Exploration of Antifouling Potential of the Brown Algae Laminaria ‘Sanhai’
2018-08-28LIXiangminLIFengchaoJIANHuiminandSURongguo
LI Xiangmin, LI Fengchao, JIAN Huimin, and SU Rongguo
Key Laboratory of Marine Chemistry Theory and Technology, Ministry of Education, Ocean University of China,Qingdao 266100, China
(Received April 14, 2017; revised October 20, 2017; accepted November 13, 2017)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2018
Abstract Seaweeds are one of the largest producers of biomass in the marine environment. It has been well known that marine algae, especially brown algae was a rich source of biogenic compounds with antifouling potential that could be ideal alternatives of tributyltin (TBT). In this paper, antifouling potential of the brown algae Laminaria ‘sanhai’ was explored. Firstly, the dried alga was extracted and the antialgal and antilarval activities were investigated. The EC50 and LC50 values of crude extract of Laminaria ‘sanhai’ against diatom (Skeletonema costatum) and barnacle larval (Chthamalus challengeri) were 8.9 μg mL-1 and 12.0 μg mL-1 respectively. Then, guided by bioassay, the bioactive substances were isolated by liquid-liquid extraction. The antialgal and antilarval activities of isolated fraction were improved with the EC50 value of 7.4 μg mL-1 against S. costatum and LC50 value of 9.7 μg mL-1 against C. challengeri larvae. Identification by IR, Q-TOFMS and GC-MS of the isolated bioactive substances revealed the abundance of fatty acids. These fatty acids, most with 16, 18 or 20 carbon atoms, contained myristic, hexadecanoic, oleic, linolenic, arachidonic and eicosapentaenoic acids. The results indicated that both the crude extract and the isolated bioactive substances had high antialgal and antilarval activities with no highlighted cytotoxicity which made the brown algae Laminaria ‘sanhai’ a promising source of the environmentally friendly antifoulants.
Key words antifouling; artificial breeding; brown algae; crude extract; fatty acids; IR Q-TOFMS and GC-MS
1 Introduction
Biofouling, a natural phenomenon, causes many problems to engineered structures such as ships, offshore rigs and jetties by way of increased use of manpower, fuel,material and dry-docking time (Callow et al., 2002;Chambers et al., 2006). Since the early 1960s, tributyltin(TBT) has been used widely in marine paint formulations,but evidence of harmful effects to many non-target organisms was followed (Evans et al., 1995; Omae, 2003;Yebra et al., 2004). Due to the environmental pressure caused by TBT, the International Maritime Organisation(IMO) banned the use of triorganotin-based paints worldwide (Champ, 2000). In order to develop effective but environmentally friendly natural antifoulants, work is progressing worldwide (Hellio et al., 2002; Sipkema et al.,2005; Raveendran et al., 2008).
Among these investigations, marine natural products have been highlighted as promising environmental friendly antifoulants (Kjelleberg and steinberg, 1994; Yebra et al., 2004; Raveendran et al., 2008). Many organisms including marine seaweeds, sponges, corals, ascidians and others are believed to produce rich natural antifouling substances that could get rid of fouling organisms(Kharchenko et al., 2012). Some novel natural products for defense purposes had been isolated and investigated,and they all showed antifouling activities with less toxic and more effective compared to the organotin compounds(Proksch et al., 2002; Qian et al., 2010), which indicated the strong antifouling potential of marine natural products.For example, Seaweeds, one of the most studied marine organisms for the isolation of AF compounds, were found to produce a variety of bioactive compounds with high antifouling activity, including fatty acids, lipopetides,amides, alkaloids, terpenoids, lactones, pyrroles and sterols (Bhattarai et al., 2006) .
The brown macroalgae, one widespread species of seaweeds, had been paid much attention for its high antifouling potential and some antifouling compounds were isolated and identified successfully, such as diterpene alcohols from Dictyota menstrualis (Schmitt et al., 1995,1998), diterpenes from Canistrocarpus cervicornis (Bianco et al., 2009), and fatty acids from Sargassum (Hossain et al., 2003; Kornprobst, 2005).
In this paper, the antifouling potential of the brown macroalga Laminaria ‘sanhai’ was explored. The Laminaria ‘sanhai’, belonging to Phaeophyceae, Laminariales,Laminariaceae, Saccharina, was achieved by artificial breeding (Laminaria japonica ♀ × Saccharina latissima♂) with excellent qualities on the basis of the original varieties such as high-temperature-resistance and high production, and it was widely planted in China and commercially available. We investigated the antialgal and antilarval activities of the crude extract and isolated bioactive substances from Laminaria ‘sanhai’, and identified their chemical structures by IR, Q-TOFMS and GC-MS.
2 Materials and Methods
2.1 Sample Pretreatment
The Laminaria ‘sanhai’ was provided by Culture Collection of Seaweed of the Ocean University of China. The Laminaria ‘sanhai’ sample was rinsed in sterile water and 5% ethanol in order to remove associated micro flora.Cleaned samples were then surface dried by pressing it briefly between the sheets of filter papers and air dried under shade at room temperature in order to prevent photolysis and thermal degradation of metabolites. The dried seaweeds were ground to powder and packed in polyethylene bags and stored in moisture free place before use.
2.2 Extraction and Purification
2.2.1 The crude extract
The dried algae was suspended by stirring in 95% ethanol (200 g in 300 mL) for 4 h at 4℃, the resultant pellet was re-extracted five times in the same way. The alcoholic extracts were combined and evaporated under vacuum at low temperature (< 40℃). Distilled water (100 mL)and dichloromethane (400 mL) were then added, shaked well and stratified. The organic phase was collected, left dry in presence of Na2SO4for 24 h, filtered and concentrated under vacuum at low temperature until the volume reaches 5 mL to 10 mL. Following that, the concentrated extract was transferred to a 50 mL centrifuge tube and dried by blowing N2(Hellio et al., 2001). Then the dried crude extract was weighted, finally the dried crude extract dissolved in 0.5 mL DMSO and diluted with Milli-Q water to obtain a 50 mg mL-1stock solution stored in refrigerator before use.
2.2.2 Purification of the crude extract
The crude extract was applied in a C18 solid phase extraction (SPE) column developed with methanol and eluted with n-hexane and dichloromethane (100%n-hexane, 5:5, 100% dichloromethane) into three fractions. The individual fractions were screened for antialgal activities. Based on the results of antialgal activities, the fractions that was eluted using n-hexane: dichloromethane (5:5) and 100% dichloromethane were combined and concentrated under vacuum (< 40℃), and then solubilized in 20 mL CH2Cl2and partitioned by NaOH (0.2 mol L-1) three times. Then the aqueous phases were combined, neutralized by H2SO4(1.0 mol L-1) and extracted by dichloromethane. The finally organic phases resulting from this neutralization were collected and concentrated under vacuum at low temperature (< 40℃) and then dissolved in 0.2 mL DMSO and diluted into solution (10 mg mL-1) using Milli-Q water before use. Following each step of purification procedure, the antialgal tests were performed to make sure that the bioactive substances were kept.
2.3 Bioassays
In this study, diatom Skeletonema costatum (S. costatum) and barnacle larval Chthamalus challengeri (C.challengeri) were used in antifouling activity tests. Diatoms were the main components of slime films in marine environments. It attached to and reproduced on all but the most toxic surface (Marsalek et al., 1979; Baier, 1980;Callow et al., 1986). Barnacle larval was also a common kind of fouling organism worldwide (Swain et al., 1998;Brady Jr et al., 2000). They were often used as test organisms for antifouling activities of antifouling compounds. In these experiments, we had made sure that the DMSO volume content (< 0.1%) was safe to the target fouling organism (Okumura et al., 2001). The filtered fresh sea water (GF/F Filter, 0.45 μm) was used in the whole experiment.
2.3.1 Antialgae activities
The marine diatom S. costatum was obtained from the Marine Eco-Pollution Chemistry Laboratory of the Ocean University of China. The S. costatum was cultivated in 250 mL Erlenmeyer flasks containing Guillard’s F/2 medium under continuous illumination (5000 lux white fluorescent lamps) at 20℃ with a 12 h:12 h light:dark cycle.Culture medium were all autoclaved (120℃, 20 min) and then inoculated with 20 mL cultivated microalgae in the exponential growth phase in 5 L Erlenmeyer flasks containing 3 L media under aseptic conditions. The cultures were shaken three times daily by hand to allow sufficient gas exchange until the microalgae were incubated to the exponential growth period (3 or 4 d) and reached a dentistry of 105cells mL-1, then they were collected at the light cycle time for the test.
All the experiments were carried out in triplicate. Tests for bioactivity were performed in 250 mL Erlenmeyer flasks filled with 100 mL S. costatum suspension in exponential growth period. The chlorophyll-a fluorescence intensities (FI) were determinated by a F4500 fluorometer(Hitachi, Japan) as an indicator of algal biomass (Suggett et al., 2011). A decrease in chlorophyll-a FI indicated algae growth inhibition by the extract. The percentage of growth inhibition was calculated as:
% inhibition = (FIC- FIT)/ FIC*100,
where FICwas the mean chlorophyll-a fluorescence intensity of the controls and FITwas the mean chlorophyll-a fluorescence intensity of the test samples.
2.3.2 Antilarval activities
The Chthamalus challengeri were collected from the intertidal zone in Zhanqiao, Qingdao. After collected,washed the sludge and other organisms away and dried in shade, the Chthamalus challengeri was cultured with fresh filtered seawater. Under light irradiation, the adult barnacle released larvae. The larvae were collected, cultured for 24 h, and then could be used in the antilarval experiments.
The anti-larval experiments were conducted in sterile 3 mL 24-well polystyrene plates. The filtered seawater(0.45 μm) was used as control. Each well contained about 30 barnacle larvae in 3 mL of the test solution. The plates were incubated at 25℃ for 24 h in plant growth chamber under in the dark. All tests were performed in triplicate.The antilarval activities were determined by counting the dead and live individuals and expressing the result as a proportion of the total number of larvae in the well (the percentage of larval death).
2.4 Statistical Analysis
The data from antialgal and antilarval tests were analyzed by SPSS Version 11.5 software package. The differences between the experimental treatments and the control samples were determined using one-way ANOVAs followed by Dunnett’s test at 95% confidence level. The EC50s were calculated as the concentration at 50% of the maximal effect of S. costatum compared with the control and the LC50s as the concentration at which 50% of larvae were dead compared with the control. And they were computed from the test results using probit analysis.
2.5 Identification of the Isolated Bioactive Substances
The isolated bioactive compounds were analyzed and identified by IR, Bruker maXis quadrupole time-of-flight mass spectrometry (Q-TOFMS) and ITQ-1100 gas chromatography-mass spectrometry (GC-MS).
3 Results and Discussion
3.1 Antialgal and Antilarval Activities of the Crudextract
For algal inhibiting test, the proper volumes of the crude extract stock solution were added to algae cultures so that its concentrations were 0, 5.0, 7.5, 10.0, 15.0, and 25.0 μg mL-1, respectively, and the chlorophyll-a FI were recorded every 24 h for 96 h. Based on ANOVA and Dunnett’s analyses, the crude extract, when tested at 7.5 μg mL-1, displayed significant toxicity when compared to the control (P < 0.05). The crude extract with concentrations below 7.5 μg mL-1did not inhibit the growth of S. costatum. But when the crude extract concentration was increased up to 10.0 μg mL-1, the algal growth was significantly inhibited (Fig.1). The EC50of the crude extract against S. costatum was 8.9 μg mL-1.
A series of aqueous solutions were prepared for antilarval activity test by diluting the crude extract stock solution with the filtered fresh sea water, and the concentrations were set at 0, 5.0, 7.5, 10.0, 20.0 and 30.0 μg mL-1,respectively. After 24 h exposure, the crude extract induced C. challengeri larvae death in a dose-dependent manner (Fig.2). Based on ANOVA and Dunnett’s analyses,the crude extract, when tested at 7.5 μg mL-1, displayed significant toxicity when compared to the control (P <0.05). When the concentration of the crude extract was above 20.0 μg mL-1, more than half of test barnacle larvae were killed and the LC50of the crude extract against C.challengeri larvae was 12.0 μg mL-1.
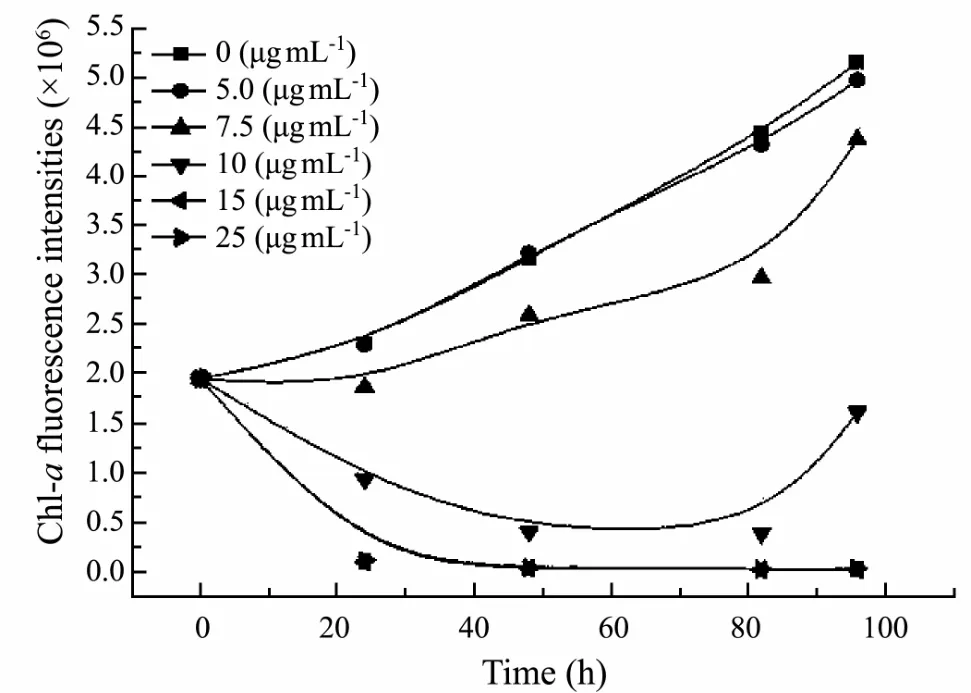
Fig.1 Growth curve of S. costatum subjected to different concentrations of the crude extract during a 96 h growth period.
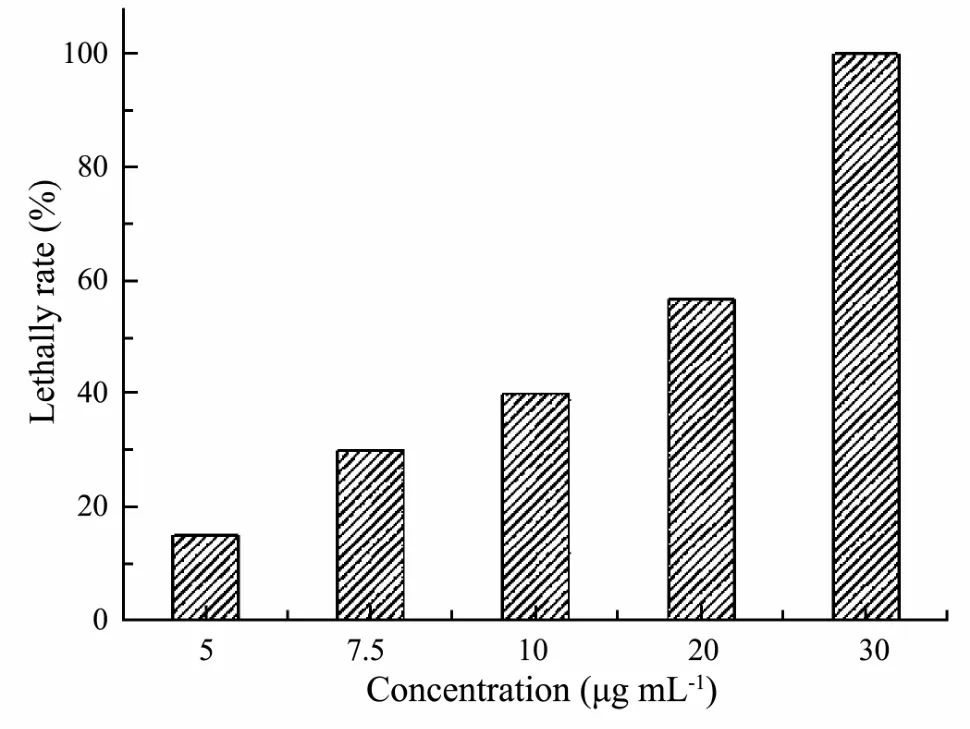
Fig.2 Lethal effects of the crude extract against C. challengeri larvae after 24 h exposure.
Hellio et al. (2002) reported that the dichloromethane extract of Ulva lactuca could inhibit the growth of the diatom Phaeodactylum tricornutum with EC50of above 300.0 μg mL-1. They (Hellio et al., 2001) also found that the crude extract of brown algae Bifurcaria brassicaeformis had antialgal activity and when its concentration was up to 30 μg mL-1, more than 60% attachment of spores of macroalgae (such as Enteromorpha intestinalis)was inhibited. Águila- Ramírez et al. (2012) presented that the MIC (the minimum inhibitory concentrations)values of crude extracts of Ulva lactuca and Laurencia johnstonii against some species of temperate marine microalgae were ranged between 10 and 25 μg mL-1. The antifouling activities of the dichloromethane extract of Himanthalia elongata and crude extract of Lyngbay ma-juscula were also reported with with an LC50of 96.2 μg mL-1against Amphibalanus amphitrite (Maréchal and Hellio, 2011) and an LC50of 20 μg mL-1against Amphibalanus amphitrite, respectively (Tan et al., 2010).Comparatively, the crude extract of Laminaria ‘sanhai’had significantly higher antialgal and antilarval activities.
3.2 Antialgal and Antilarval Activities of the Purified Bioactive Substances
The stock solution of purified substances was added to algae cultures to make its concentrations 0, 2.5, 5.0, 7.5,10.0, and 20.0 μg mL-1, respectively, and the chlorophyll-a FI were recorded every 24 h for 96 h (Fig.3).Based on ANOVA and Dunnett’s analyses, the purified substances, when tested at 7.5 μg mL-1, displayed significant toxicity when compared to the control (P < 0.05). The purified substances at concentration below 5.0 μg mL-1had no obvious algal growth inhibition and when the concentration of the purified substances reached up to10.0 μg mL-1, the FI of S. costatum was too weak to be detected. The EC50of purified substances toward S. costatum was determinated as 7.4 μg mL-1.
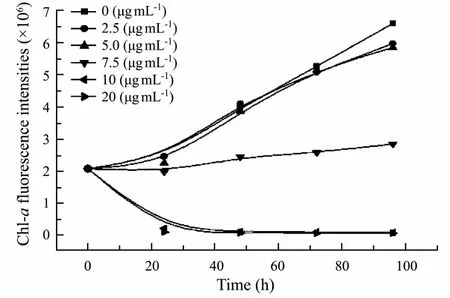
Fig.3 Growth curve of S. costatum subjected to different concentrations of purified substances during a 96 h growth period.
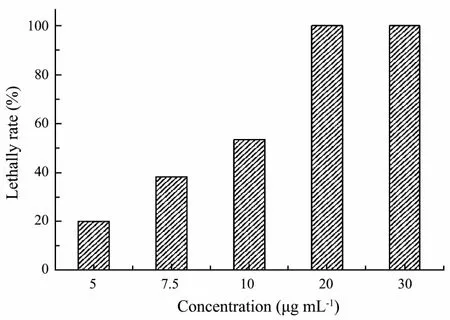
Fig.4 Lethal effects of the purified substances against C.challengeri larvae after 24 h exposure.
Aqueous solutions of 0, 5.0, 7.5, 15.0, 25.0 and 30.0 μg mL-1purified substances were prepared for antilarval activity test. Exposure to the purified substances for 24 h also increased the C. challengeri larvae death in a dosedependent manner (Fig.4). Based on ANOVA and Dunnett’s analyses, the purified substances, when tested at 7.5 μg mL-1, displayed significant toxicity when compared to the control (P < 0.05). More than half of the larvae were dead at the concentration of 15.0 μg mL-1compared to the control. And when the concentration of the purified substance reached up to 25.0 μg mL-1, all of the tested larvae died. The LC50of purified substances toward C. challengeri larvae was 9.7 μg mL-1.
Some researchers had reported some natural bioactive compounds with antifouling potential. The palmitic acids from macroalgae Sargassum muticum could inhibit the growth of the diatom Cylindrotheca closterium with an EC50value of 45.5 μg mL-1(Bazes et al., 2009). Two fatty acids, 9, 12-Octadecadienoic acid) and n-Hexadecanoic acid, isolated from Laurencia brandenii, showed antilarval acitivities with the EC50of 0.912 mg mL-1and LD50of 1.48 mg mL-1against Balanus amphitrite settlement, respectively (Manilal et al., 2010), the bis-1-oxaquinolizidine alkaloids isolated from marine sponge Haliclona exigua showed settlement inhibition activity against barnacle cypids with LC50of 18.07 μg mL-1(Raveendran et al., 2008), and the linear diterpenes from brown algae Bifurcaria bifurcate had a LC50value of 38.3 μg mL-1against cyrids of Balanus amphitrite (Maréchal et al.,2004). Recently, Cho reported that the chromanol class isolated from brown algae Sargassum horneri with EC50of 0.08-1.79 μg mL-1against diatom Navicula annexa and EC50of 1.4-6.4 μg mL-1agaist mussel larvae settlement(Cho, 2013). From the above, the purified bioactive substances from Laminaria ‘sanhai’ had high antialgal and antilarval activities and could be considered as a promising source of natural antifouling compounds.
3.3 Identification of the Bioactive Compounds
The bioactive substances with high antialgal and antilarval activities was isolated through the bioassay-guided isolation procedure, in which, the alkali washing and extraction under acidic conditions were key steps that indicated that the bioactive substances should be fatty acid compounds. After bioassays, the isolated bioactive substances were subjected to IR, Q-TOFMS and GC-MS analysis for chemical structure elucidation.
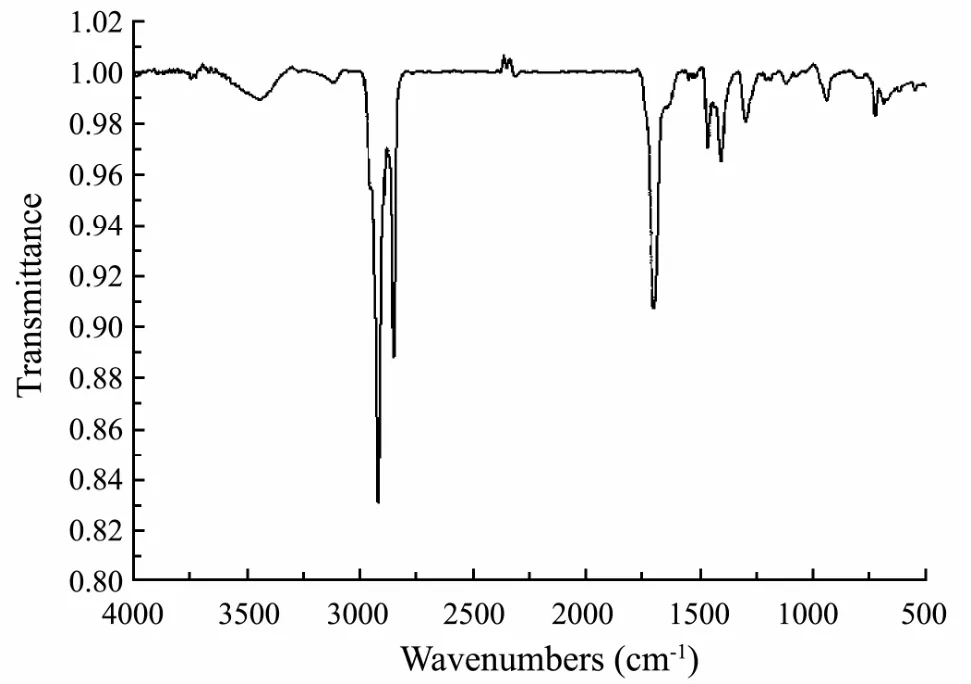
Fig.5 IR analysis of the bioactive compounds.
3.3.1 IR identification of the bioactive compounds
The IR analysis result was shown in Fig.5. There were three strong peaks. Two of the peaks’ wave-numbers were about 3000 which was in accord with the IR wave number of the carboxylic acid hydroxyl (3400-2500 cm-1).The wave number of about 1700 was the IR characteristic peak of carbonyl group in carboxylic acid. So the IR detection confirmed the existence of carboxyl group and the isolated bioactive substances should be mainly composed of fatty acid compounds.
3.3.2 Q-TOFMS identification of the bioactive compounds
Negative mode ionization that removed a hydrogen ion was developed to screen for possible candidate compounds (Fig.6). SmartFormula3D was used to identify the fragment and give the sum formula information in the analysis. The bioactive compounds contained 16, 18 or 20 carbon atoms based on the molecular formula (Table 1).So the isolated bioactive compounds should be a series of unsaturated and saturated fatty acids that contains 16 or 18 carbon atoms based on the molecular formula provided by Q-TOFMS and the functional group provided by the IR. The exact chemical structures of the isolated bioactive compounds would be given by the following GCMS analysis.
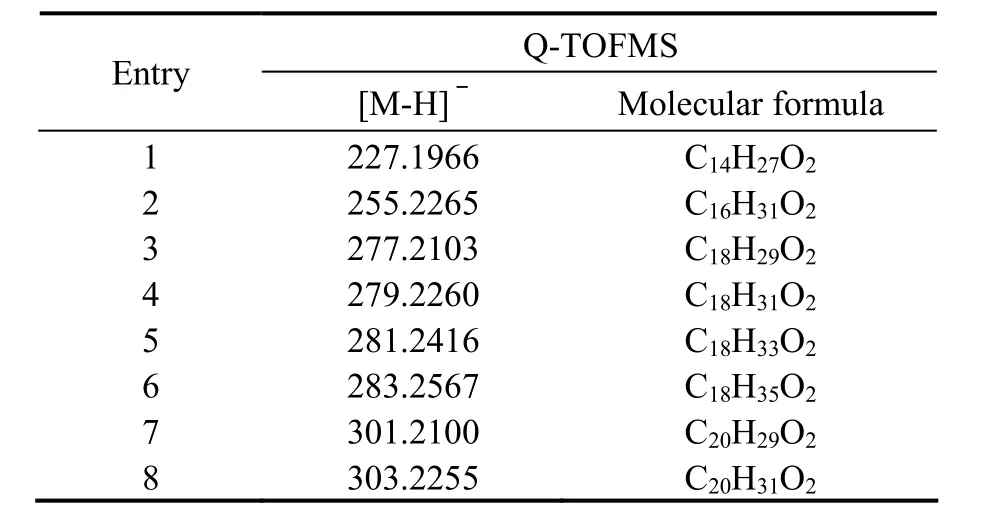
Table 1 the molecular formulae, average mass accuracies of the observed fragments
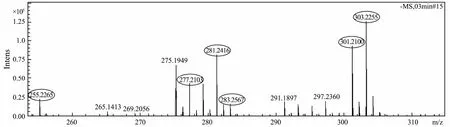
Fig.6 Negative mode ionization of the bioactive compounds provided by the Q-TOFMS.
3.3.3 GC-MS identification of the bioactive compounds
Results from the optimized GC-MS analysis indicated the main active ingredients of the isolated bioactive substances were ten fatty acids, oxalic acid, myristic acid,hexadecanoic acid, octadecanoic acid, 9-octadecenoic acid, 9,12-octadecadienoic acid, 6,9,12-octadecatrienoic acid, 9,12,15-octadecatrienoic acid, 6,9,12,15-Eicosatetraenoic acid, 5,8,11,14-eicosatetraenoic acid and cis-5, 8,11, 14, 17-eicosapentaenoic acid (Fig.7), they were a series of unsaturated and saturated 16, 18 or 20 carbon fatty acids. Among those fatty acid compounds, Octadecanoic acid accounts for 21.64% by GC peak area integration with total area detected normalized to 100%, hexadecanoic acid for 20.11%, oxalic acid for 18.49%, oleic acids for 8.86%, and myristic acid for 6.29% (Table 2).
Some fatty acids could exert a high level of inhibition against certain species of microorganism (Willett and Morse, 1966; Miller et al., 1977) and people have attached much importance to fatty acids as a promising alternative to TBT (Russel, 1991; Bhattarai et al., 2006). Hexadecanoic acid, isolated from macroalgae Sargassum muticum, showed good antialgal activity against the diatomCylindrotheca closterium with an EC50value of 45.5 μg mL-1(Bazes et al., 2009). Octadecanoic acid, 5,8,11,14-Eicosatetraenoic acid, and 5,8,11,14,17-Eicosapen-taenoic acid, isolated from two congeneric sponges from Hong Kong and the Bahamas, had been proved with highly antibacterial activities (Lee et al., 2007). Subergorgic acid, produced by a gorgonian, inhibited settlement of Balanus amphitrite larvae with LC50value of 200 μg mL-1(Qi et al., 2008). Myristic and hexadecanoic acids, isolated from marine fungus Aureobasidium pullulans,showed high inhibition effects against Skeletonema costatum and Amphibalanus amphitrite larval with an EC50of 49.4 μg mL-1and LC50of 22.2 μg mL-1respectively(Gao et al., 2013). Comparatively, the fatty acid compounds isolated from Laminaria ‘sanhai’ exerted much higher antifouling activity against S. costatum and C.challengeri.
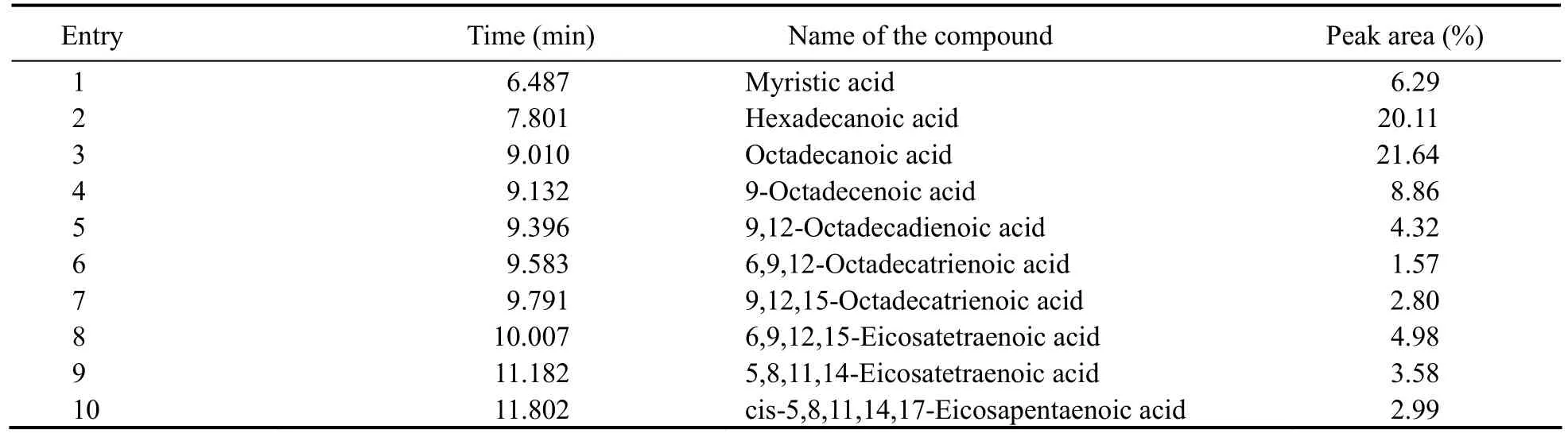
Table 2 The compounds identified by GC-MS analysis
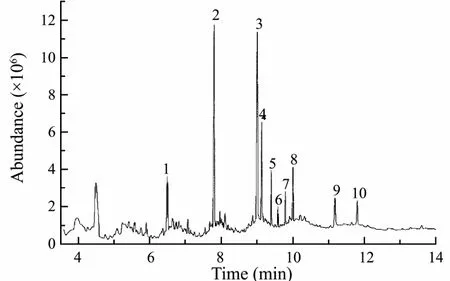
Fig.7 GC-MS analysis of the bioactive compounds.
4 Conclusions
In conclusions, the bioactive substances isolated from artificial breeding Laminaria ‘sanhai’ had high antialgal and antilarval activities, and eleven fatty acid compounds were identified as the main active ingredients. Unlike the high toxicity and environmental persistency of the organotin compounds, those natural bioactive compounds are reversible toxicity and non-residual effect, which made them promising sources of environmental friendly antifoulants.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 41376106), the National Key Research and Development Program (No.2016YFC1402101) and the research program from National Marine Hazard Mitigation Service (No.2014AA060). We are thankful to Prof. Tao Liu of College of Marine Life of Ocean University of China for providing the Laminaria ‘sanhai’.
杂志排行
Journal of Ocean University of China的其它文章
- Effect of Different Dietary Protein and Lipid Levels on the Growth, Body Composition, and Intestinal Digestive Enzyme Activities of Juvenile Yellow Drum Nibea albiflora (Richardson)
- Modelling Wave Transmission and Overtopping Based on Energy Balance Equation
- Extreme Sea Level Rise off the Northwest Coast of the South China Sea in 2012
- Spatial Distribution and Seasonal Variation of Explosive Cyclones over the North Atlantic
- The Influence of Two Kinds of El Niño Events on the Strong Tropical Cyclone Generation and Strength in the Pacific Ocean
- Sea State Bias Estimation with Least Absolute Shrinkage and Selection Operator (LASSO)
