A nomogram to predict prognosis for gastric cancer with peritoneal dissemination
2018-09-14ShiChenXijieChenRuncongNieLiyingOuYangAihongLiuYuanfangLiZhiweiZhouYingboChenJunshengPeng
Shi Chen, Xijie Chen, Runcong Nie, Liying Ou Yang, Aihong Liu, Yuanfang Li,Zhiwei Zhou, Yingbo Chen, Junsheng Peng
1Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, the Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou 510655, China; 2Department of Gastrointestinal Surgery, the Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou 510655, China;3Department of Gastropancreatic Surgery, Sun Yat-sen University Cancer Center, Guangzhou 510060, China; 4Department of Intensive Care, Sun Yat-sen University Cancer Center, Guangzhou 510060, China
Abstract Objective: To identify independent prognostic factors to be included in a nomogram to predict the prognosis of gastric cancer patients with peritoneal dissemination.Methods: This is a retrospective study on 684 patients with a histological diagnosis of gastric cancer with peritoneal dissemination from the Sun Yat-sen University Cancer Center as the development set, and 62 gastric cancer patients from the Sixth Affiliated Hospital of Sun Yat-sen University as the validation group. Chi-square test and Cox regression analysis were used to compare the clinicopathological variables and the prognosis of gastric cancer patients with peritoneal dissemination. The Harrell’s concordance index (C-index) and calibration curve were determined for comparisons of predictive ability of the nomogram.Results: Univariate and multivariate analyses showed that serum carcinoembryonic antigen (CEA) level(P=0.032), ascites grading (P=0.008), presence of extraperitoneal metastasis (P<0.001), seeding status (P=0.016) and performance status (P=0.009) were independent prognostic factors for gastric cancer patients with peritoneal dissemination in the development set. The nomogram model was constructed using these five factors. Internal validation showed that the C-index of the model was 0.641. For the external validation, the C-index of this model was 0.709.Conclusions: We developed and validated a nomogram to predict the prognosis for gastric cancer patients with peritoneal dissemination. This nomogram may play an important clinical role in guiding palliative therapy for these types of patients, although it may need more data for optimization.
Keywords: Gastric cancer; prognosis; peritoneal dissemination; nomogram
Introduction
Despite the decrease in the incidence of gastric cancer over the past few decades, it remains the second most common cause of cancer-related death among carcinomas in the world, with 738,000 deaths globally, mainly in Latin America, Eastern Europe and Eastern Asia (1-4). In many countries, including China, gastric cancer patients are diagnosed at a relatively advanced stage (5). In addition,30%−60% of gastric cancer patients were found to have peritoneal dissemination at the time of diagnosis and have a median survival time of only 3−6 months (6).
Despite the progress in surgery, chemotherapy and radiotherapy, the prognosis of gastric cancer is still unsatisfactory (7). Recently, the molecular targeting of genes, such as human epidermal growth factor receptor 2(Her2), has been the focus of many gastric cancer investigations, and several molecular targeting agents have been approved for use in clinic practice due to their beneficial effects on the short-term survival of gastric cancer patients with or without peritoneal dissemination(8,9). However, the effects of Trastuzumab on the subgroup of patients with peritoneal dissemination have been unclear.
In the clinical setting, the 7th version of the TNM Staging System of the American Joint Committee on Cancer (AJCC) is used to stage gastric cancer patients and predict their prognosis. However, other important clinicopathological features, including the gross type and patient age, among others, are not taken into consideration in this system. We and other investigators have found that gastric cancer patients may have a different prognosis even when they are classified in the same stage. Above all, a more reliable tool is needed to predict the prognosis of patients so better treatments can be administered. A nomogram is an efficient tool to quantify risks by combining all known prognostic factors using statistical software and has demonstrated practical use in the diagnosis of several diseases. There are already a few nomograms that have been developed to estimate the prognosis of gastric cancer, for example, the Memorial Sloan Kettering Cancer Center (MSKCC) nomogram for gastric cancer, which is used to predict the 5-year and 9-year disease-specific survival (DSS) after an R0 resection without any other therapy, has been widely validated and proven to be accurate (10-21). We have observed that some patients with gastric cancer with peritoneal dissemination(GCPD) achieved a better prognosis after receiving palliative gastrectomy, chemotherapy or hyperthermic intraperitoneal chemotherapy (HIPEC). However, it is difficult to identify these patients clinically. In this study,we tried to develop a nomogram model to predict the prognosis of gastric cancer patients with peritoneal dissemination by months of survival, for the purpose of screening out appropriate patients for more aggressive treatment.
Materials and methods
Ethics statement
All the patients provided written informed consent for their information to be stored in the hospital database of the Sixth Affiliated Hospital of Sun Yat-sen University and the Sun Yat-sen University Cancer Center. We obtained separate consent for the use of this information for research. Study approval was obtained from the independent Ethics Committees of the Sixth Affiliated Hospital of Sun Yat-sen University and the Sun Yat-sen University Cancer Center. This study was performed in accordance with the ethical standards of the World Medical Association Declaration of Helsinki.
Patient inclusion and exclusion criteria
The inclusion criteria were as follows: 1) histologically proven adenocarcinoma of the stomach with synchronous peritoneal dissemination at the time of surgery or biopsy;and 2) no other synchronous or metachronous cancers. The exclusion criteria were as follows: 1) incomplete or important data censored; 2) patients with mental disorders or severe dysfunction of the liver and/or kidneys; or 3) no pathological diagnosis of peritoneal dissemination.
Peritoneal seeding status
We classified peritoneal seeding status according to the first English edition of the Japanese Classification of Gastric Carcinoma (22). The classification was as follows:P0, no peritoneal seeding; P1, disseminated metastasis to the region directly adjacent to the peritoneum of the stomach (above the transverse colon, including the greater omentum); P2, several scattered metastases to the distant peritoneum and ovarian metastasis only; and P3, numerous metastases to the distant peritoneum.
Follow-up
After treatment, the patients were monitored every month for the first year, every 3 months for the second year, and every 6 months thereafter, with regular follow-up assessments. We always followed up using telephone interviews, a follow-up letter, a short message platform and an email.
End points
In our research, the end point is the overall survival of patients with peritoneal dissemination. We followed up all the patients until June 2017 or the death of patients.
Statistical analysis
All data were analyzed using IBM SPSS Statistics (Version 20.0; IBM Corp., New York, USA) and R software (version 3.4.2; R Foundation for Statistical Computing, Vienna,Austria). The clinicopathological parameters between the development cohort and the validation cohort were analyzed using a Chi-square test or Fisher’s exact test.Then, we performed a Kaplan-Meier analysis and Cox regression analysis to evaluate the independent prognostic factors for overall survival of the gastric cancer patients with peritoneal dissemination.
A nomogram was developed as a tool to predict the prognosis of gastric cancer patients with peritoneal dissemination by the months of survival. It graphically presents the patient prognosis. The nomogram and the calibration curve were displayed using the package of Regression Modeling Strategies in R. The predictive performance of this model was evaluated in the test group using the concordance index (C-index) (11). P<0.05 was considered statistically significant.
Results
Patient characteristics
From January 2000 to December 2014, a total of 746 patients with histologically diagnosed GCPD were included in the present study: 684 consecutive patients from Sun Yat-sen University Cancer Center comprised the development cohort, and the other 62 consecutive patients from the Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, China comprised the validation cohort. The flow chart is shown inFigure 1. All cases of peritoneal dissemination were proven clinicopathologically at surgery. The clinicopathological factors for the development set and validation set are shown inTable 1.Among the 684 patients from Sun Yat-sen University Cancer Center, 31 patients received gastrectomy +perioperative chemotherapy; 195 patient received gastrectomy + postoperative chemotherapy; 70 patients received gastrectomy only; 31 patients received bypass surgery + postoperative chemotherapy; 37 patients received bypass surgery only; another 226 patients received palliative chemotherapy only, and the remaining 94 patients did not receive any therapy. In addition, among the 62 patients from the Sixth Affiliated Hospital of Sun Yat-sen University, 3 patients received gastrectomy +perioperative chemotherapy; 45 patient received gastrectomy + postoperative chemotherapy; 3 patients received bypass surgery only; another 3 patients received palliative chemotherapy only; and the remaining 8 patients did not receive any therapy. Regarding hyperthermic intraperitoneal perfusion, 24 patients in the development group and 16 patients in the validation group accepted this treatment after surgery. None of the patients received targeted chemotherapy according to our records. Patient survival was measured from the diagnosis of peritoneal dissemination. The median follow-up time and median survival for all patients were 12.5 (range: 1−152) months and 11.5 [95% confidence interval (95% CI): 10.4−12.6]months, respectively.
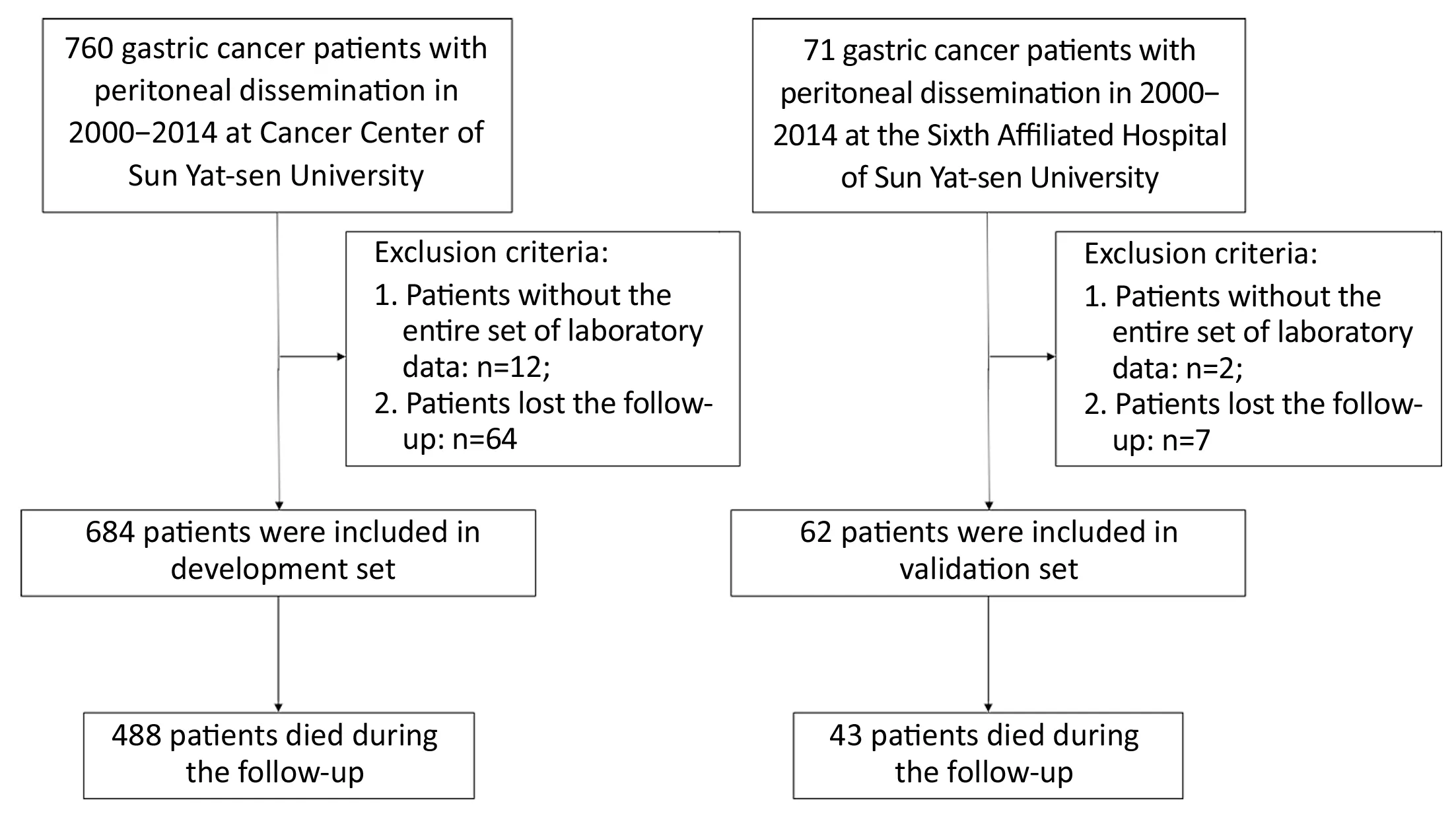
Figure 1 Flow chart of patient selection.
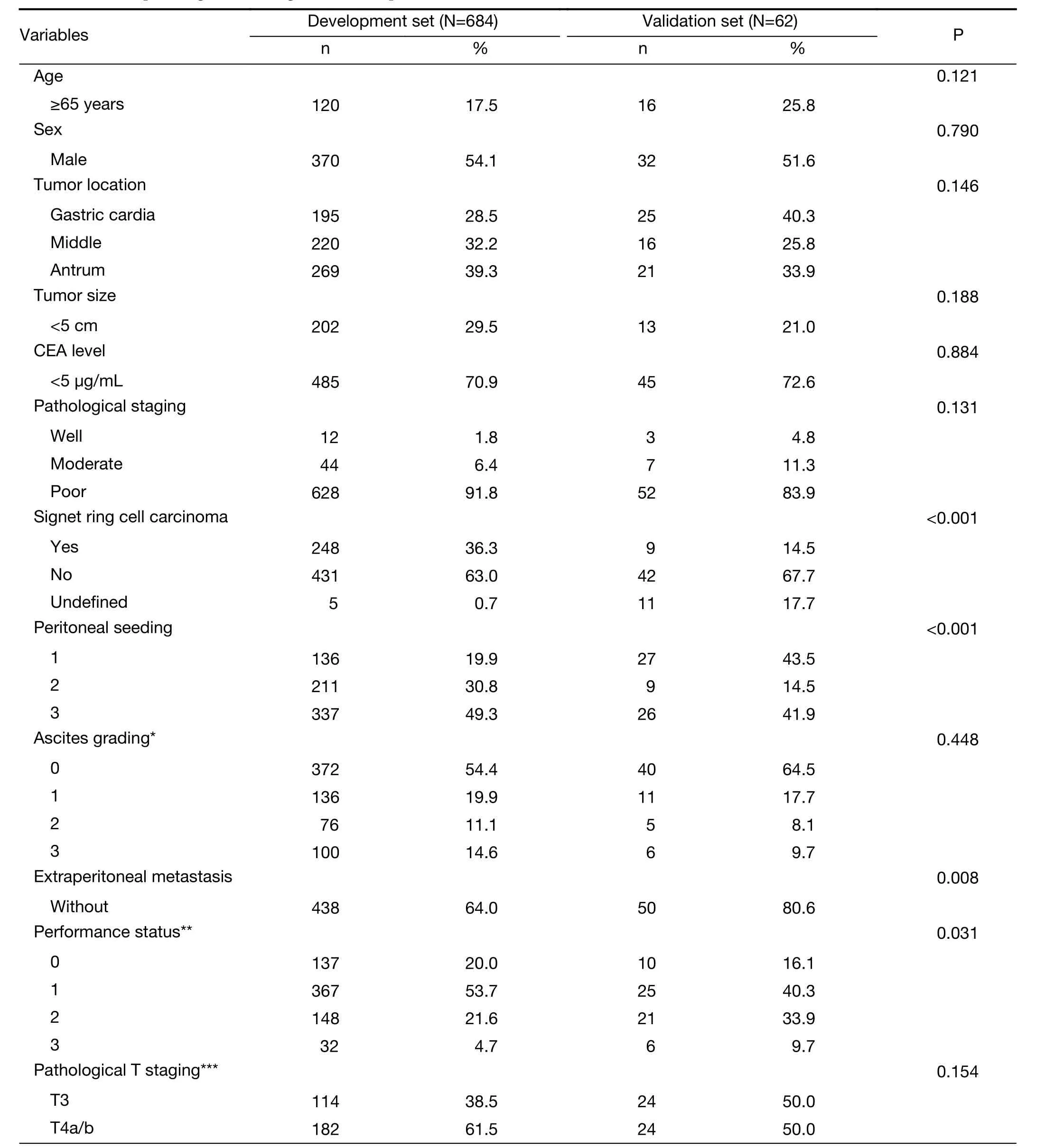
Table 1 Clinical pathological data of gastric cancer patients (N=746)

Table 1 (continued)
Univariate and multivariate analyses (Cox’s regression) of gastric cancer patients in development set
Univariate analysis showed that tumor size, serum carcinoembryonic antigen (CEA) level, ascites grading, presence of extraperitoneal metastasis, seeding status and performance status were prognostic factors for these patients. We included these clinicopathological factors identified as prognostic factors in the univariate analysis (P<0.05) for the Cox regression model. Multivariate analysis showed that serum CEA level, ascites grading, presence of extraperitoneal metastasis, seeding status and performance status were independent prognostic factors for gastric cancer patients with peritoneal dissemination in the development set. The results of the univariate and multivariate analyses are shown inTable 2.
Development and internal validation of nomogram model to predict prognosis of gastric cancer patients with peritoneal dissemination
Along with the visualized tool provided by the nomogram,we then used the Cox regression model to predict the prognosis by months of survival of the gastric cancer patients with peritoneal dissemination. The nomogram model is shown inFigure 2.
We performed an internal validation to validate this nomogram and found that the concordance index was 0.641, which closely corresponded to the actual survival.The internal calibration curves for half-, 1- and 2-year survival are shown inFigure 3. As shown inFigure 3, the black line represents the predicted values of the nomogram,while the gray line represents the actual values. The less discrepant they are, the more precise the predictive capability of the model is. For the internal calibration, the black lines fluctuated above and below the gray lines, to identify a reliable predictive capability of the nomogram.
External validation of nomogram model of gastric cancer patients from the Sixth Affiliated Hospital of Sun Yat-sen University
We used an external validation set, consisting of 62 gastric cancer patients from the Sixth Affiliated Hospital of Sun Yat-sen University, to characterize the discrimination of the newly developed nomogram model. The C-index was 0.709, and the external calibration curves for half-, 1- and 2-year survival are shown inFigure 4. As shown inFigure 4,the goodness of fit indicated relatively satisfactory predictive values in the external validation, even though the predicted half-year survival and the predicted 2-year survival were not as fit as the predicted 1-year survival.
Discussion
Peritoneal dissemination is one of the most common forms of metastasis for gastric cancer. Palliative chemotherapy has been the main treatment administered to gastric cancer patients with peritoneal dissemination. In the past few decades, researchers have devoted efforts toward the development of new treatments for gastric cancer patients with peritoneal dissemination, including radiotherapy,chemotherapy, and molecular targeted drugs (23).Unfortunately, the results of clinical trials have been unsatisfactory, although some patients were reported to experience prolonged survival after they received intraperitoneal perfusion of chemotherapy or combination treatment with chemoradiotherapy (24). Clinically, it is important to predict the prognosis of gastric cancer patients with peritoneal dissemination to ensure that they receive the most optimal treatment.
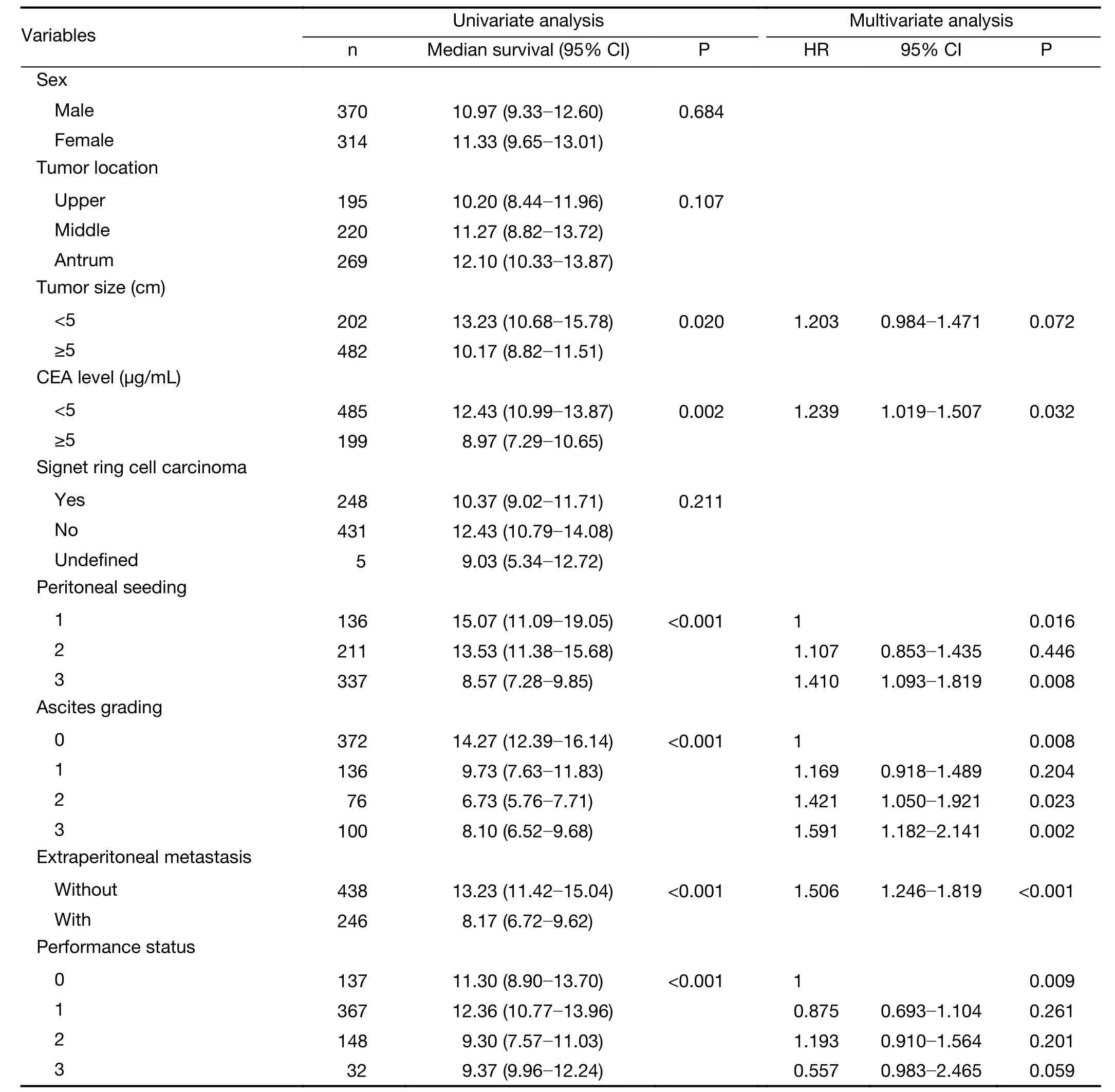
Table 2 Univariate and multivariate analysis of gastric cancer patients in development cohort (N=684)

Figure 2 Nomogram predicting half-, 1- and 2-year overall survival for gastric cancer patients with peritoneal dissemination (GCPD). The nomogram is used by adding up the points identified on the points scale for each variable. The total points projected on the bottom scales indicate the probability of half-, 1- and 2-year survival. Serum carcinoembryonic antigen (CEA) level: 0, negative; 1, positive;extraperitoneal metastasis: 0, without; 1, with.

Figure 3 Internal calibration curve to validate nomogram model for which C-index was 0.641.
Currently, the 7th Edition of the AJCC Staging System is used clinically to predict the survival of gastric cancer patients. However, this system places all the gastric cancer patients with peritoneal dissemination into stage IV. In a previous report, it was found that the patient age, tumor location, total number of lymph nodes retrieved,postoperative recurrence, adjuvant radio/chemotherapy,the Lauren classification, etc. were related to the prognosis of gastric cancer patients (10-14,16-18,25-27). In our present study, we identified serum CEA level, ascites grading, presence of extraperitoneal metastasis, seeding status, and performance status as independent prognostic risk factors for GCPD patients by a Cox regression model.In our research, all the patients were gastric cancer patients with synchronous peritoneal dissemination. The prognostic influence of some proven clinicopathological factors,Lauren type, lymph node metastasis, lymphovascular and perineural invasion might be inconsequential in the presence of distant metastasis. In our previous study, we found that palliative gastrectomy can prolong the survival of GCPD patients without extraperitoneal metastasis when combined with more than five cycles, and particularly more than eight cycles, of first-line chemotherapy (28-37). In another study, it was shown that preoperative or postoperative chemotherapy on the basis of S-1, alone or combined with other chemotherapeutic agents such as cisplatin (SP therapy), paclitaxel or oxaliplatin as first-line chemotherapy can prolong patient survival, with one patient still alive three years after chemotherapy (38-40).

Figure 4 External calibration curve to validate nomogram model for which C-index was 0.709.
In support of the practicality of our model, the C-indexs were 0.641 and 0.709 in the internal and external validation subsets. This finding means that the model was still not precise enough and needed to be optimized, which might be related to the following reasons. First, the treatment strategies in this group of patients were complex. As aforementioned, there were 6 treatment strategies in our data: 1) gastrectomy + perioperative chemotherapy; 2)gastrectomy + postoperative chemotherapy; 3) gastrectomy only; 4) bypass surgery + postoperative chemotherapy; 5)bypass surgery + postoperative chemotherapy; 6) palliative chemotherapy only; and 7) no therapy. In addition, some patients received HIPEC. Different treatments may affect the prognoses of the patients. Second, we only included the preoperative variables to construct the model to make it fit clinically. However, the effects of the treatments were disregarded in the model. Finally, this study had bias because it was a retrospective study; moreover, for the validation group, more patients from multiple centers will be needed for external validation to fully examine this model. However, the calibration was unsatisfactory, which we believe may be related to the different treatment strategies accepted in the validation group compared with those in the development group. The percentage of patients in the validation group who received chemotherapy was lower than that of the development group.This observation may account for the lower fitness of the predicted half-year survival and of the predicted 2-year survival. In addition, the number of patients in the validation group is too small, which also may have caused bias in the calibration curve. In conclusion, we have reasons to believe that the nomogram we constructed has prognostic potential accordingly.
We therefore believe that this model can be used to predict the prognosis of GCPD. Chemotherapy is currently the mainstay of treatment for GCPD patients.However, some of these patients were shown to have a better prognosis under comprehensive therapy that included surgery and other approaches. This model could be practical for suggesting the optimal clinical treatment for these patients. For example, for the patients predicted to have a better prognosis, aggressive treatments including surgery or HIPEC should be considered. For the patients predicted to have a poor prognosis, only palliative therapy should be administered. We are the first to identify possible risk factors for patients with peritoneal dissemination in a nomogram and believe that this model should permit individualized survival prediction and provide better treatment allocation than the existing systems. We believe that our nomogram may assist surgeons in selecting the appropriate treatment for gastric cancer patients with regard to the probability of a survival benefit.
As shown inTable 1, there were several clinicopathological factors that differed significantly between the development group and the validation group, such as signet-ring cell carcinoma, peritoneal seeding, extraperitoneal metastasis,performance status, and palliative chemotherapy. Among them, peritoneal seeding, extraperitoneal metastasis and performance status were included in the nomogram model,which may demonstrate the validity of the model for two different groups of patients. However, the ratio of the palliative chemotherapy group may cause a deviation of the prediction from the model because different treatment strategies may cause survival differences. Another group of patients who had treatment strategies similarly proportional to those of the development group may be needed to validate the accuracy of the model.
There are some limitations associated with our study.First, although we constructed a robust nomogram model for prognostic prediction, more patients from multiple centers will be needed for external validation to fully examine this model. In addition, we removed some patients who had been lost to follow-up or discontinued follow-up for various reasons. Other patients were also removed because of missing data, such as tumor size, precise pathological staging and performance status. Second, the findings may not be generalizable to gastric cancer in Western countries.
Conclusions
We have constructed a nomogram to estimate the survival of gastric cancer patients with peritoneal dissemination.The internal and external validations showed that this model can effectively predict the prognosis of gastric cancer patients with peritoneal dissemination. However,the number of subjects included in the external validation set was too small. More data are needed to validate and modify this model.
Acknowledgements
This work was supported in part by a major special project grant from Guangzhou Health and Medical Collaborative Innovation (No. 15570006) and the Tianhe District Science and Technology Project, Guangdong, China (No.201434KW020).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Evaluation of menopausal status among breast cancer patients with chemotherapy-induced amenorrhea
- Increased incidence of chemoport-related thrombosis in patients with colorectal cancer receiving bevacizumab: A singleinstitutional experience
- Annual cost of illness of stomach and esophageal cancer patients in urban and rural areas in China: A multi-center study
- A radiomic nomogram based on an apparent diffusion coefficient map for differential diagnosis of suspicious breast findings
- Differential study of DCE-MRI parameters in spinal metastatic tumors, brucellar spondylitis and spinal tuberculosis
- Radiomic analysis of pulmonary ground-glass opacity nodules for distinction of preinvasive lesions, invasive pulmonary adenocarcinoma and minimally invasive adenocarcinoma based on quantitative texture analysis of CT
