Increased incidence of chemoport-related thrombosis in patients with colorectal cancer receiving bevacizumab: A singleinstitutional experience
2018-09-14JwaHoonKimJeongEunKimYongSangHongSunYoungKimKyupyoKimKiEunChoiJiHoonShinTaeWonKim
Jwa Hoon Kim, Jeong Eun Kim, Yong Sang Hong, Sun Young Kim, Kyu-pyo Kim, Ki Eun Choi,Ji Hoon Shin, Tae Won Kim
1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 138-736, Republic of Korea; 2Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 138-736, Republic of Korea
Abstract Objective: Chemoport-related thrombosis (CRT) is a serious complication that causes morbidities and interrupts administration of intravenous cancer therapy. We investigated the incidence and risk of CRT in colorectal cancer(CRC) patients treated with bevacizumab (BEV).Methods: We retrospectively reviewed 1,534 CRC patients who received chemotherapy with or without BEV using a chemoport between 2014 and 2016.Results: The participants had a median age of 58 (18−85) years, and 60.3% were male. All participants were stratified into three groups: adjuvant chemotherapy (AC) (n=670), palliative chemotherapy (PC) without BEV(n=356), and PC with BEV (n=508). The median follow-up was 20.19 (interquartile range, 14.07−27.19) months.CRT occurred in 3.8% of all patients; incidence of symptomatic and asymptomatic CRT was 2.9% and 0.9%,respectively. CRT occurred more in patients with BEV (5.7%) than in patients without BEV (2.9%, P=0.008). The cumulative incidence of CRT in patients administered PC with BEV was significantly higher than that in those administered AC (P=0.011) and there was a trend toward increased CRT in patients administered PC with BEV compared with those administered PC without BEV (P=0.044). Multivariate analysis found that BEV treatment was the only variable that was significantly associated with CRT (hazard ratio, 2.06; 95% confidence interval, 1.24−3.43;P=0.006).Conclusions: BEV treatment was significantly associated with increased incidence of CRT in CRC patients.
Keywords: Bevacizumab; central venous catheters; thrombosis; colorectal neoplasms
Introduction
Implantable central venous ports (chemoports) are used to administer conventional chemotherapy or moleculartargeted agents and for nutrition, transfusions, and blood sampling (1,2). Chemoports are safe for long-term venous access in cancer patients, but complications such as chemoport-related thrombosis (CRT) occur (3). CRT causes loss of central venous access and 10%−15% of cancer patients with CRT may experience a lifethreatening pulmonary embolism (4,5). Vessel injury resulting from multiple venipunctures, peripheral infusion of cytotoxic chemotherapy following loss of central venous access, or attempts to insert another central venous catheter may increase morbidity and delay cancer treatment (5).
Bevacizumab is a monoclonal antibody targeting vascular endothelial growth factor (VEGF) (6) and it has been approved for the first line and second line treatment of metastatic colorectal cancer (CRC) (7-10). Despite its survival benefits, adverse effects of bevacizumab have been reported such as arterial hypertension, arterial thromboembolic events, gastrointestinal perforation and bleeding,cardiovascular events, and proteinuria (7-10). Regarding venous thromboembolism (VTE), there has been controversy whether bevacizumab increase risk of VTE or not in meta-analyses (11,12). In a meta-analysis of 8,000 patients with various cancers receiving bevacizumab, the incidence of all-grade VTEs was 11.9% and the relative risk was 1.33 [95% confidence interval (95% CI),1.13−1.56; P<0.001] (11), whereas the other meta-analysis including 6,055 patients with various cancers reported that the incidence of all-grade VTEs was 10.9% with bevacizumab [odd ratio (OR), 1.14; 95% CI, 0.96−1.35;P=0.13] (12). The discrepancy might be due to different number and heterogeneous studies included. Incidences of VTE showed significant heterogeneity among the included studies and there were differences in sample size, tumor type, concomitant chemotherapy, and other potential risk factors among these included studies. The association between bevacizumab and VTE remains still unclear.
The pathogenesis of bevacizumab-induced thrombosis may be related to endothelial cell damage and apoptosis associated with anti-VEGF activity (13). The catheter insertion procedure and the continued presence of an indwelling catheter, which induce endothelial erosion,together with venous stasis, may trigger the development of mural thrombi (2). The prothrombotic state induced by a central catheter can be exacerbated by bevacizumab treatment. Incidence of VTE also varied according to locations and tumor types as a significant risk factor (11).The highest incidence of VTE was reported as 19.1% in patients with CRC treated with bevacizumab (11) and risk of VTE increased in only CRC patients who received more bevacizumab (OR, 2.17; 95% CI, 1.16−4.08; P=0.016) (14).Taken all together, there may be a strong association between bevacizumab treatment and limited CRT in patients with single-type tumor, CRC.
This study investigated the incidence of CRT and factors associated with the risk of CRT in a series of patients with CRC treated with or without bevacizumab.
Materials and methods
Patients and data collection
We retrospectively reviewed the medical records of 1,586 patients with histologically confirmed CRC who received chemotherapy at Asan Medical Center, Seoul, Korea,between March 2014 and 2016. Fifty-two patients without a chemoport were excluded, and the remaining 1,534 patients with conventional chemotherapy with or without targeted agents (cetuximab and bevacizumab) were included in the analysis. Sixty-three patients with two or three chemoport insertions were only analyzed at the time of the first chemoport insertion. Therefore, a total of 1,534 patients with no previous history of chemoport or CRT were included in the three groups (Figure 1): adjuvant chemotherapy (AC) group (n=670), palliative chemotherapy (PC) without bevacizumab (n=356), and PC with bevacizumab (n=508). Patient baseline characteristics,clinical setting, chemotherapy regimen, with or without targeted agents and previous relevant medical history were obtained from the patients’ medical records. Chemoports were implanted by an experienced radiologist using ultrasound-guided venous access and placed in the internal jugular vein or subclavian vein of upper extremities.Patients were instructed regarding the maintenance and care of the chemoports. Baseline blood test and coagulation function test was performed before chemotherapy administration. Anticoagulation prophylaxis was not given.The study protocol was approved by the Institutional Review Board of the Asan Medical Center. All procedures followed the ethical guidelines of the institutional and national committees on human experimentation and the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute was obtained from all patients before being included in the study.
Evaluation of CRT
Patients with clinical symptoms including swelling or pain at the chemoport site or ipsilateral neck were evaluated using ultrasound or contrast-enhanced computed tomography (CT). Asymptomatic CRT was incidentally diagnosed on CT scans during routine disease evaluation,but routine screening of asymptomatic CRT was not performed. CRT was diagnosed on CT as an intraluminal filling defect of a venous segment in two or more views.
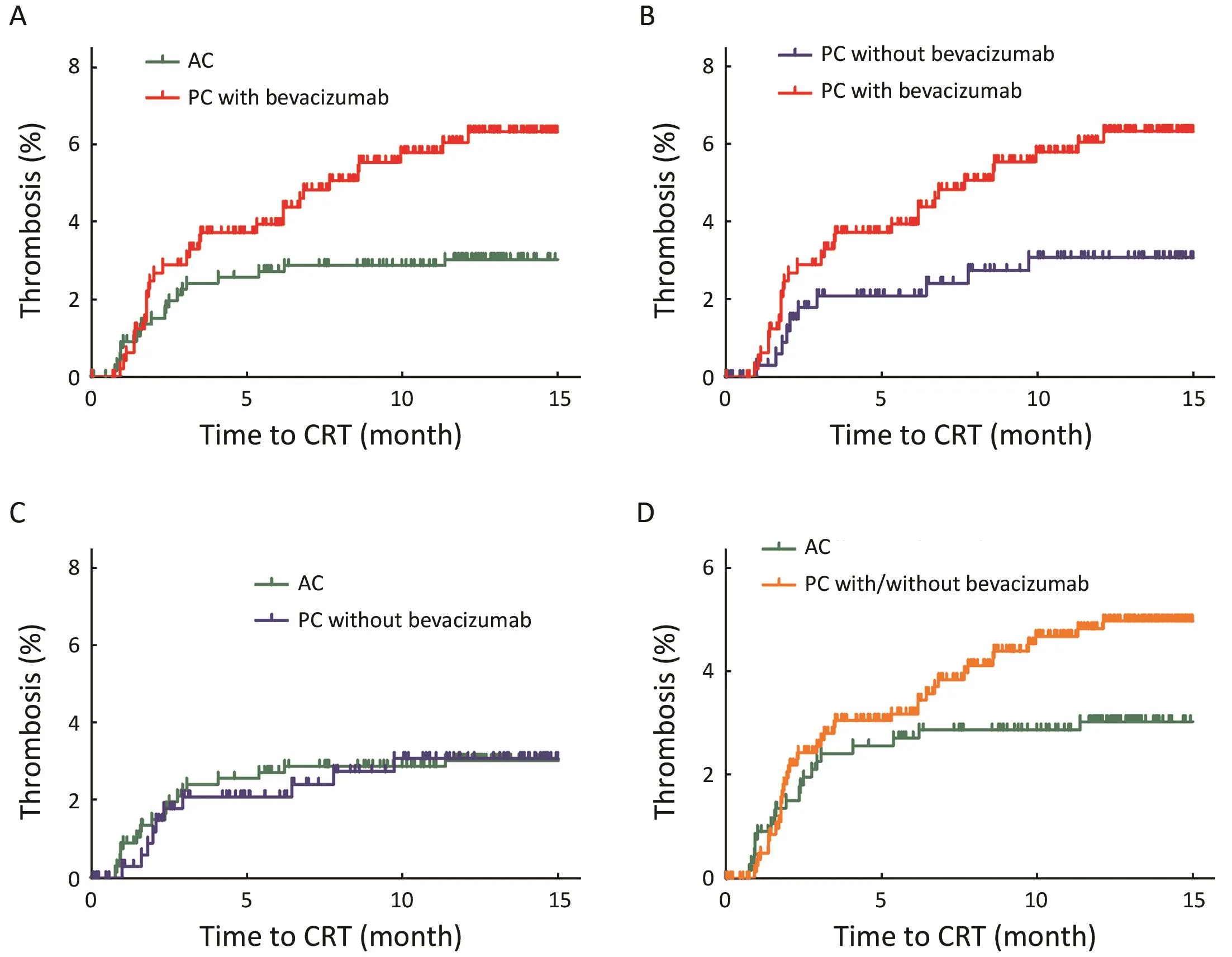
Figure 1 Cumulative incidence of chemoport-related thrombosis (CRT) in three study groups. P<0.017 (0.05/3) was considered significant using Bonferroni correction. (A) Cumulative incidence of CRT in palliative chemotherapy (PC) with bevacizumab was significantly greater than that of CRT in adjuvant chemotherapy (AC) (P=0.011); (B) There was a trend toward increased CRT in PC with bevacizumab compared with PC without bevacizumab (P=0.044); (C) There was no significant difference in cumulative incidence of CRT between patients administered PC without bevacizumab and those administered AC (P=0.999); (D) There was no significant difference in cumulative incidence of CRT between patients administered PC with or without bevacizumab and those administered AC (P=0.074).
Statistical analysis
Baseline characteristics assessed as potential risk factors for thrombosis were compared between the three study groups(AC, PC without bevacizumab, and PC with bevacizumab)using the Chi-square/Fisher’s exact tests for categorical variables and the one-way analysis of variance (ANOVA) or nonparametric test for continuous variables. The incidence of CRT was reported as a percentage of study patients.Kaplan-Meier analysis with log-rank test was used to compare the cumulative incidence of CRT observed in the three groups. Time to CRT was calculated from the date of chemoport insertion to the date of CRT diagnosis. Patients who were lost to follow-up and patients who did not experience CRT were censored at their last date of followup with chemoport. Risk factors for CRT were identified using Cox proportional hazard regression models.Multivariate analysis included factors considered significant(P<0.1) in univariate analysis. Only factors with P<0.05 in multivariate analysis using backward elimination model were considered significant. In Kaplan-Meier analysis with multiple comparisons, P<0.017 (0.05/3) was considered significant using Bonferroni correction. All statistical analyses were performed using the SPSS 18.0 software(SSPS Inc., Chicago, IL, USA).
Results
Patient characteristics
Table 1shows the clinical characteristics of the patients in the three study groups. FOLFIRI (folinic acid, fluorouracil and irinotecan) was the most frequent regimen for PC with or without bevacizumab. The most frequent regimen for AC was FOLFOX (folinic acid, fluorouracil and oxaliplatin;P<0.001). The percentage of patients with a body mass index (BMI) of >30 kg/m2was larger in patients administered PC without bevacizumab than in the other patients (P=0.011). Within patients for PC with or without bevacizumab, although number of metastatic sites was larger in PC with bevacizumab than in PC without bevacizumab, the percentage of prior surgery or priorchemotherapy was similar or even lower in PC with bevacizumab. Almost patients also received FOLFIRI or FOLFOX regimen as the first line treatment with or without bevacizumab. There were no other significant differences in baseline characteristics. Twenty patients(1.3%) received chemotherapy with or without bevacizumab taking aspirin or clopidogrel or warfarin because of cardiovascular disease. All patients except for 3 patients (0.2%) taking warfarin had normal coagulation function test and there were no coagulopathy and no
suspicious symptoms or signs of thromboembolism at baseline. Prothrombin time (PT) international normalized ratio (INR) was in therapeutic range (2−3) in 3 patients taking warfarin.
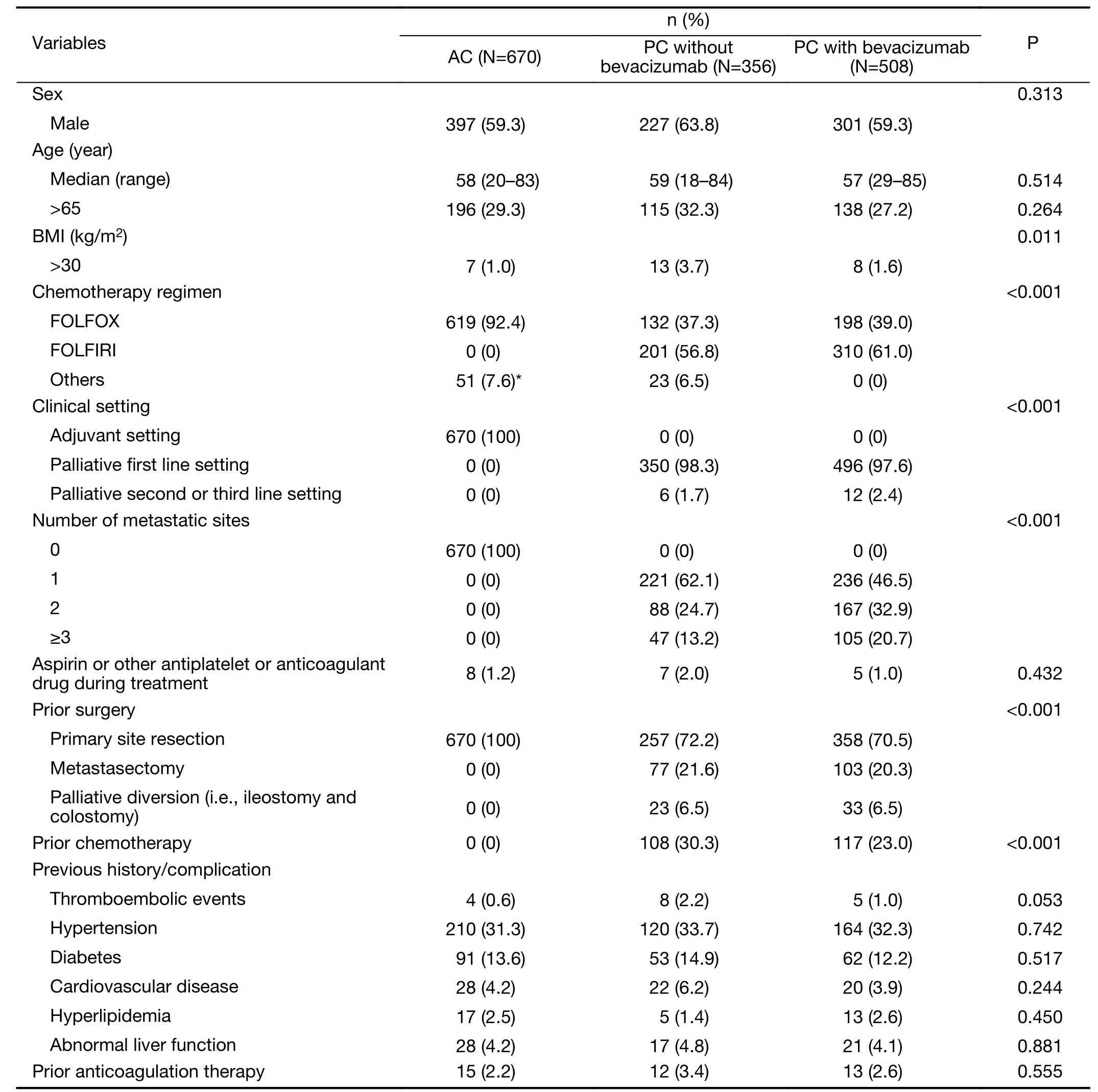
Table 1 Baseline characteristics of patients given AC or PC with or without bevacizumab (N=1,534)
Incidence of CRT
CRT occurred in 59 patients (3.8%) at a median follow-up of 20.19 [interquartile range (IQR), 14.07−27.19] months;symptomatic and asymptomatic CRT (2.9% and 0.9%,respectively). Among 59 patients with CRT, most 45 (76.3%) patients had symptoms such as swelling or pain in the chemoport site or ipsilateral neck. The median time to CRT was 2.33 (IQR, 1.59−5.79) months, with a median of five chemotherapy cycles before CRT (range, 2−23 cycles).Table 2summarizes characteristics of CRT. The incidence of CRT in PC with bevacizumab was 5.7%(29/508 patients), and it was higher than that with AC(2.9%, 20/670 patients) or PC without bevacizumab (2.8%,10/356 patients) (P=0.008). The overall incidence of CRT in PC with or without bevacizumab was 4.5% (39/864 patients). The cumulative incidence of CRT was significantly higher in patients administered PC with bevacizumab than in those administered AC (Figure 1A,P=0.011) and there was a trend toward increased CRT in PC with bevacizumab compared with PC without bevacizumab (Figure 1B, P=0.044). There was no significant difference in cumulative incidence of CRT between patients administered PC without bevacizumab and those administered AC (Figure 1C, P=0.999) and between patients administered PC with or without bevacizumab and those administered AC (Figure 1D,P=0.074).
Only in patients with symptomatic CRT, its cumulative incidence was significantly higher in patients administered PC with bevacizumab than in those administered AC(Figure 2A, P=0.015). There was no significant differencebetween patients administered PC with bevacizumab and those administered PC without bevacizumab (Figure 2B,P=0.158) and between those administered PC without bevacizumab and those administered AC (Figure 2C,P=0.565) and between those administered PC with or without bevacizumab and those administered AC(Figure 2D, P=0.055).
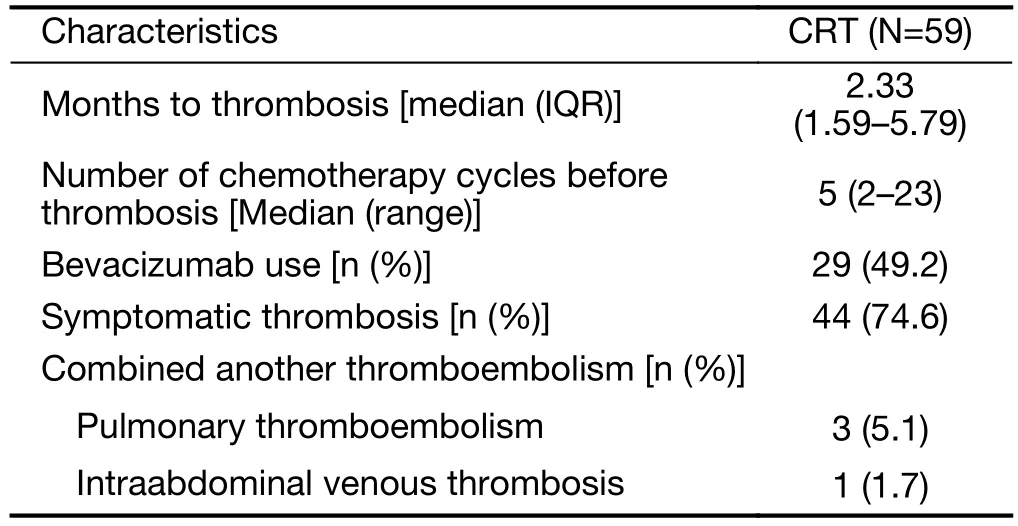
Table 2 Characteristics of CRT
Risk factors for CRT
Univariate analysis (Table 3) revealed that prior thromboembolism, prior anticoagulation therapy, presence of metastasis, administering bevacizumab regardless of treatment settings, and PC with bevacizumab either compared with AC or PC without bevacizumab were significant risk factors for CRT (P<0.1). Following multivariate analysis (Table 3), only the addition of bevacizumab remained significantly associated with CRT[hazard ratio (HR), 2.06; 95% CI, 1.24−3.43; P=0.006].
Discussion
The overall incidence of CRT was 3.8%. The incidence of 5.7% (29/508) in patients administered bevacizumabwas was significantly higher than the incidence of 2.9%(30/1,026) in patients not administered bevacizumab(P=0.008). The cumulative incidence of CRT in PC with bevacizumab was significantly higher than that in AC(P=0.011) and there was a trend toward increased CRT in PC with bevacizumab compared in PC without bevacizumab (P=0.044). Only in patients with symptomatic CRT, its incidence was significantly higher in PC with bevacizumab than that in AC (P=0.015). Bevacizumab treatment was the only factor significantly associated with the risk of CRT (HR, 2.06; 95% CI, 1.24−3.43; P=0.006)after multivariate analysis, independent of age, sex, BMI,presence of metastasis, and other diseases affecting thrombosis risk.
The reported incidence of CRT has been as high as 28%, but the most recently reported incidence is 1%−5%(2,4,5). One study found the incidence of symptomatic and asymptomatic CRT to be 4%−5% and 30%, respectively(4). The discrepancies among studies can be attributed to differences in the definition of CRT, patient baseline characteristics associated with thrombosis risk, and whether CRT was routinely screened. This study did not routinely screen for asymptomatic CRT and incidence of overall CRT (3.8%) or symptomatic CRT (2.9%) was similar to that reported in recent studies. However, 5.7% in patients administered bevacizumab was numerically higher than that reported in previous studies, and only in patients with PC, there was a trend toward increased CRT in PC with bevacizumab compared with PC without bevacizumab(P=0.044). After multivariate analysis, bevacizumab treatment (HR, 2.06; 95% CI, 1.24−3.43; P=0.006)significantly increased CRT in our CRC patients.
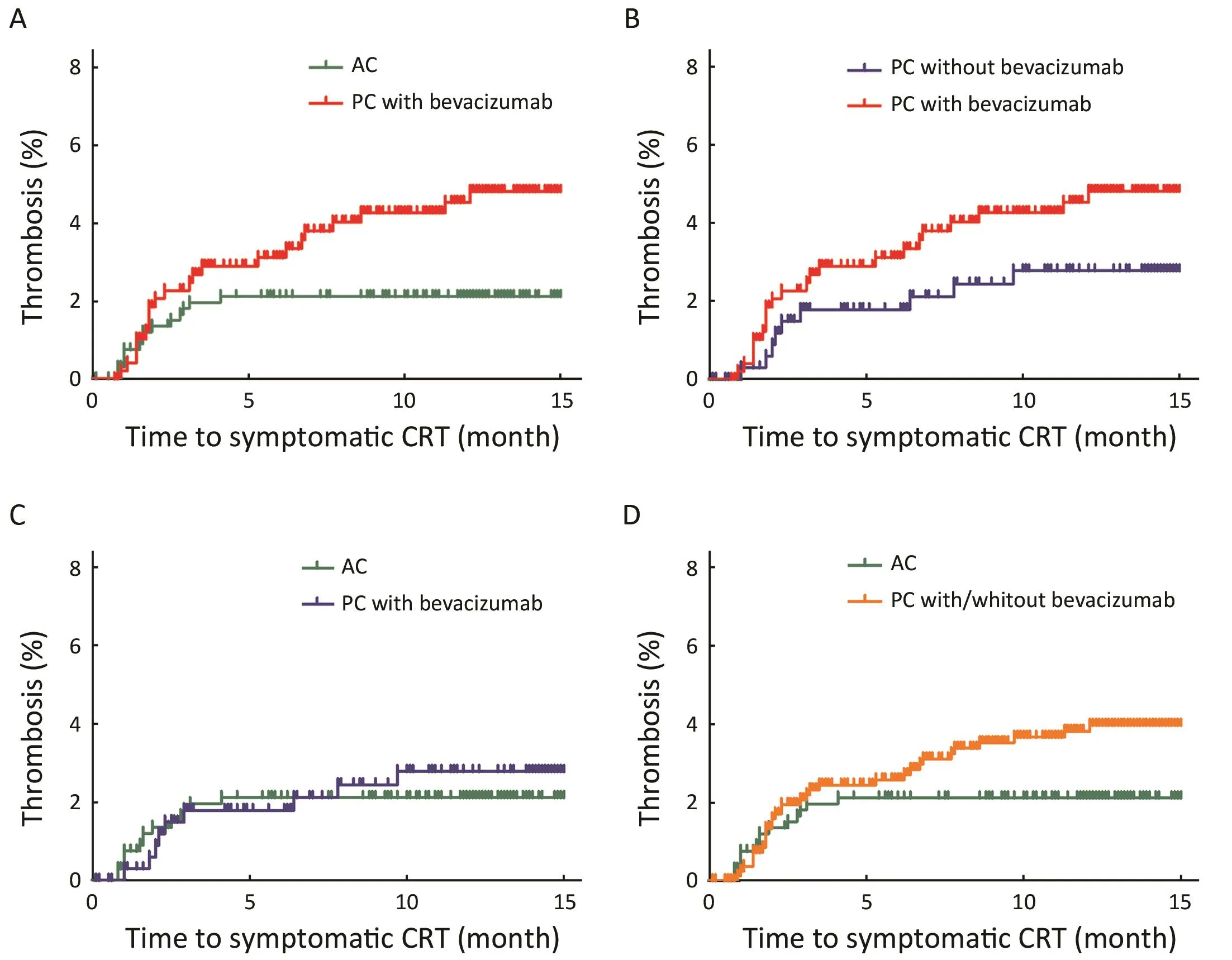
Figure 2 Cumulative incidence of symptomatic chemoport-related thrombosis (CRT) in three study groups. P<0.017 (0.05/3) was considered significant using Bonferroni correction. (A) Cumulative incidence in palliative chemotherapy (PC) with bevacizumab was significantly greater than that of CRT in adjuvant chemotherapy (AC) (P=0.015); (B) There was no significant difference in cumulative incidence of symptomatic CRT between patients administered PC with bevacizumab and those administered PC without bevacizumab(P=0.158); (C) There was no significant difference in cumulative incidence of symptomatic CRT between patients administered PC without bevacizumab and those administered AC (P=0.565); (D) There was no significant difference in cumulative incidence of symptomatic CRT between patients administered PC with or without bevacizumab and those administered AC (P=0.055).
The mechanism of bevacizumab-associated thrombosis remains poorly understood. Anti-VEGF activity may be associated with thrombosis through the damage or apoptosis of endothelial cells (15). There may be synergic effects with chemoport, which could induce erosion of endothelial cells, and CRT may occur. Another hypothesis reported bevacizumab-immune complex can directly activate platelets and cause thrombosis via platelet Fcgamma-RIIa IgG receptor (16). Notably, bevacizumabimmune complex was thrombotic in presence of heparin and heparin promoted bevacizumab-immune complex deposition on to platelets (16). Furthermore, this activity was enhanced by small amounts of heparin (17). Peripheral blood level of circulating heparin in 28 cancer patients with chemoport was obtained within 5 min of port flushing with heparin and it was significantly higher than controls(P<0.001) (17). It suggested that repeated exposures to port flushing heparin could induce bevacizumab-associated thrombosis (17). Further studies are needed to understand the association between anti-VEGF activity and thrombosis in future.
Patient or therapy- and catheter-related factors have been identified as risk of CRT (2,5,18-20). Catheterrelated factors have been mainly investigated and may be more important to development of CRT. Previous history of chemoport insertion or CRT and several attempts of chemoport insertion were significant factors for CRT and several catheter characteristics (i.e. material, tip position)were associated with CRT (2,5,18-20). In this study,although several catheter characteristics were not investigated, all chemoports were inserted by experienced radiologists in the same method at a single-institution and we also excluded patients with previous history of chemoport insertion or CRT. Meanwhile, we found thatpresence of metastasis could not play role in our CRC patients with CRT. Presence of metastasis was not a significant factor for CRT in multivariate analysis and cumulative incidence of CRT in PC without bevacizumab was not significantly different from that in AC (P=0.999). It was supported that extent of malignancy was not significantly different between various cancer patients with CRT and without CRT (P=0.65) (5). Although tumor burdens could contribute to thrombosis, CRT may be a local complication and catheter-related factors may play a more crucial role.
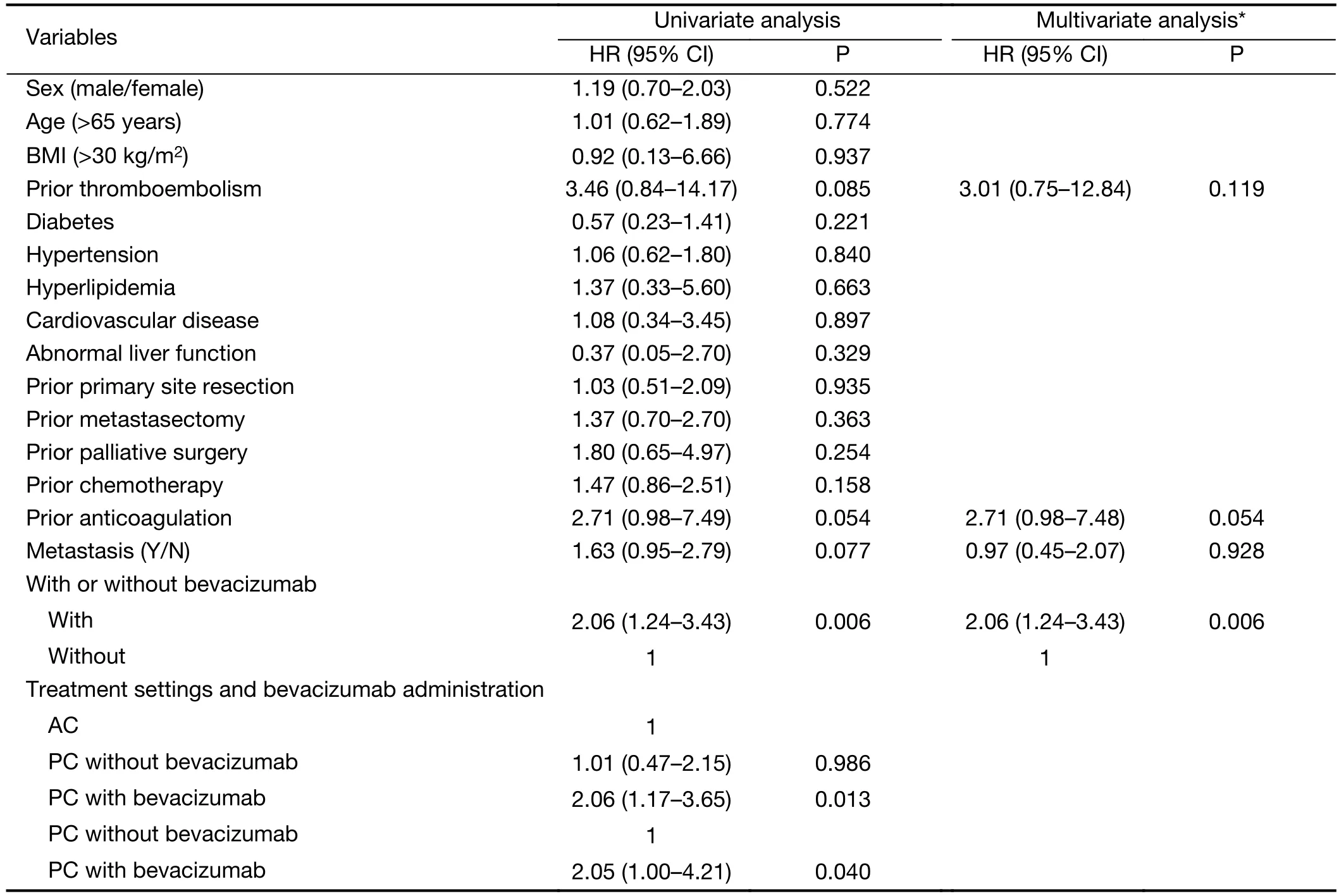
Table 3 Univariate and multivariate analyses for risk factors of CRT
This single-institution study was limited by differences in types of catheters used and differences in insertion technique and catheter care in other hospitals. Patients might have variations of internal diameter of the vein where the chemoports were inserted, which could contribute to CRT. Moreover, the retrospective design of the study could not account for different baseline characteristics and thrombosis risk factors. However, to the best of our knowledge, this is the largest study limited to homogeneous patients with CRC and CRT to demonstrate the independent association of bevacizumab and CRT in multivariate analysis.
Conclusions
CRT is a serious complication that causes morbidities and interrupts administration of intravenous cancer therapy.Bevacizumab was associated with an increased incidence of CRT in patients with CRC.
Acknowledgements
This study was supported by a grant from the Asan Institute for Life Sciences, Asan Medical Center, Seoul,Korea (No. 2016-0733).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Evaluation of menopausal status among breast cancer patients with chemotherapy-induced amenorrhea
- A nomogram to predict prognosis for gastric cancer with peritoneal dissemination
- Annual cost of illness of stomach and esophageal cancer patients in urban and rural areas in China: A multi-center study
- A radiomic nomogram based on an apparent diffusion coefficient map for differential diagnosis of suspicious breast findings
- Differential study of DCE-MRI parameters in spinal metastatic tumors, brucellar spondylitis and spinal tuberculosis
- Radiomic analysis of pulmonary ground-glass opacity nodules for distinction of preinvasive lesions, invasive pulmonary adenocarcinoma and minimally invasive adenocarcinoma based on quantitative texture analysis of CT
