Evaluation of menopausal status among breast cancer patients with chemotherapy-induced amenorrhea
2018-09-14BailinZhangJinqiWuRongshouZhengQianZhangMargaretZhuoerWangJunQiHaijingLiuYipengWangYangGuoFengChenJingWangWenyueLyuJidongGaoYiFangWanqingChenXiangWang
Bailin Zhang, Jinqi Wu, Rongshou Zheng, Qian Zhang, Margaret Zhuoer Wang, Jun Qi,Haijing Liu, Yipeng Wang, Yang Guo, Feng Chen, Jing Wang, Wenyue Lyu, Jidong Gao, Yi Fang,Wanqing Chen, Xiang Wang
1Department of Breast Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China; 2Department of Breast Surgery, Cancer Hospital of Huanxing Chaoyang District Beijing, Beijing 100122, China; 3Office for Cancer Registry, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China; 4Department of Epidemiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China; 5Pritzker School of Medicine, University of Chicago, Chicago 60637, USA; 6Department of Clinical Laboratory, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China; 7Pharmacy Department, Kailuan General Hospital, Tangshan 063000, China;8Surgery Department, Maternal and Child Health Care Hospital of Yanqing District Beijing, Beijing 102100, China; 9Office of Cancer Screening,National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China
Abstract Objective: In patients with chemotherapy-induced amenorrhea (CIA), the menopausal status is ambiguous and difficult to evaluate. This study aimed to establish a discriminative model to predict and classify the menopausal status of breast cancer patients with CIA.Methods: This is a single center hospital-based study from 2013 to 2016. The menopausal age distribution and accumulated incidence rate of CIA are described. Multivariate models were adjusted for established and potential confounding factors including age, serum concentration of estradiol (E2) and follicle-stimulating hormone (FSH),feeding, pregnancy, parity, abortions, and body mass index (BMI). The odds ratio (OR) and 95% confidence interval (95% CI) of different risk factors were estimated.Results: A total of 1,796 breast cancer patients were included in this study, among whom, 1,175 (65.42%) were premenopausal patients and 621 (34.58%) were post-menopause patients. Five hundred and fifty patients were included in CIA analysis, and a cumulative CIA rate of 81.64% was found in them. Age (OR: 1.856, 95% CI:1.732−1.990), serum concentration of E2 (OR: 0.976, 95% CI: 0.972−0.980) and FSH (OR: 1.060, 95% CI:1.053−1.066), and menarche age (OR: 1.074, 95% CI: 1.009−1.144) were found to be associated with the patients’menopausal status. According to multivariate analysis, the discriminative model to predict the menopausal status is Logit (P)=−28.396+0.536Age−0.014E2+0.031FSH. The sensitivities for this model were higher than 85%, and its specificities were higher than 89%.Conclusions: The discriminative model obtained from this study for predicting menstrual state is important for premenopausal patients with CIA. This model has high specificity and sensitivity and should be prudently used.
Keywords: Breast neoplasms; drug therapy; amenorrhea; menopause; logistic models
Introduction
Breast cancer is the most commonly diagnosed female cancer in China, and ranks the fifth cause of cancer deaths in Chinese females (1). Chemotherapy and endocrine therapy are increasingly recommended by guidelines worldwide for younger women with hormone-sensitive breast cancer. Data from a national retrospective study showed that 62.91% of the breast cancer patients in China were premenopausal and that 85.77% of them received chemotherapy (2). As chemotherapy agents induce amenorrhea by interfering with follicular maturation with or without depletion of primordial follicles, many premenopausal patients face an unclear status of menstruation after chemotherapy treatment. The incidence rate of chemotherapy-induced amenorrhea (CIA) ranged from 35% to 97% in patients aged 40 years and older (3).Patient age and chemotherapy regimens are important factors in the CIA characteristics, such as the incidence rate, duration and recovery rate of menstruation (4-6).
Clinical trials have shown that endocrinotherapy can significantly reduce the risk of recurrence and metastasis and increase the overall survival rate of breast cancer patients. Aromatase inhibitors (AIs) are increasingly used for the treatment of postmenopausal patients with hormone receptor-positive breast cancer, based on evidence of superiority to tamoxifen (7-11). AIs alone, however, are contraindicated in women with functioning ovaries (12). In patients with CIA, menopausal status is ambiguous and difficult to evaluate, which makes it difficult for oncologists to determine whether to use AIs among them, which has been shown to be highly effective for contradicted premenopausal breast cancer patients. Therefore, it is important to be able to estimate when these patients truly enter menopause.
Menopause is defined as the permanent cessation of menses with permanent decrease in ovarian estrogen synthesis (13). Amenorrhea time, however, is not a reliable indicator of menopausal status for premenopausal women who then received chemotherapy. We hypothesized that the menstruation status of patients with CIA could be detected using a model based on patient age and serum concentrations of follicle stimulating hormone (FSH) and estradiol (E2). The aim of this study is to evaluate the distribution of menopausal age and CIA rate in breast cancer patients in China and then, to establish a model that predicts and classifies the menstruation status of breast cancer patients with CIA.
Materials and methods
Eligibility criteria
This is a hospital-based study of female patients with breast cancer. Inclusion criteria included patients diagnosed with stage I to III breast cancer with a complete medical record of menses. Childbearing and breastfeeding history were then collected. Potential baseline covariates included smoking, drinking, education level, parity, menarche age,body mass index (BMI) and serum concentration of E2 and FSH. Notable exclusion criteria were as follows: pregnancy or lactation at the time of diagnosis, a history of other cancers (include previous contralateral breast cancer),previous chemotherapy, oophorectomy (unilateral or bilateral), hysterectomy, current or previous usage of exogenous hormone medications known to affect ovarian function, including gonadotropin-releasing hormone(GnRH) analogs, within twelve months before study recruitment, and current usage of oral contraceptives.
This study was approved by the Ethics Committee of Cancer Institute and Hospital, Chinese Academy of Medical Sciences (CICAMS) and Peking Union Medical College (PUMC) (Approval No. 13-080/756). It is registered in Chinese Clinical Trial Registry (Registration No. ChiCTR-OCS-13003848). Informed consents were obtained from each participant.
Definitions of postmenopausal, premenopausal,perimenopausal periods and CIA
Menopausal status was assessed based on the final menstrual period (FMP) from the patient’s medical record.Patients with amenorrhea for more than 12 months and FSH and E2 levels within the postmenopausal range were considered postmenopausal (13). Natural menopause was defined for women without a history of hysterectomy and/or bilateral oophorectomy, and who did not report any use of exogenous hormones one year after FMP.Premenopausal patients were defined as women with regular menses or amenorrhea less than 8 weeks before receiving chemotherapy. Perimenopausal patients in this study were defined as those whose FMP occurred within 8 weeks to 12 months before receiving chemotherapy.Perimenopausal patients were excluded from CIA analysis,because naturally occurring menopause could not be distinguished from CIA. Premenopausal patients with CIA were defined as those experiencing absence of menses for at least three consecutive months after beginning chemotherapy. Pre-/perimenopausal patients were both calculated as premenopausal patients during the statistical analyses of menopausal age distribution.
Treatment and follow-up
Patients included in CIA analysis received chemotherapy as adjuvant or neoadjuvant treatment. Patients with hormone receptor-positive breast cancer received endocrinotherapy after the chemotherapy. Among them, tamoxifen or toremifene was used in premenopausal patients and AIs were used in postmenopausal patients. The menopausal statuses were defined according to their situation before chemotherapy. Patients received radiotherapy if their clinical or pathological stages were more advanced than T3 or N2. Routine follow-ups with imaging and laboratory examinations were performed in this group. Serum concentrations of E2 and FSH were measured before chemotherapy as baseline and monitored during each cycle of chemotherapy and the follow-up period. The first follow-up started at the third month after the chemotherapy. The interval of follow-ups was three months in the first two years and six months from the third to the fifth years after chemotherapy. Data of twelve to eighteen months of follow-ups in this group were obtained and analyzed.
Serum hormone assays and measures
Fasting blood samples were collected at each time point during the chemotherapy period and follow-up. All serum samples were measured at the Department of Clinical Laboratory of Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College(PUMC). Serum concentrations of E2 and FSH were tested with a FSH and E2 test kit (Roche Diagnostics GmbH, Germany) using a Cobas® e601 analyzer (Roche Diagnostics GmbH, Sandhofer Strasse 116, 68305 Mannheim, Germany). The average inter- and intra-assay coefficients of E2 variation were 9.8% and 4.6%,respectively, and the lower limit of detection (LLD) was 17.15 pmol/L. Inter- and intra-assay coefficients of FSH variation were 5.1% and 1.9%, respectively, and the LLD was 0.1 IU/L.
Data collection and data management
A questionnaire to collect patient demographic data was used at baseline. Patient information was collected by trained staff. All of the questionnaires were checked by at least two staff members.
Statistical analysis
SAS software (Version 9.4; SAS Institute Inc., Cary, NC,USA) was used for statistical analysis. Chi-square test andt-test were used to compare categorical and continuous variables between premenopausal and postmenopausal groups. Logistic models for univariate analysis of different variables were established, and the accuracy of each model in predicting the status of menopause was evaluated. The hazard ratios and 95% confidence interval (95% CI) of different risk factors were estimated. All statistical tests were two-sided using a 0.05 significance level. Multivariate models were adjusted for established and potential confounding factors including age, feeding, pregnancy,parity, abortions and BMI, and the backward method was used for screening variables. As recommended by WHO,the BMI (the weight in kilograms divided by the square of the height in meters) categorization for Asian populations was underweight (<18.5 kg/m2), increasing but acceptable risk (18.5−22.9 kg/m2), increased risk (23.0−27.5 kg/m2),and high risk (>27.5 kg/m2) (14).
Results
Patients, menopausal age distribution and incidence rate of CIA
From 2013 to 2016, 1,796 breast cancer patients were included in this study, after being confirmed to meet the eligibility criteria (Figure 1). Among these patients, 1,175(65.42%) were premenopausal with a median age of 46(range: 16−60) years and 621 (34.58%) were postmenopausal with a median age of 57 (range: 45−86)years. The mean age of natural menopause was 49.99±3.24 years with the youngest age of menopause being 37 years and the oldest 60 years. The mean age of menarche was 15.26±1.96 years for postmenopausal patients and 14.62±1.76 years for premenopausal patients.The cumulative proportion of postmenopausal patients was low among patients aged 35 to 44 years old (4.83%),and rapidly increased among patients of 45 to 55 years old(97.42%) and was stable at 100% among patients of 55 years old or older (Figure 2). As patients older than 60 years were defined as postmenopausal (13) and a low proportion of postmenopausal patients was found among people under 45 years old, the target population for menopausal status estimation was 1,142 patients aged 45 to 60 years old in this study. In postmenopausal patients, the median serum concentrations and interquartile ranges(IQR) of E2 and FSH were 18.57 (18.35−41.39) pmol/L and 58.97 (46.17−76.88) IU/L, respectively. In premenopausal patients, the median serum concentrations and IQR of E2 and FSH were 204.30 (52.22−429.60)pmol/L and 11.69 (5.94−32.81) IU/L, respectively.
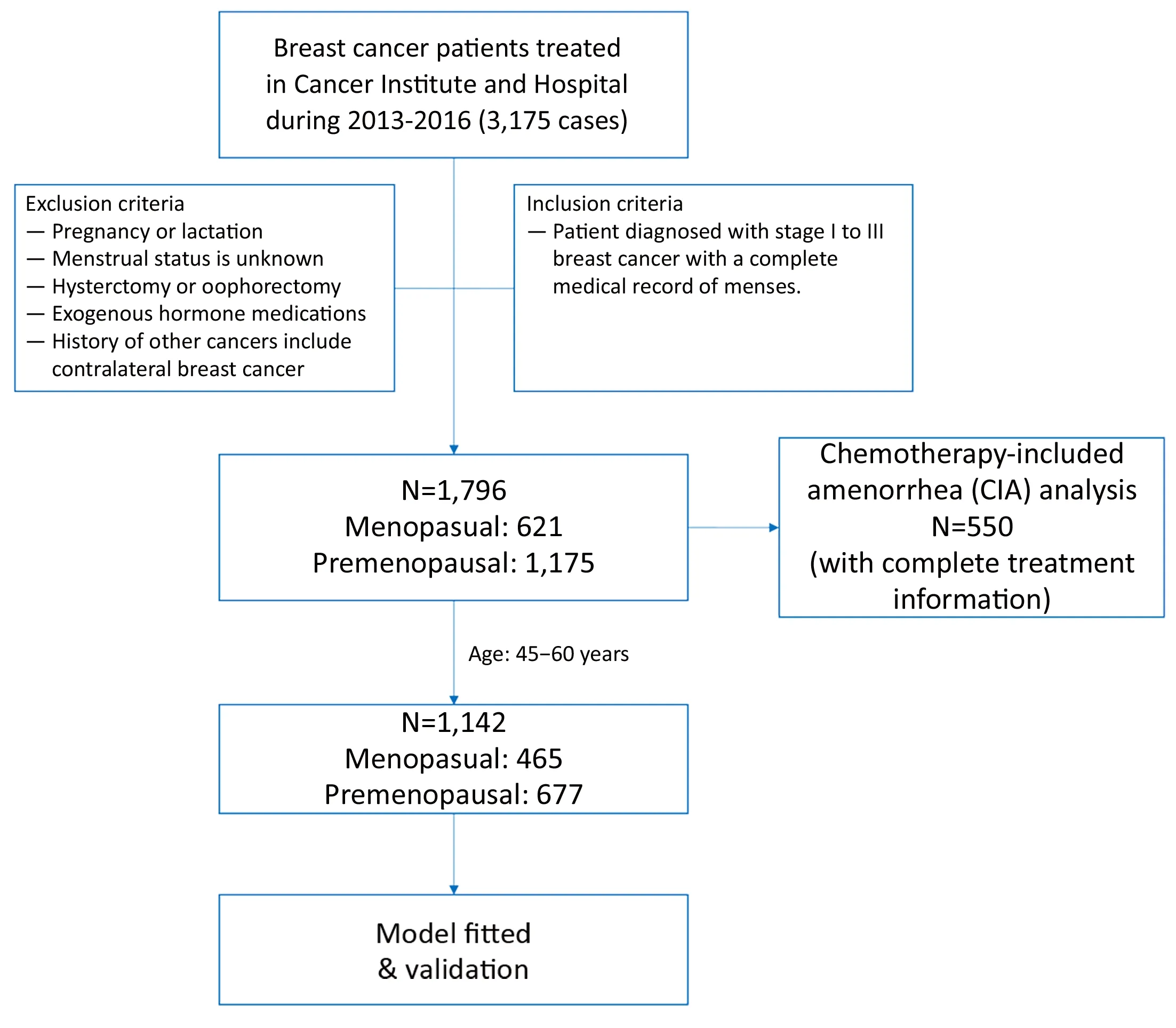
Figure 1 Flow diagram.
Five hundred and fifty premenopausal patients treated with chemotherapy and having complete medical record of chemotherapy and follow-up were included in CIA analysis. Among them, 449 patients (81.64%) had CIA.The cumulative proportion of CIA increased rapidly among patients aged 40 to 50 years old, with 59.69% at 45 years old, 89.53% at 50 years old and 99.55% at 55 years old (Figure 2).
Variation patterns of E2 and FSH serum concentrations in patients treated with chemotherapy
The variation patterns of E2 and FSH serum concentrations in postmenopausal patients showed slight fluctuation after completion of chemotherapy (Figure 3A).The concentrations of E2 in premenopausal patients,however, gradually decreased after starting chemotherapy,and achieved a steady low state after three cycles of chemotherapy. The serum concentration of FSH gradually increased after the start of chemotherapy and reached the highest level after the end of chemotherapy. The levels of E2 and FSH showed opposite changes after chemotherapy was completed. Serum levels of E2 quickly recovered from the lower level to its original level before chemotherapy, at about 6 months (at the second follow-up) after cessation of chemotherapy. The serum level of FSH gradually returned to its pre-chemotherapy level from its highest level at the third follow-up (Figure 3B).
Variable selection and structure of the prediction model
A total of 1,142 patients aged 45−60 years old in this cohort were selected for the establishment of the prediction model. Of these patients, 465 (40.72%) were postmenopausal, and 677 (59.28%) patients were premenopausal. All patients had documented medical history, such as BMI, history of feeding, pregnancy and abortion (Table 1). BMI, baseline serum concentrations of E2 and FSH, number of pregnancies and abortions were the main factors considered in the model to predict the menopause, according to literature search and clinical judgment. Age was found to be associated with menopausal status (OR: 1.856, 95% CI: 1.732−1.990) (Table 1). Other associated factors included serum concentrations of E2(OR: 0.976, 95% CI: 0.972−0.980) and FSH (OR: 1.060,95% CI: 1.053−1.066), menarche age (OR: 1.074, 95% CI:1.009−1.144) and number of abortions (OR: 0.829, 95%CI: 0.736−0.933). BMI, breast feeding, number of pregnancies and parity were not found to have significant relevance (Table 1).

Figure 2 Proportion of patients with menopause and chemotherapy-induced amenorrhea (CIA) by age.
Variables with significant relevance by univariate logistic regression analysis were selected into the multivariate analysis, along with other variables clinically judged to be significant, such as number of parity.
According to multivariate analysis (Table 2), menopausal status was correlated with age, baseline serum concentrations of E2 and FSH. The model was established to predict the sensitivity and specificity using patient age and baseline serum concentrations of E2 and FSH. The model is Logit (P)=−28.396+0.536Age−0.014E2+0.031FSH, which can also be expressed as follows:
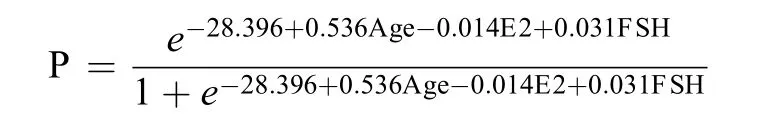
When P is greater than 0.5, then the patient’s menstrual state will be classified as postmenopausal.
Validation of discriminative model
To examine the performance of this model, sensitivity analyses and model validation were conducted using external test sets, including Leave-One-Out Cross Validation test, 10%, 20%, 30%-fold cross validation check, and back substitution check. Sensitivity, specificity and the positive predictive value (PPV) were used in the evaluation as indices. Sensitivity is the proportion of true positive cases that are predicted correctly by the test, and specificity is the proportion of negatives cases that are correctly identified by the test. The areas under the ROC curve (AUC) were 0.922, 0.856 and 0.877 for the individual factors of age, E2 and FSH, respectively, but the comprehensive model’s AUC reached 0.968 (Figure 4).This model was verified by different methods in order to ensure the stability and reliability of the prediction results.The results show that the sensitivities for different methods were higher than 85%, and the specificities were higher than 89% (Table 3).
Discussion
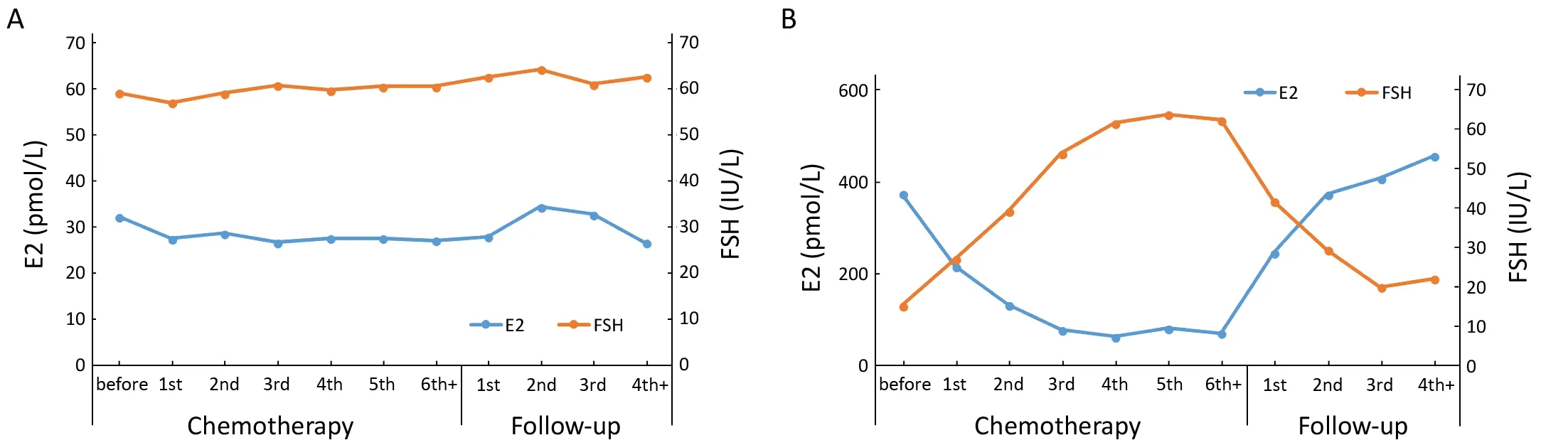
Figure 3 Changes in serum concentration of estradiol (E2) and follicle-stimulating hormone (FSH) levels during chemotherapy periods and follow-up period by status of menstruation. (A) postmenopausal stage; (B) premenopausal stage. During the period of chemotherapy, 6th+means the 6th and the following cycles of chemotherapy; and during the period of follow-up, 4th+ means the 4th and the following follow-up.
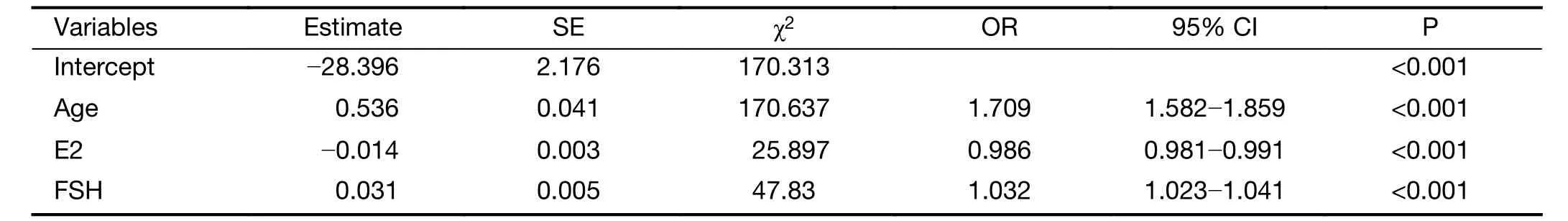
Table 2 Multivariate logistic analysis

Figure 4 Receiver operating characteristic (ROC) curves and the areas under the ROC curve (AUC) for serum concentration of estradiol (E2), follicle-stimulating hormone (FSH) and age.
Patient age consistently predicted amenorrhea in women treated with anthracycline-based regimens, as rates of CIA were not associated with dose intensity (15,16). Data from our study showed that the cumulative proportion of CIA tended to increase with age (Figure 2). Chemotherapy agents interfered with the ovarian function, leading to amenorrhea. These changes, however, are usually not permanent (17). Because of the difficulty in obtaining follow-up information and inconsistency in follow-up intervals, it is hard to describe the recovery of ovarian function without resumption of menses (18,19). The menstrual state of patients after CIA is an important parameter for oncologists to decide on the endocrine therapy plan. In some patients without resumption of menses, the function of their ovarian might have recovered after chemotherapy, with residual ovarian function for a long time without the recovery of menses. In the National Comprehensive Cancer Network (NCCN) guidelines, it is emphasized that for premenopausal women starting adjuvant chemotherapy or endocrinotherapy, amenorrhea time is not a dependable indicator of menopausal status, as ovarian function may still be intact or resume during chemotherapy-induced anovulation/amenorrhea (13). So, it is necessary to develop an assistive tool for the determination of menstrual status of patients with CIA.
In fact, the ovarian failure of many patients with CIA would be temporary. It is difficult to determine when these patients will develop menopause. The remaining follicles,however, may still be recruited from the primordial pool.Accordingly, estrogen and gonadotropin levels may return to normal. The impairment and recovery of the ovarian function in this study were measured by assaying the serum concentrations of E2 and FSH of the patients before,during, and after chemotherapy. With these data, the variation pattern of these hormones was depicted, and then the physiological status of ovarian function was illustrated.There was a trend of progressively decreasing serum concentration of E2 and increasing level of FSH in premenopausal patients with chemotherapy (Figure 3B).The changes in hormone concentrations indicated damaged ovarian function and disrupted feedback regulation of the hypothalamic-pituitary-gonadal axis (20).After stopping chemotherapy, serum concentrations of E2 and FSH reversed to the original levels before chemotherapy. Although the menses of many pre-menopausal patients did not recover after chemotherapy, the reversal of hormone concentrations indicated the potential of recovery of the ovarian function after chemotherapy cessation. At the same time, hormone concentrations of postmenopausal patients were not influenced by chemotherapy and were stable throughout the course of chemotherapy (Figure 3A). Chemotherapeutic agents do not affect the hormone levels of postmenopausal patients in the absence of ovarian function (20-22). As the hypothalamic-pituitary-gonadal hormonal feedback loop could be described by changes in serum levels of E2 and FSH, biochemical alterations can be used in predicting and diagnosing menopause or amenorrhea (22). Therefore, our model for predicting menstruation status in patients with CIA could be established based on baseline serum concentrations of E2 and FSH before chemotherapy.Following hormone levels, age of the patient is the second most important factor in the predicting procedure. This finding conforms with the reports by van Hellemondv (23)and Henry (21) that the ovarian function of young people is more likely to recover than that of elder patients.
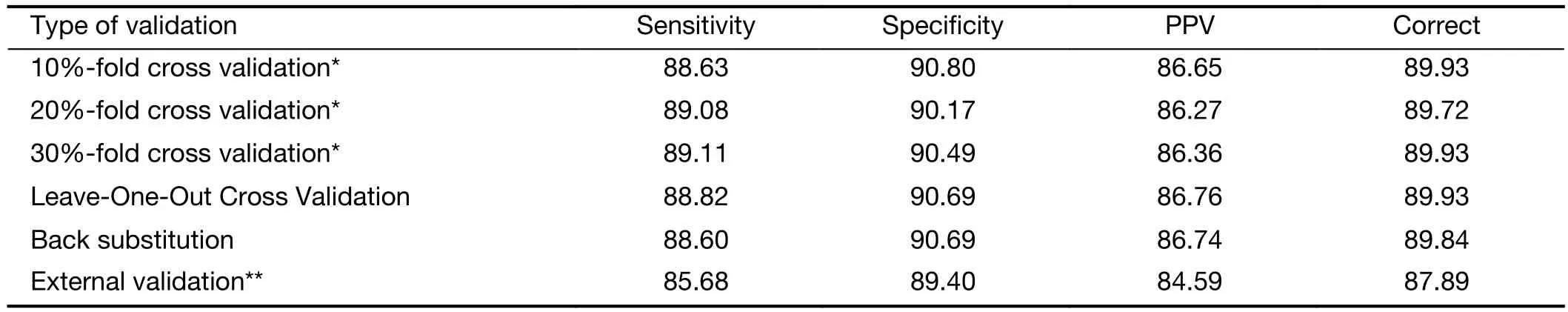
Table 3 Cross validation for different methods (%)
However, patients with tamoxifen therapy-induced amenorrhea were not included in this study. Tamoxifen treatment serves as the first line endocrinotherapy in some patients with early-stage breast cancer. Some of these patients would have the problem of amenorrhea like CIA.Whether this model could be used in determining the menstruation status of patients with tamoxifen treatmentinduced amenorrhea should be validated with cohort studies in these patients.
Considering the multiple of blood test systems(equipments and reagents) in different hospitals, we did not try to use data from other center. As the result from the single center study, the limitation of the representative should be considered. Because there was no reliable model to predict the menopausal status of breast cancer patients after chemotherapy, to some extent, we hope this predictive model can help oncologists when they try to distinguish the menopausal status. In addition, the E2 level of some patients might be higher than normal during the period of tamoxifen treatment. The family history of menstruation should also be considered.
Conclusions
The model shown in this study for predicting menstrual state is important for premenopausal breast cancer patients with CIA. We found that patients aged 45−60 years were the target population for this model. This multivariate model for the prediction of menstrual state in these patients has high specificity and sensitivity. However, as the result of single center study and some other limitations from this study, this prediction model can only be prudently used when decided the menopausal status of patients after chemotherapy.
Acknowledgements
This work was supported by Chinese Medical Foundation(CMF, No. 313.2215).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Radiomics approach for preoperative identification of stages I-II and III-IV of esophageal cancer
- Computed tomography-based radiomics for prediction of neoadjuvant chemotherapy outcomes in locally advanced gastric cancer: A pilot study
- Radiomic analysis of pulmonary ground-glass opacity nodules for distinction of preinvasive lesions, invasive pulmonary adenocarcinoma and minimally invasive adenocarcinoma based on quantitative texture analysis of CT
- Differential study of DCE-MRI parameters in spinal metastatic tumors, brucellar spondylitis and spinal tuberculosis
- A radiomic nomogram based on an apparent diffusion coefficient map for differential diagnosis of suspicious breast findings
- Annual cost of illness of stomach and esophageal cancer patients in urban and rural areas in China: A multi-center study
