Anticancer activity of crude acetone and water extracts of Tulbaghia violacea on human oral cancer cells
2018-10-12SamkelisoTakaidzaArumugamMadanKumarCorneliusCanoSsemakaluNagabishekSirpuNateshGayathriKaranamMichaelPillay
Samkeliso Takaidza, Arumugam Madan Kumar, Cornelius Cano Ssemakalu, Nagabishek Sirpu Natesh, Gayathri Karanam, Michael Pillay✉
1Department of Biotechnology, Vaal University of Technology, Private Bag X021, Vanderbijlpark, 1900, South Africa
2Cancer Biology Lab, Molecular and Nanomedicine Research Unit, Sathyabama Institute of Science and Technology, Chennai, India
Keywords:Anticancer activity Antioxidant Apoptosis Caspase Cell cycle Tulbaghia
ABSTRACT Objective: To evaluate the anticancer activity of crude acetone and water leaf extracts of Tulbaghia violacea on a human oral cancer cell line (KB). Methods: The antioxidant activity of the leaf extracts was evaluated by using the DPPH assay while the anti-proliferative activity was assessed by using the MTT assay. The morphological characteristics of apoptotic cells were examined by using the dual acridine orange/ethidium bromide staining. Flow cytometry was used to evaluate the induction of multi-caspase activity and changes in the cell cycle.Results: The acetone and water extracts exhibited antioxidant activity in a concentration dependent manner. The extracts inhibited the growth of the KB cell line with IC50 values of 0.2 mg/mL and 1 mg/mL, respectively for acetone and water. Morphological changes such as cell shrinkage, rounding and formation of membrane blebs were observed in the treated cells.In acridine orange/ethidium bromide staining, the number of apoptotic cells increased as the concentration of the extracts increased. The activation of multi-caspase activity in KB cells treated with Tulbaghia violacea extracts was concentration dependent, leading to cell death by apoptosis and cell cycle arrest at the G2/M phase. Conclusions: The acetone and water extracts of Tulbaghia violacea appear to have anti-cancer activity against human oral cancer cells and need to be investigated further.
1. Introduction
Many types of cancer are known to affect the human population[1].Oral cancer is the 8th most common cancer worldwide, with a high prevalence in South Asia[2] and Eastern and Southern Africa[3] with a higher occurrence in males than in females[4]. Oral cancer can result from poor life style choices such as smoking tobacco and consumption of alcohol that are considered as major risk factors in the development of this type of cancer[5,6]. Tobacco contains known carcinogens such as N’-nitrosonornicotine and aromatic hydrocarbon benzo-pyrene, N-nitrosomine (4-nitrosomethylamino)-1-(3-pyridyl)-1-butanone (NNK). These compounds have been linked to oncogenesis capable of inducing tumours of the oral and nasal cavities, lungs, oesophagus and pancreas[7]. Biological agents can also cause oral cancer. Herrero[8] showed that the human papilloma virus was able to cause oral carcinoma. Genetic predisposition is another important risk factor in the development of oral cancer. Evidence shows that certain individuals inherit genetic dispositions that result in the inability to metabolize carcinogens as well as repair DNA damage thus causing cancer[7]. Treatment of oral cancer has been achieved through the use of either surgery or radiation or both depending on the severity of the disease. These treatment options result in side effects such as vomiting, nausea, hair loss, fatigue, mouth sores and complications like mucositis[9]. In addition, these cancer treatment options are expensive and inaccessible to people living in resource limited or poor communities. Due to the inaccessibility and cost of cancer treatment, many communities rely on medicinal plants to treat the disease. For example, anecdotal evidence reveals that many people in the rural parts of the Eastern Cape Province in South Africa rely on Tulbaghia species to treat cancer with various degrees of success[10,11].Therefore it is imperative that empirical evidence to substantiate such claims be established.
Tulbaghia is a genus of herbaceous perennial bulbous plant in the family Amaryllidaceae. It is predominantly found in Southern Africa[10]. One of the species Tulbaghia violacea (T. violacea) known as wild garlic, wilde knoffel (Afrikaans), isihaqa (Zulu), or itswele lomlambo (Xhosa) is widely distributed and commonly used to treat oesophageal cancer and other ailments in traditional medicine[11].The plant is rich in sulphur-containing compounds[12,10] which may be contributing to its characteristic odour and medicinal properties.Nonetheless, the mechanism of action through which Tulbaghia extracts are able to slow down cancerous growth remains unclear. To understand the role of T. violacea in inhibiting cancerous growth, one needs to understand cancer at the cellular level.
Cancer development is due to the inability of cells to undergo apoptosis[13]. One of the ways of treating cancer is to restore apoptosis. Strategies aimed at inhibiting cell proliferation through the activation of apoptosis, suppressing angiogenesis and metastasis is key strategy towards the discovery of anticancer drugs[14]. Of these, apoptosis has been widely studied and has been recognised as an ideal way to eliminate malignant cells[15]. The key process in apoptosis is the stimulation of a caspase cascade of signalling events,which is controlled via both the extrinsic and intrinsic apoptosis pathways[16,17]. Apoptotic cell death is marked by morphological changes such as cell shrinkage, chromatin condensation, membrane blebbing and fragmentation of DNA[18,19]. These morphological and biochemical markers of apoptosis enable its distinction from other forms of cell death[20]. The induction of apoptosis in tumour cells is considered to be useful in the management, treatment and prevention of cancer. Screening apoptotic inducers, either in the form of crude extracts or as purified bioactive compounds, is a crucial step toward cancer treatment[21]. This study investigated the ability of crude acetone and water leaf extracts of T. violacea to suppress the proliferation, activate apoptosis and initiate cell cycle arrest in a human oral cancer cell line (KB).
2. Materials and methods
2.1. Plant materials
Samples of T. violacea were purchased from an indigenous plant nursery in Gauteng, South Africa and kept in the greenhouse at the Vaal University of Technology, Vanderbijlpark, South Africa as in the study by Takaidza et al.[22]. The plant was authenticated by Professor Stefan Seibert at North West University, Potchefstroom,South Africa. A voucher specimen (ST0008) was deposited in the AP Goossens Herbarium.
2.2. Preparation of T. violacea acetone and water crude extracts
The preparation of acetone and water extracts of T. violacea was done following the protocol previously described by Takaidza et al.[22]. Crude leaf extracts were prepared by homogenizing 10 g of T. violacea leaves in 100 mL of absolute acetone or sterile distilled water. The acetone homogenate was macerated for 24 h and then filtered through No. 1 Whatman filter paper. The acetone filtrate was allowed to evaporate in a fume hood and the obtained extract was kept at 4 ℃ until required for further analysis. The water homogenate was boiled for 10 min in a water bath at 100 ℃,cooled down and then filtered through a No. 1 whatman filter paper.Thereafter the filtrate was kept at -20 ℃ for 24 h, lyophilized and then the powder stored at 4 ℃ in an airtight container for further use.
2.3. Antioxidant activity
One mL of either acetone or water crude leaf extract at concentration ranging from 50 μg/mL to 500 μg/mL was mixed with 1 mL of 0.12 mM DPPH solution. Thereafter 300 μL of the mixture was dispensed in triplicate into 96 well plates. The 96 well plates were incubated at room temperature in the dark for 30 min,following which the absorbance was measured at 517 nm using a microplate reader (Perklin Elmer, Walthman, MA). L-ascorbic acid was used as a positive control. The radical scavenging activity was expressed as percentage inhibition and calculated using the below formula:
% scavenging activity = [(Acontrol-Atest)/Acontrol] × 100
Where Acontrolis the absorbance of the control (DPPH solution without test sample) and Atestis the absorbance of the test sample(DPPH solution plus antioxidant). The inhibitory concentration(IC50) value denotes the concentration of sample which is required to scavenge 50% of DPPH free radicals.
2.4. Establishment of human oral cancer cell line
A human oral cancer (KB) cell line was procured from NCCS,Pune, India and maintained at a cancer biology laboratory at Sathyabama Institute of Science and Technology, Chennai, India.This cell line was grown in complete culture medium consisting of high glucose DMEM (GE Health Life Sciences, Logan, UT)supplemented with 10% FBS (ThermoScientific, Cramlington,Northumberland) and 1 × penicillin at 37 ℃ in a humidified atmosphere at 5% CO2(Galaxy 170 S-CO2Incubator, Eppendorf,Hamburg, Germany).
2.5. Inhibition of KB cell growth
The MTT assay was used to assess the inhibitory effects of the crude acetone and water leaf extracts of T. violacea on the oral cancer cells. The KB cells were seeded in 96-well plates and incubated for 24 h at 37 ℃ in a humidified 5% CO2incubator. After 24 h the cells were washed three times with PBS buffer. The cells were then treated with acetone and water extracts prepared in culture media at concentrations ranging between 0.003 mg/mL and 2 mg/mL.Culture medium was used as a negative control. The cells were incubated for 24 h after which the media were aspirated and the cells washed twice with PBS buffer. Thereafter, 100 μL of the culture media was added into each well followed by addition of 10 μL of MTT solution (5 mg/mL in phosphate-buffered saline).The plates were then incubated for 4 h after which 85 μL of the media was removed from each well and 100 μL of DMSO was added. The plates were then gently shaken to solubilize the formazan. The amount of formazan produced was then measured at 570 nm using a microplate reader (Perklin Elmer). The percentage viability of KB cells was calculated as follows:Cell viability (%) = [(absorbance of untreated control - absorbance of treated sample)/absorbance of untreated control)] × 100.
The inhibitory concentration (IC50) was calculated from the straight line graph plotted in the Excel software using percentage viability.These IC50values were used in subsequent experiments.
2.6. Morphological observations
The morphological features of the treated KB cells were examined using microscopy. The KB cells were seeded in 12-well plates and incubated for 24 h at 37 ℃. After 24 h the cells were treated with T.violacea acetone and water crude leaf extracts at 0.5 × IC50, 1 × IC50and 2 × IC50. The 12-well plates were then incubated at 37 ℃ in a humidified 5% CO2incubator. After 24 h images were taken using a microscope (Evos-FL-AMG, Life Technologies, Bothwell, WA) at 20 ×magnification.
2.7. Acridine orange/ ethidium bromide (AO/EB)
Dual AO/EB staining was used to visualise cells undergoing apoptosis.The KB cells were treated as explained in section 2.6. Untreated cells were taken as negative control while positive control cells were treated with melphalan. After 24 h the cells were washed with PBS twice and 100 μL of PBS was then added to all the wells. Thereafter, 5 μL of acridine orange (100 μg/mL) was added into each well followed by the addition of 5 μL ethidium bromide (100 μg/mL) after 5 min. The plates were covered with foil and incubated for 10 min at room temperature before visualising and imaging under a fluorescence microscope (Evos-FL- AMG, Life Technologies, Bothwell, WA).
2.8. Multicaspase activity
The multicaspase activity in the KB cells stimulated with either acetone or water extracts of T. violacea as in section 2.6 was evaluated using the Multicasapase SR kit (Cat. No. 4500-0500,Merck KGaA, Darmstadt, Germany) following the manufacturer’s instructions. In brief, 10 μL of the caspase working solution was added to 100 μL of 1 × 106cells/mL and incubated for 1 h at 37 ℃in a CO2incubator. Thereafter, 100 μL of 1× apoptosis wash buffer was added to each tube. The samples were then centrifuged for 5 min at 300 × g and the supernatant was discarded. Exactly 200 μL of 1× apoptosis wash buffer was added to the tubes, the sample was homogenised, then centrifuged for 5 min at 300 × g and the supernatant was discarded. The cells were then suspended in 200 μL of the caspase 7- aminoactinomycin D (7-AAD) working solution and incubated for 10 min at room temperature. Each sample was analysed with the Guava Easy Cyte 12HT flow cytometer (EMD Millipore Corp, Bellerica, MA ) using 10 000 events.
2.9. Cell cycle analysis
The cell cycle analysis was conducted to determine the percentage of cells in G0/G1, S and G2/M phases based on DNA content. The Guava cell cycle reagent (cat # 4500- 0220, MERCK) was used following the manufactures’ instructions. Briefly, about 200 KB cells per microliter were treated with either T. violacea acetone or water leaf extracts as in section 2.6 for 24 and 48 h. Untreated cells were taken as negative control while positive control cells were treated with melphalan. After each time point, both the treated and controls were harvested and washed with 1× PBS twice. The cells were fixed with ice-cold ethanol and kept at 4 ℃ for at least 12 h before staining. The cells were stained with propidium iodide. The samples were transferred to 96 well plates and then incubated at room temperature for 30 min before acquiring 10 000 events using the Guava Easy Cyte 12HT system. The experiment was done in triplicate.
2.10. Statistical analysis
The experimental results were expressed as mean ± standard error(SE) of three replicates. P values less than 0.05 were considered statistically significant. Microsoft Excel 2010 statistical package was used.
3. Results
3.1. Antioxidant activity of crude acetone and water extracts of T. violacea
The free radical scavenging activity of the crude acetone and water extracts of T. violacea was assessed using the DPPH assay.Figure 1 shows that there was a concentration dependent free radical scavenging activity by both extracts ranging from 49% to 73%. The acetone crude extract exhibited a radical scavenging activity which increased from 51% to 68% as the concentration of the extract increased while for the water extract it increased from 49% to 73%.Although the water extract showed a slightly greater ability to scavenge free radicals (Figure 1) than that of acetone, there was no significant difference (P > 0.05) between the scavenging activities of the two extracts. The free radical scavenging ability of ascorbic acid which was approximately 100%, remained significantly higher(P < 0.05) than that of both extracts irrespective of the concentration(Figure 1). The IC50values for ascorbic acid, acetone extract and water extract were found to be 0.002 μg/mL, 207.33 μg/mL and 168.88 μg/mL respectively.
3.2. Effect of acetone and water leaf extracts on viability of KB cells
The inhibitory effect of crude acetone and water leaf extracts of T.violacea was evaluated using the MTT assay. The acetone extract exhibited a dose dependent inhibitory effect on the treated KB cells as shown in Figure 2. At concentrations 0.003 mg/mL to 0.1 mg/mL there was no significant difference (P > 0.05) in the viability of the control and the treated cells while for concentrations ranging from 0.25 mg/mL to 2 mg/mL a significant difference was observed (P <0.01). In a similar manner, there was no significant difference (P >0.05) in the cells treated with water extracts at concentrations between 0.003 mg/mL and 0.05 mg/mL compared to the control. A significant difference (P < 0.05) was observed between the control and the treated cells when the concentration of the water extracts was increased from 0.1 mg/mL to 2 mg/mL.
Treatment with acetone showed a higher inhibitory effect compared to water extract. The IC50values for acetone and aqueous extracts were 0.2 mg/mL and 1 mg/mL, respectively. These IC50values were used in subsequent experiments.

Figure 1. Percentage free radical scavenging activity of acetone and water crude leaf extracts from T. violacea examined using the DPPH assay.

Figure 2. Cell viability of KB cells assessed using the MTT assay.
3.3. Morphological observations
When the KB cells were treated with acetone extracts at 0.5 ×IC50(0.1 mg/mL), 1 × IC50(0.2 mg/mL) and 2 × IC50(0.4 mg/mL),a decrease was observed in the number of cells with increased concentration of the extract (Figure 3). Cell shrinkage, formation of membrane blebs and rounding of the cells were observed in all treatments with acetone extracts at 0.5 × IC50, 1 × IC50and 2 × IC50(Figure 3). Similar observations were made in treatments involving the water extracts at 0.5 × IC50(0.5 mg/mL), 1 × IC50(1 mg/mL)and 2 × IC50(2 mg/mL). A concentration dependent reduction in the number of cells was observed. At 0.5 × IC50only cell shrinkage was observed. Cell shrinkage, formation of membrane blebs and rounding of the cells were observed in water extracts at concentrations of 1 ×and 2 × IC50(Figure 3).
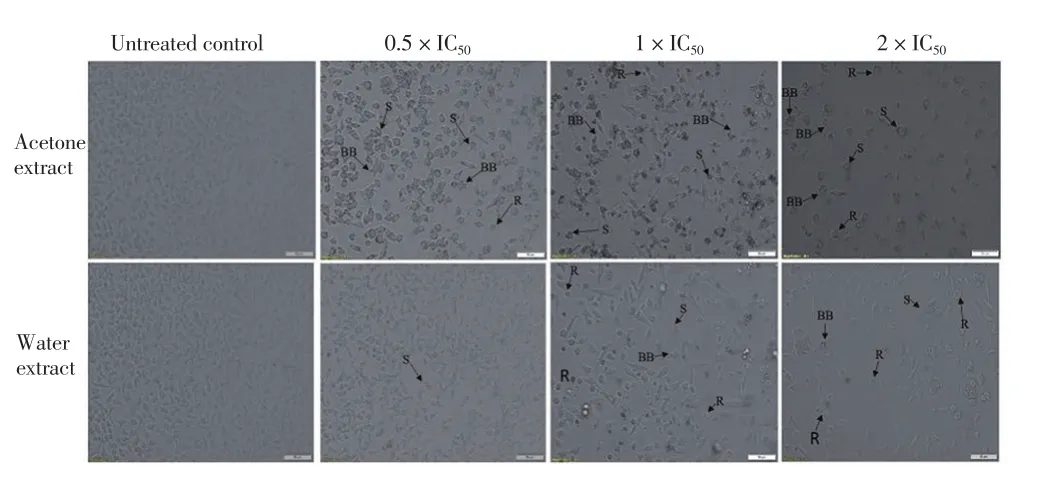
Figure 3. Microscopy images of KB cells treated with acetone and water extracts at inhibitory concentrations (IC50), 0.5 × IC50 (0.1 mg/mL), 1 × IC50(0.2 mg/mL), 2 × IC50 (0.4 mg/mL) and 0.5 × IC50 (0.5 mg/mL), 1 × IC50 (1 mg/mL), 2 × IC50 (2 mg/mL), respectively.
3.4. AO/EB staining
Microscopic images of the KB cells treated at 0.5 × IC50of acetone(0.1 mg/mL) and water (0.5 mg/mL) extracts showed yellow/green staining, indicating the induction of apoptosis (Figure 4). The number of apoptotic cells increased, as indicated by yellow staining of cells, as the concentration of the extracts increased (Figure 4). At concentrations of 1 × IC50for acetone (0.2 mg/mL) and water (1 mg/mL) extracts there was a higher number of cells staining bright yellow compared to those at lower concentrations. At a higher concentration of 2 × IC50, the number of cells that stained red increased, indicating necrosis of the cells. In general, there were a higher number of apoptotic cells in the acetone treatment compared to those treated with water.
3.5. Multicaspase activity
The apoptotic effect of the acetone and water extracts was examined on KB cells using the Multicaspase SR kit. Flow cytometry analysis showed that the acetone and water extracts induced apoptosis in KB cells (Figure 5A). Although, the acetone extract induced apoptosis in a concentration dependent manner (9.8% at 0.1 mg/mL, 14.9% at 0.2 mg/mL and 18.7% at 0.4 mg/mL), it also induced necrosis in a similar manner at higher percentages (23.3% at 0.1 mg/mL, 39.2% at 0.2 mg/mL and 48.2% at 0.4 mg/mL). On the other hand, treatment of KB cells with water extract at 0.5 mg/mL induced greater levels of apoptosis (6.8%) compared to necrosis (1.7%). The percentage of apoptosis was 22.6% and 30.5% at concentrations of 1 mg/mL and 2 mg/mL, respectively whereas the necrosis levels were 1.9% and 0.9% for the same extract concentrations, respectively. Both extracts significantly (P < 0.05) induced apoptosis in a dose dependent manner compared to the untreated control (Figure 5B).

Figure 4. Illustration of dual AO/EB staining images of KB cells treated with crude acetone [0.5 × IC50 (0.1 mg/mL), 1 × IC50 (0.2 mg/mL) and 2 × IC50(0.4 mg/mL)] and water [0.5 × IC50 (0.5 mg/mL), 1 × IC50 (1 mg/mL) and 2 ×IC50 (2 mg/mL)] leaf extracts from T. violacea.

Figure 5. Multicaspase activity result.
3.6. Cell cycle analysis
Treatment of the KB cells with crude acetone extracts at concentrations of 0.1 mg/mL, 0.2 mg/mL and 0.4 mg/mL for 24 h resulted in a decrease in the percentage of cells in the G0/G1phase of the cell cycle and a dose dependent increase in the G2phase (Figure 6A). After 48 h treatment with the same concentrations of the extract,a significant (P < 0.05) decrease in the G1/G0and an increase in the G2phase were observed (Figure 6B). In a similar manner, 24 h treatment of the KB cells with crude water extract at concentrations of 0.5 mg/mL, 1 mg/mL and 2 mg/mL resulted in a reduction in the percentage of cells in the G0/G1phase and an increase in the cell population in the G2phase as the concentration of the extract increased (Figure 6C). After 48 h treatment, a more pronounced decrease in the G1/G0and an increase in the G2phase of the cell cycle were observed (Figure 6D). The 24 h and 48 h treatment of the KB cell line with melphalan, a positive control, showed an increase in S phase cell population.
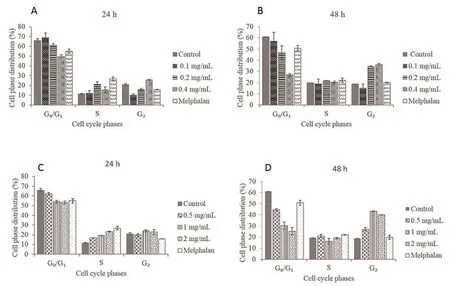
Figure 6. Effect of T. violacea acetone and water extracts on cell cycle in KB cells.
4. Discussion
Bioactive compounds such as phenolic acids, terpenoids, lignans,tannins, flavonoids, coumarins, quinones, and alkaloids which show significant antioxidant and anti-inflammatory activities from certain medicinal plants, have played an important role in treatment of cancer[23]. Although T. violacea is widely used in traditional medicine,little research on the anticancer activity of the plant is available. In this study, the antioxidant activity of crude acetone and water extracts of T.violacea at a concentration of 500 μg/mL was approximately 68% and 73%, respectively. Phyto-active compounds such as phenols, flavonoids and saponins are present in T. violacea[24] and are probably responsible for the level of antioxidant activity observed in this study. Secondary metabolites such as phenolic components are potential antioxidants and free radical terminators[22]. Free radicals such as reactive oxygen species (ROS) contribute to the initiation of oncogenesis[25].Therefore, inhibition of these free radicals can potentially aid in the prevention of cancer progression[26]. The inhibitory effect of acetone and water extracts of T. violacea on the growth of human oral cancer cells was observed to be dose dependent with IC50values of 0.2 mg/mL and 1 mg/mL, respectively. Cell death of KB suggests the potential of the crude extracts of T. violacea to inhibit the progression of cancer. Cell death can occur via apoptosis or necrosis. To determine the cause of cell death, morphological and biochemical markers were examined. Morphological features of cells undergoing apoptosis include cell shrinkage, rounding of the cells and the formation of membrane blebs. All these morphological changes were observed in the KB cells, indicating that the KB cells did undergo apoptosis. Staining of the cells with the AO/EB dual stain is a further way of indicating apoptosis or necrosis. Acridine orange is a fluorescent dye that stains nuclear DNA when the cell membrane is intact whereas ethidium bromide stains cells that have lost membrane integrity[27]. Treatment of KB cells with acetone and water extracts of T. violacea resulted in an increase in the number of apoptotic cells in a dose dependent manner. The cells treated with acetone and water extracts at concentrations 0.4 mg/mL and 2 mg/mL, respectively, showed a higher number of cells that stained yellow and red indicating membrane disruption. Similar results were observed with the positive control, melphalan. The untreated cells had an even distribution of bright green staining which suggests that they did not undergo apoptosis. Multicaspase activity and cell cycle arrest were the molecular markers that were examined to further confirm the apoptotic effects of the acetone and water extracts on the KB cells. The activation of multicaspase activity in the KB cells treated with T. violacea acetone and water extracts was examined by flow cytometry. The human oral cells were stained with caspase reagent and 7-AAD which can enter the cells only when the plasma membrane is damaged. This allows early apoptosis cells (Caspase reagent +, 7 -AAD -) to be distinguished from late apoptosis (Caspase reagent +, 7-AAD +) and necrotic cells (Caspase Reagent -, 7-AAD+). The percentage of apoptotic cells following treatment with the acetone and water extracts at concentrations of 0.5 × IC50, 1 × IC50and 2 × IC50was 9.8%, 14.9%, 18.7% and 6.8%, 22.6%, 30.5%,respectively, compared to 1.8% in the untreated control. These results suggest that both the acetone and water extracts of T. violacea induced apoptosis in a dose dependent manner. However, it was observed that the acetone extract also induced necrosis as the concentration of the extract increased. Cysteine-dependent aspartate-specific proteases(Caspases) are triggered during the early stages of apoptosis. The caspases are synthesized as inactive zymogens; but once turned on,they can initiate a proteolytic deluge, resulting in the breakdown of key cellular components required for normal cellular function[28].Elevated caspase activity is regarded as a marker for apoptosis[29]and the observed cell death in this study most likely occurred through apoptotic induction.
Loss of DNA content is a common feature of apoptosis[30]. This is verified by staining cells with propidium iodide and enumerating the cells at different phases of the cell cycle. In this study, cell cycle analysis after treatment for 24 h and 48 h with both the acetone and water extracts of T. violacea showed an increase in the percentage of cells in the G2/M phase and a reduction in the G0/G1phase while no major changes were observed in the S phase. These results suggest that the extracts caused cell cycle arrest at the G2/M phase in the KB cells while the positive control, melphalan, induced S phase arrest. Recent studies have indicated that cancer can be viewed as a disease of the cell cycle due to inefficient cell cycle checkpoint control[28,31,32]. Cell cycle arrest of cancer cells is thus considered as one of the target mechanisms in cancer treatment. The continuous dividing ability of cells can be interrupted by blocking cells in G1, S,G2or M phases of the cell cycle. Therefore the ability of T. violacea extracts to arrest the cell cycle at G2/M phase in the human oral cancer cells is of significance. According to Teiten et al.[31] allicin metabolites and diallyl polysulfides found in Allium sativum have been reported to be strong activators of early mitotic arrest, aberrant tubulin depolymerisation that prevents the synthesis of normal spindle microtubules and interruption of microtubular dynamics,therefore leading to G2/M arrest. The genetic relationship between Allium sativum and Tulbaghia species may suggest that similar mechanisms of cell disturbances may be present in the species under consideration in this study.
The apoptotic effect of the T. violacea extracts observed in this study is in agreement with the findings of Saibu et al.[33] who demonstrated induction of apoptosis in a panel of four cancer cell lines treated with T. violacea water extract. The anticancer activity of the extracts of T. violacea can be attributed to some secondary metabolites such as phenolic compounds which have been shown to inhibit the formation of tumours by interfering with the growth,proliferation, metastasis as well as pro-apoptotic effects by blocking proteasome activity and suppressing angiogenesis[21].
In conclusion, acetone and water extracts of T. violacea exhibited inhibitory effects against the human oral cancer cells. This research provides strong evidence to suggest that death of the human oral cancer cells occurred through the induction of apoptosis and alteration of the cell cycle. Most cells showed cell cycle arrest at G2/M phase.This study also suggests that acetone and water extracts of T. violacea may have potential anticancer activity against human oral cancer cells, supporting the traditional use of T. violacea in cancer treatment.Future studies are required to isolate the specific compound(s) with anticancer activity from T. violacea.
Conflict of interest statement
The authors declare they have no conflict of interest.
Acknowledgements
This work was supported by NAM S & T Centre Research Training Fellowship for Developing Country Scientists, Sathyabama University, India and Vaal University of Technology, South Africa.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Spatial distribution of sand flies (Diptera: Psychodidae; Larroussius group), the vectors of visceral leishmaniasis in Northwest of Iran
- Effects of physicochemical factors on development and survival of Opisthorchis viverrini uterine eggs
- Identification of commonly regulated protein targets and molecular pathways in PC-3 and DU145 androgen-independent human prostate cancer cells treated with the curcumin analogue 1,5-bis(2-hydroxyphenyl)-1,4-pentadiene-3-one
- Add-on therapy of herbal formulation rich in standardized fenugreek seed extract in type 2 diabetes mellitus patients with insulin therapy: An efficacy and safety study
- Conocarpus erectus L., a plant with a high content of structural sugars, ions and phenolic compounds, shows antioxidant and antimicrobial properties promoted by different organic fractions
