subsequently, 40 μL of the Sabourand Dextrose broth medium was added to all wells, except the first,which was filled with 200 μL of the culture medium, corresponding to the sterility control. Positive control used was fluconazole (64 μg/mL) in the second well. Finally, the fungal suspension (80 μL; 106CFU/mL) was added in the second well to the last well in the row.The third well (containing microorganisms in the absence of the sample) corresponded to the 100% growth control. The plates were incubated at 36 ℃ and the optical density was measured at time zero and after 24 hours of incubation using a spectrophotometer(600 nm). The MIC50corresponded to the lowest concentration of the sample capable of promoting the reduction of ≥ 50% of CFU,comparing the optical density of the sample with that of the growth control.
For the determination of MFC, 10 μL of the wells with samples containing concentrations of ≥ MIC50were inoculated in Petri dishes containing Sabourand Dextrose agar medium, which were subsequently incubated at 36 ℃ for 24 h. The MFC corresponded to the lowest concentration of the sample capable of reducing the number of CFUs by 99.9% in relation to the initial inoculum. Each assay was performed in triplicate and three independent experiments were performed.
2.12. Antibacterial assays
2.12.1. Preliminary assay
The plate-hole diffusion assay described by Shan et al.[19], with changes, was used to determine the growth inhibition of bacteria by the organic fractions. Strains were cultivated in plate at 37 ℃. After 24 h,a plate-hole diffusion assay was performed using test microorganism strains from the Microorganism Culture Collection of Departamento de Antibióticos, Universidade Federal de Pernambuco (UFPEDA).Species included for testing were: Pseudomonas aeruginosa (P.aeruginosa); Staphylococcus aureus (S. aureus); Escherichia coli (E.coli); Shigella flexneri (S. flexneri); Enterococcus faecalis (E. faecalis);Salmonella enteritidis (S. enteritidis) and Klebsiella pneumoniae (K.pneumoniae). Nutrient medium (7 mL) was added to the Petri dishes,after refining, 25 mL of Mueller Hinton agar medium containing 106 cells of the strains were added, where four 6 mm diameter holes were formed with the aid of a mold forming the wells. The wells were filled with 20 μL of the fractions to be tested.
2.12.2. Determination of MIC and minimum bactericidal concentration (MBC)
Antimicrobial activity was determined by microdilution in plates of 96 wells, according to the methods prescribed by the Clinical and Laboratory Standards Institute[20]. Mueller Hinton broth was distributed in wells and fractions were added at concentrations ranging from 1 600 to 6.25 μg/mL. Then, 10 μL of standardized microbial inoculum, containing 1.5 ×106CFU/mL of S. aureus;E. faecalis; E. coli; P. aeruginosa; S. flexneri; S. enteritidis and K.pneumoniae, was added. Microplates were cultured at 37 ℃ for 24 h.Microplates were then stained with 0.01% resazurin and incubated for 1 to 4 h to observe color changes. The MBC was determined by establishing subculture wells on Petri dishes containing the Mueller Hinton agar and incubating at 37 ℃ for 24 h.
3. Results
3.1. Characterization of leaves biomass
Structural and chemical compositions found in leaves from C.erectus, showed a high concentration of cellulose (28.69 ± 0.08)%and lignin (20.77 ± 1.07)%. The concentrations of hemicellulose(18.71 ± 0.50)% and pectin (15.51 ± 0.04)% also were identified.Moreover, (9.95 ± 0.01)% of extractives of this plant were distributed between primary and secondary metabolites and (5.22 ± 0.21)%constituted the total ashes.
The determination of sugars, organic acids and products of degradation, showed the presence of glucose (0.45 g/L), xylose (0.3 g/L), galacturonic acid (0.12 g/L), hydroxymethylfurfural (0.002 3 g/L) and furfural (0.001 1 g/L) in leaves from C. erectus. Moreover,other compounds like formic and acetic acids, cellobiose and arabinose were not found in our analyses.
Metal ions quantification of leaves of C. erectus L., demonstrated the high presence of Na [(55.126 ± 18.65) μg/L] and K [(31.163± 6.88) μg/L) ions. The presence of Zn [(0.350 ± 0.435) μg/L], Fe[(0.291 ± 0.159) μg/L] and Cu [(0.244 ± 0.065) μg/L] ions, and absence of Mn, Cr, Cd, Pb and Ni were also identified.
3.2. Results obtained from the organic fractions of leaves of C. erectus
3.2.1. Extraction yield and phenolic compounds obtained in organic fractions
Analysis of extraction yield of three organic extracts of leaves of C. erectus showed high yield of ethanolic fraction (37.86% of dry weight) in relation to others. Moreover, this same fraction also showed higher phenolic compounds amounts (340.53 mg/g GAE)followed by ethyl acetate and hexanic fractions, respectively (Table 1).
The phytochemical study identified important compounds in the hexanic, ethyl acetate and ethanolic fractions. These compounds were analyzed through mass spectrometry attached with UPLC-MS and GC-MS. The name, retention time, area, structure molecular and mass of the compounds of the hexanic, ethyl acetate and ethanolic fractions are shown in the Table 2 , 3 and 4 respectively,corresponding to the peaks identified in the chromatograms illustrated in Figure 1A-C.

Table 2 GC-MS spectral analysis of hexanic fraction of C. erectus leaves.
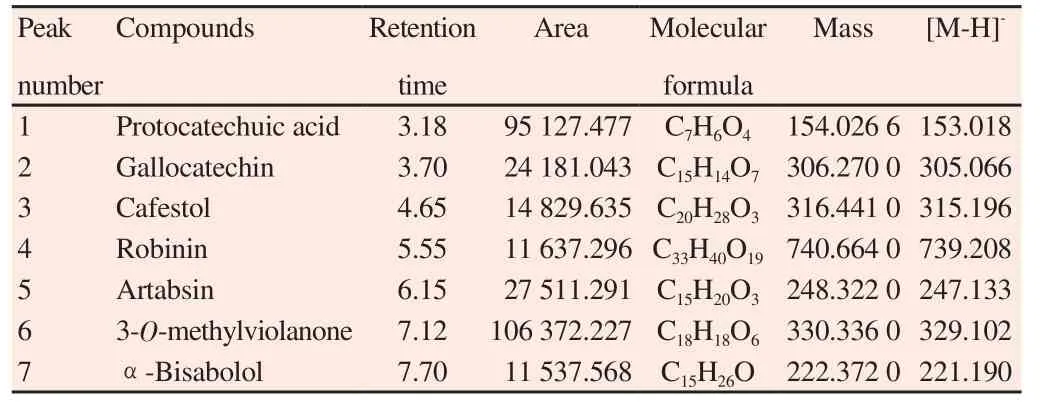
Table 3 UPLC-MS spectral analysis of ethyl acetate fraction of C. erectus leaves.

Table 4 UPLC-MS spectral analysis of ethanolic fraction of C. erectus leaves.
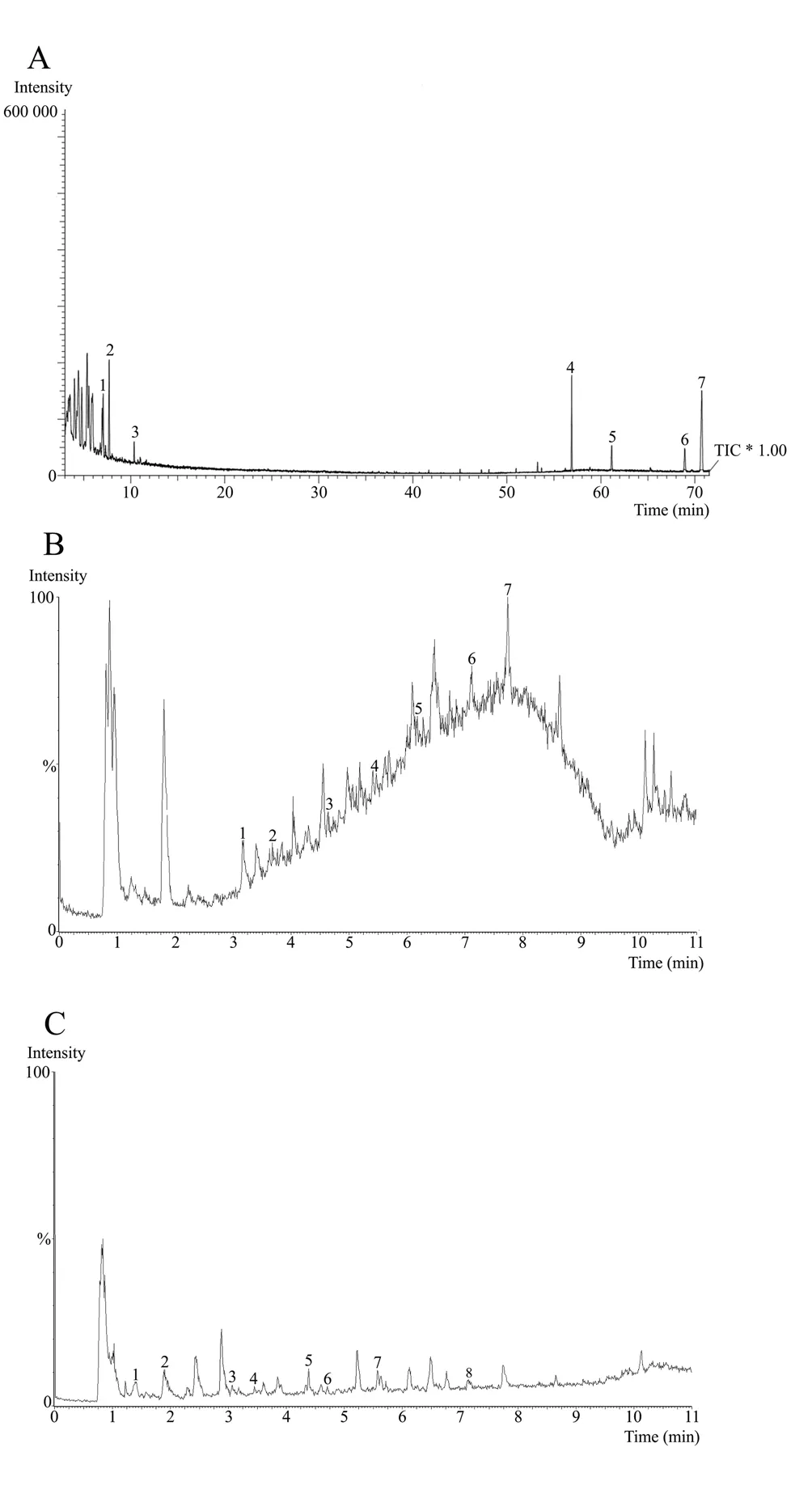
Figure 1. GC-MS analysis of hexanic fraction of C. erectus leaves (A),which shows the resulting total ion current chromatogram (TIC); UPLC-MS chromatogram of ethyl acetate fraction of C. erectus leaves (B); UPLC-MS chromatogram of ethanolic fraction of C. erectus leaves (C).
3.2.2. Antioxidant profile promoted by organic fractions
The hexanic fraction exhibited no antioxidant activity measured by the stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) and exhibited low total antioxidant activity (2.55 ± 0.01)% in phosphomolybdenum technique. However, the ethanolic and ethyl acetate fractions presented good antioxidant activities as illustrated in Table 5 .

Table 5Antioxidant activities promoted by organic fractions of C. erectus leaves.
3.2.3. Antimicrobial action promoted by organic fractions
The ethanolic fraction from C. erectus leaves showed high fungistatic potential for all Candida species tested, as shown in Table 6. Hexanic and ethyl acetate fractions showed moderate inhibitory activity with MIC50= 0.5 mg/mL, for C. glabrata. However, no fungicidal activity was detected for the three fractions.
Regarding to antibacterial effect, the ethanolic fraction showed to be the most active presenting inhibition halos between 14.5 and 27.5 mm for the strains tested. In addition, this fraction has proven to have moderate bacteriostatic and bactericidal activity for the S.aureus strain. E. faecalis, S. flexneri and S. enteritidis also showed sensitivity to ethanolic extract according to Table 7. The ethyl acetate fraction presented significant inhibition halos, between 19 and 22 mm. However, it showed low bacteriostatic activity with MIC50>1.6 mg/mL. P. aeruginosa strain presented resistance to both organic extracts. No antibacterial activity was detected to the hexanic fraction in the agar diffusion test, thus the microdilution assays were not performed for this fraction.

Table 6 Minimum inhibitory concentrations (MIC50) and minimal fungicidal concentrations (MFC) of organic fractions obtained from C. erectus leaves(mg/mL).
4. Discussion
The leaves of C. erectus showed to be rich sources of insoluble dietary fiber, being constituted mainly by cellulose (28.69 ± 0.08)%and lignin (20.77 ± 1.07)%. In fact, lignins are present in a wide variety of foods being particularly abundant in cereal bran[21].Moreover, cellulose also has been applied in several industrial sectors, including the food, pharmaceutical, cosmetic and plastic industries[22]. Hemicellulosic polysaccharides (like glucose and xylose) also have been suggested as a potential functional food ingredient and are involved with modulation of colon microflora and stimulation of immunological cells[12]. High levels of metallic ions found in C. erectus also corroborates with functional food idea, because sodium and potassium are elements associated with biochemical processes such as organic hydroelectrolyte balance[23].Leaves from Avicennia schaueriana, Laguncularia racemosa and Rhizophora mangle, native plants of the mangrove ecosystem, also demonstrated to be rich in Na and K ions, and we suggest that this parameter (high presence of ions) is common in plants of this ecosystem type[24].
Total phenolic compounds were found in high concentrations in organic fractions investigated in this study, specially the ethanolic[(340.53 ± 0.84) mg/g GAE] and ethyl acetate [(221.26 ± 1.84) mg/g GAE] fractions. These values were higher compared with the study results from Raza et al.[25] who performed studies investigating the ethanolic extract obtained of aerial parts of C. erectus (58.23 mg/g GAE), and from Abdel-Hameed et al.[10] who investigated ethyl acetate fraction obtained from leaves of this plant [(186.21 ± 6.89)mg/g GAE]. Although hexanic fraction of this study has shown low values of phenolic compounds [(10.04 ± 0.24) mg/g GAE], the values were still higher than those from Raza et al.[25] studies with the same fraction [(0.43 ± 0.01) mg/g GAE].
Flavonoids content was not detected in hexanic fraction of this study. However, ethyl acetate fraction [(182.65 ± 1.72) mg/g QE]followed by ethanolic fraction [(32.56 ± 0.12) mg/g QE] presented significant amounts. Raza et al.[25] did not found detectable amounts of flavonoids in their hexanic fraction and showed lower values to ethyl acetate fraction in relation to our results (5.41 mg/g QE). Moreover,other authors presented low concentration of flavonoids in defatted methanolic fraction of fruits of C. erectus [(29.1 ± 0.7) mg/g QE][26].This variation in the concentrations of secondary metabolites identified in the extracts may have occurred due to extraction method, solvent used, type of plant samples and environmental factors[27].
The GC-MS and UPLC-MS analysis reinforce these phytochemical findings and showe new compounds. In the hexanic fraction, we can identify alkenes and triterpenoids compounds (peak number 1-5 and 6-7, respectively). These classes of compounds are known for their anti-inflammatory, immunomodulatory, antimicrobial and virostatic actions[28]. In the ethyl acetate fraction, we identify a phenolic acid (peak number 1), flavonoids (peak numbers 2, 4 and 6) and terpenoids (peak numbers 3, 5 and 7). The ethanolic fraction was the fraction that showed more different compounds identified.The classes of compounds were sesquiterpene alcohol, phenolic acid, terpenoid, flavonoid (peak numbers 1 to 4, respectively),flavones (peak numbers 5 and 7), diterpenoid (peak number 6) and polyphenol (peak number 8).
Numerous phenolic compounds have been studied for their biological properties and benefits to human health like antioxidant,antimicrobial, antiviral and anti-inflammatory[29]. These compounds act on the microbial cell altering the cellular permeability, damagingthe cytoplasmic membrane and interfering with the energy generation system, finally leading to cell death[30]. Terpenoids represent a class of secondary metabolites and just like the plant polyphenols, have attracted interest of the researchers for their benefits to medicinal chemistry due to its pharmacological potential[31,32]. Phenolic acids as well as the flavonoids also show antioxidant, antitumor and antimicrobial properties[29].

Table 7 Inhibition halos, minimum inhibitory concentrations (MIC50) and minimal bactericidal concentrations (MBC) of the ethyl acetate and ethanolic fractions of C.erectus leaves.
Antioxidant results in this study showed that ethyl acetate and ethanolic fractions at 0.5 mg/mL concentration were able to sequester approximately 71% of the DPPH free radical, presenting a similar result showed by Raza et al.[25], who evaluated the same ethanolic extract of C. erectus, but worked at 1 mg/mL concentration.The total antioxidant activity detected by the phosphomolybdenum method in ethyl acetate and ethanolic fractions [(10.89 ± 0.05)%and (9.17 ± 0.03)%, respectively] was approximately the twice of the antioxidant activity of BHT (4.12 ± 0.10)%, the most commonly used synthetic antioxidant in the food and pharmaceutical sectors[33]. The hexanic fraction was not able to sequester DPPH free radical but showed a half value in relation to BHT standard in the phosphomolybdenum method. This antioxidant profile of C. erectus is also related to the phenolic compounds present in the plant[34].
According to Vieitez et al.[30], extracts of plants can be classified,considering the MIC, as strong inhibitors: MIC50≤ 0.5 mg/mL,moderate inhibitors: MIC50between 0.6 and 1.6 mg/mL or weak inhibitors: MIC50> 1.6 mg/mL. In this study, different strains for both bacteria and fungi were treated with three organic fractions.Antifungal results demonstrated that ethanolic extract presented high inhibitory action against all Candida strains tested (fungistatic activity between 0.125 and 0.25 mg/mL) and, ethyl acetate and hexanic fractions had high fungistatic activity against C. glabrata with MIC50= 0.5 mg/mL for both. Terças et al.[35] investigated the antifungal action promoted by ethanolic extract from leaves of Terminalia catappa, also belonging to Combretaceae family, which showed weak activity against C. tropicalis (3 mg/mL) and moderate activity against C. albicans (1.5 mg/mL), C. glabrata (0.75 mg/mL)and C. krusei (0.75 mg/mL).
In the opinion of Estevam et al.[36], zones of inhibition ≥ 10 mm have been considered relevant for assays with plant extracts. Because of this, plants which present this value or superior, are considered as antimicrobial agents of moderate action. Although hexanic fraction not showed antibacterial activity, our antibacterial results point to a moderate antibacterial activity of ethanolic fraction against S.aureus and high activity against other strains investigated (zones of inhibition between 14.5 and 27.5 mm. The ethanolic fraction also developed moderate bacteriostatic and bactericidal activity on S.aureus, S. flexneri and S. enteritidis and, the ethyl acetate fraction did not present significant values of MIC50and MBC.
Several studies have demonstrated the antibacterial potential of extracts, fractions and molecules isolated from the leaves of C.erectus. Abdel-Hameed et al.[10] showed that the methanol extract from leaves can form inhibition halos of (21.5 ± 0.31) mm for S.aureus and Santos et al.[9] showed that the aqueous extract from leaves can form inhibition halos of 10 mm for multidrug resistant S. aureus isolated from cutaneous wounds. In addition, Shohayeb et al.[26] showed similar results for ethanolic extract of leaves,with significant values of MIC50[(0.67 ± 0.29) mg/mL] and MBC[(1.33 ± 0.58) mg/mL] against S. aureus. However, different from our results, these same authors showed high values in ethyl acetate fraction against S. aureus (MIC50= 0.21 mg/mL).
In conclusion, C. erectus can be used as a source of functional food,and shows antioxidant and antimicrobial properties, with consequent health benefits. These findings can contribute to the knowledge about the effectiveness of the plant use and in development of the future phytotherapic compound.
Conflict of interest statement
The authors declare there is no conflict of interests.
Acknowledgements
We would like to thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for the grant of a master’s degree scholarship during the entire research period. We also would like to thank CETENE (Centro de Tecnologias Estratégicas do Nordeste)that granted us technical support to carry out the analyzes in UPLCMS.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Spatial distribution of sand flies (Diptera: Psychodidae; Larroussius group), the vectors of visceral leishmaniasis in Northwest of Iran
- Effects of physicochemical factors on development and survival of Opisthorchis viverrini uterine eggs
- Identification of commonly regulated protein targets and molecular pathways in PC-3 and DU145 androgen-independent human prostate cancer cells treated with the curcumin analogue 1,5-bis(2-hydroxyphenyl)-1,4-pentadiene-3-one
- Add-on therapy of herbal formulation rich in standardized fenugreek seed extract in type 2 diabetes mellitus patients with insulin therapy: An efficacy and safety study
- Anticancer activity of crude acetone and water extracts of Tulbaghia violacea on human oral cancer cells
