Spatial Distributions and Potential Sources of Long Chain(C30, C32 1,15-) Alkyl Diols in Surface Sediments from Eastern China Marginal Seas
2018-08-28YUMengZHANGHailongLILiandZHAOMeixun
YU Meng , ZHANG Hailong LI Li and ZHAO Meixun ,
1) Key Laboratory of Marine Chemistry Theory and Technology (Ocean University of China), Ministry of Education,Qingdao 266100, China
2) Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China
(Received October 3, 2017; revised May 24, 2018; accepted May 29, 2018)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2018
Abstract Long chain alkyl diols have shown important potential for the reconstruction of sea surface temperature, productivities and upwelling conditions in marine or lacustrine environments. However, little is known about the distribution and sources of the diols in eastern China marginal seas (CMS), which are areas of important organic carbon sink. Here the contents of C30 and C32 1,15-diols were analyzed in 181 surface sediments from eastern CMS. The similar distribution pattern and strong linear correlation between C30 and C32 diols indicated that they had similar biological source, with a dominance of C30 diol. Their contents ranged from 7-2726 ng g-1 for C30 diol and 5-669 ng g-1 for C32 diol, and both showed higher values mainly in the mud area of the Yellow Sea,while the TOC normalized contents showed a more obvious seaward increasing trend. The similar distribution pattern and significant positive correlation between diols and the other marine biomarkers (brassicasterol, dinosterol, C37 alkenones) indicated C30 and C32 diols in eastern CMS were mainly from marine algae. This conclusion was also supported by principal component analysis (PCA).Our results also showed that sediment diol contents were generally related to marine productivity, suggesting that diols could be applied for marine productivity reconstruction in eastern CMS.
Key words long chain alkyl diols, spatial distribution, eastern China marginal seas, marine biomarkers
1 Introduction
Long chain alkyl diols (LDs) are common biomarkers both in marine and freshwater environments since their first identification in Unit I and II sediments from the Black Sea (de Leeuw et al., 1981). They have been detected in various marine settings (e.g., restricted, upwelling and open ocean) and freshwater lakes from low to high latitudes (Versteegh et al., 1997, 2000; Pinturier-Geiss et al., 2002; Xu et al., 2007; Speelman et al., 2009;Shimokawara et al., 2010; Zhang et al., 2011). In natural environment, long chain diols can be produced by distinct sources in several structurally related series. For example,eustigmatophytes are the major producers of LDs in lake surface water (Villanueva et al., 2014); while marine diatom genus Proboscia is a major source for C28, C301,14-diols (Sinninghe Damsté et al., 2003); but LDs also occurred in the cuticular waxes of some terrestrial plants(Jetter, 2000). However, as the potential producer of both 1,13- and 1,15-diols in marine settings, eustigmatophytes are rarely reported and still unclear if they are the producers of diols in marine sediments (Volkman et al.,1992, 1999; Versteegh et al., 1997).
Although their sources are not fully constrained, the wide occurrence and spatiotemporal distributions of LDs in marine sediments can provide valuable information about their biological origins and environmental change.Recently, a number of studies have shown the potential application of long chain diols for marine palaeoenvironment reconstruction. The contents of LDs in sediments have been used as proxies of phytoplanktonic productivity (Li et al., 2014; Plancq et al., 2014). The diol indexratio between C30and C321,15-diols was proposed to be potentially related to salinity and surface water conditions(Versteegh et al., 1997, 2000; Pinturier-Geiss et al., 2002).Sinninghe Damsté et al. (2003) identified Proboscia as a biological source of 1,14-diols and suggested that 1,14-diols can be applied to indicate high-nutrient conditions such as upwelling areas. Then another diol index based on the ratio of 1,14-diols and 1,13- or 1,15-diols has also been used as indicators for upwelling (Rampen et al., 2008, 2014; Willmott et al., 2010; Seki et al., 2012;Nieto-Moreno et al., 2013). Furthermore, the long chain diol index (LDI), de fi ned as the abundance of the C301,15-diol relative to those of C281,13-, C301,13- and C301,15-diol abundances, was introduced as a promising new paleothermometer (Rampen et al., 2012). The LDI has been subsequently applied in paleoclimate reconstruction studies with other organic paleothermometers which can provide more information of paleoceanographic records(Naafs et al., 2012; Rampen et al., 2012; Lopes Dos Santos et al., 2013; Smith et al., 2013; Rodrigo-Gámiz et al.,2014; Becker et al., 2015).
The eastern China marginal seas (CMS) between the Asia continent and Pacific Ocean, including the Bohai Sea (BS), the Yellow Sea (YS) and the East China Sea(ECS), are very important organic carbon sinks due to large riverine input and continuous mud area. Recently,paleoceanographic reconstructions based on lipid biomarkers have been successfully applied for sea surface temperature, marine productivity, organic matter sources and anammox activity (Xing et al., 2011a, 2011b, 2014;Tao et al., 2012; Yuan et al., 2013; Hu et al., 2016; Wu et al., 2016) etc. LDs have been detected in particulate organic matter, in marine and lake sediments mainly from southern China, e.g., South China Sea (Hu et al., 2001; Li et al., 2014; Zhu et al., 2014); Huguangyan maar Lake(Wang et al., 2013); Lugu Lake (Zhang et al., 2015).C30-32diols contents in South China Sea sediments could reflect the eustigmatophyte productivity and the diol indices recorded paleoceanographic and climate changes during the last 30000 years (Hu et al., 2001). Wang et al.(2013) found that the long-chain diol homologs (C28-32) in Huguangyan maar Lake sediments mainly came from phytoplankton in the middle water column and the diol indices in suspended particles had strong relationship with water temperature. However, few studies of LDs in eastern CMS marine surface sediments are reported. So in this study, the contents of C30and C321,15-alkyl diols(abbreviated as C30and C32diols) were analyzed in 181 surface sediments covering eastern CMS to reveal their spatial distributions. By comparing diol data with published data of other lipid biomarkers in these samples, we aimed to reveal the factors controlling diol distributions and their potential sources, and to evaluate implications for using LDs based proxies for environmental reconstruction of the eastern CMS.
2 Materials and Methods
2.1 Sample Collection
The map of the surface sediment sites is shown in Fig.1.A total of 181 surface sediment samples covering eastern CMS were all collected using a box corer and a grab sampler during the research cruises which were conducted in August 2008 (Xing et al., 2014), June 2011(Fan et al., 2014a, 2014b; Xing et al., 2016), August 2011(Zhao et al., 2013) and April 2012 (Fan et al., 2015) respectively. All samples were stored at -20℃ before analysis.
Fig.1 Sampling sites located in eastern China marginal seas.
2.2 Experimental Analysis
For lipid biomarker analyses, detailed procedures have been provided by Xing et al. (2011a). Briefly, about 5 g freeze-dried and homogenized samples were extracted four times with a mixture of dichloromethane/methanol(DCM/MeOH, 3:1, v/v) by ultrasonication, after adding n-C24D50and C19n-alkanol as internal standards. The total lipid extracts (TLE) were hydrolyzed with 6% KOH in MeOH and separated into non-polar and polar fractions by silica gel chromatography using hexane and DCM/MeOH (95:5, v/v) as eluents, respectively. The non-polar fraction containing n-alkanes was dried under N2steam before instrumental analysis. The polar fraction containing alkenones, alkanols, diols and sterols was dried and derivatized with N,O-bis(trimethylsily) trifluo- roacetamide (BSTFA) at 70℃ for 1 h before instrumental analysis. Biomarker identification was performed with a Thermo gas chromatography-mass spectrometry (GC-MS)and by comparison of retention times of standards and mass spectra with those reported in literatures. Biomarker contents were quantified with an Agilent 6890N GC by using a HP-1 capillary column (50 m × 0.32 mm × 0.17 μm)and H2as the carrier gas. The oven temperature was programmed to increase from 80 to 200℃ at 25℃ min-1, 200 to 250℃ at 4℃ min-1, 250 to 300℃ at 1.8℃ min-1, and was then held at 300℃ for 15 min (Xing et al., 2011a).The average relative standard deviation was less than 10%.
For TOC analysis, homogenized samples were acidized with 4 mol L-1HCl at room temperature for 24 h. After rinsing with deionized water to neutral and drying in an oven at 55℃, the carbonate-free samples were measured for TOC% using a Thermo Flash 2000 Elemental Analyzer, with a standard deviation of 0.02 wt.% (n = 6).
Previous studies have reported TOC%, biomarker content of brassicasterol (B), dinosterol (D), C37alkenones(A) and long chain odd number alkanes or TOC normalized biomarker contents (Zhao et al., 2013; Fan et al.,2014a, 2014b, 2015; Xing et al., 2014, 2016) of these samples.
The terrestrial and marine biomarkers ratio (TMBR)based on terrestrial and marine biomarkers was defined as follows (Xing et al., 2011a):
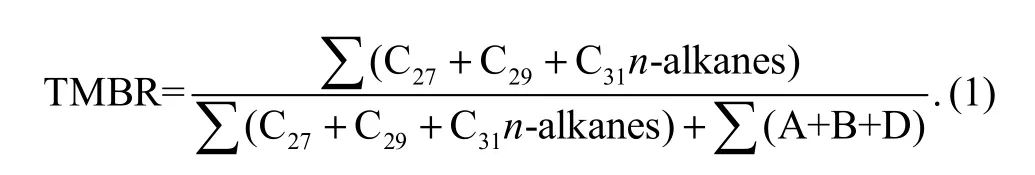
The following diol index was used in our study (Versteegh et al., 1997):
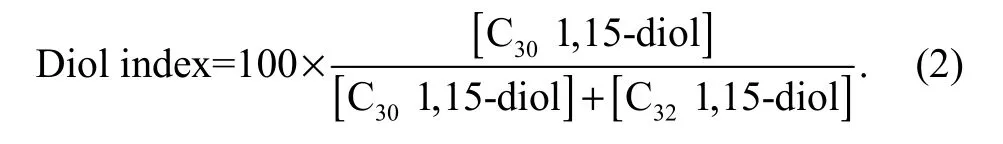
2.3 Data Analysis
The distribution contour graphs of measured parameters were plotted by using Surfer 10.0 (Golden Software).Pearson correlation analysis and principal component analysis were performed by using SPSS 19.0 (IBM SPSS software) to determine relationships between the measured parameters.
3 Results
3.1 Contents of Long Chain Alkyl Diols
As shown in Fig.2a, the content of C30diol ranged from 7 to 2726 ng g-1dry weight, with two obvious high values areas in the southern YS (SYS) central mud area and the other was in the northern YS (NYS) mud area.Low values were distributed in a broad region along the coast of the BS, YS and the ECS. Overall, it showed seaward increasing trend. The content of C32diol (Fig.2b)ranged from 5 to 669 ng g-1, with a spatial distribution pattern similar to that of C30diol. The average content of C30diols was 414 ± 498 ng g-1, which was 4 times higher than that of C32diol (104 ± 110 ng g-1) in eastern CMS sediments. It was clear that the sum of C30and C32diols(∑LD) was more abundant in the YS (ave. = 741 ± 767 ng g-1, n = 93) than that in the BS (ave. = 338 ± 175 ng g-1, n =29) and in the ECS (ave. = 254 ± 163 ng g-1, n = 59). The spatial distributions of C30and C32diols were broadly consistent with TOC content (Fig.2f), with the exception of coastal region along the Minzhe coastal mud area and near the Yellow River delta mud area where diol values were lower while TOC% values were relatively high.
The LDs contents were normalized to TOC to minimize the influences of sediment grain size, varying sedimentation rate and degradation on biomarker contents.The TOC normalized C30and C32diols content ranged from 9 to 419 (avg. = 97 ± 69) μg g-1and 4 to 102 (avg. =26 ± 16) μg g-1, respectively. They had a similar spatial pattern with an overall seaward increasing trend while high values areas were broadly from offshore Shandong Peninsula to outer shelf area in the YS and also in the outer shelf in the ECS (Figs.3a, 3b). Compared with content distribution pattern, the higher TOC normalized contents expanded to the northern part of the NYS, westward to the south of Shandong Peninsula in the SYS and occurred in outer shelf in the ECS. In addition, the normalized contents showed more clearly seaward increasing trend in eastern CMS.
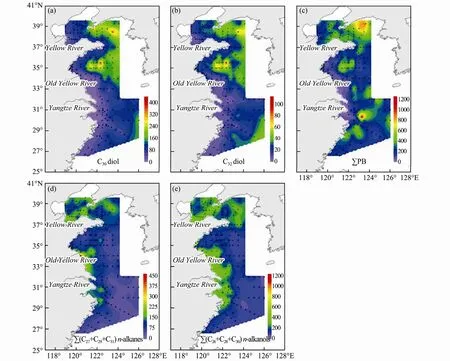
Fig.3 Spatial distributions of TOC-normalized biomarker contents (μg g-1 TOC) of (a) C30 diol; (b) C32 diol; (c) ∑PB; (d) ∑(C27 + C29 + C31) n-alkanes; (e) ∑ (C28 + C30 + C32) n-alkanols in eastern CMS.
3.2 Marine Biomarkers
The content of three marine phytoplankton biomarkers(∑PB, the sum of brassicasterol, dinosterol and C37alkenones) ranged from 29 to 5605 (avg. = 1034 ± 850) ng g-1, with high values in the NYS, the central SYS mud area, the Minzhe coastal area and the area outside the Yangtze River estuary (Fig.2c). The TOC normalized∑PB ranged from 31 to 1175 (avg. = 274 ± 164) μg g-1,with high values in the northern part of the NYS, south of Shandong Peninsula, an area outside the Yangtze River estuary (Fig.3c).
3.3 Terrestrial Biomarkers
The content of long chain odd carbon number n-alkanes (∑(C27+ C29+ C31)) and even carbon number n-alkanols (∑(C28+ C30+ C32)) ranged from 41 to 1714(avg. = 382 ± 262) ng g-1and 94 to 2367 (avg. = 772 ± 483)ng g-1respectively, both with high values near estuaries,in the NYS, the central SYS mud area and Minzhe coastal area (Figs.2d, 2e). The TOC normalized ∑(C27+ C29+C31) n-alkanes and ∑(C28+ C30+ C32) n-alkanols ranged from 30 to 456 (avg. = 104 ± 54) μg g-1and 62 to 1171 (avg.= 219 ± 125) μg g-1respectively, both with high values mainly near the estuaries and along the coast from the Old Yellow River to the Yangtze River estuary (Figs.3d,3e).
3.4 The Biomarker Based Proxies
TMBR values ranged from 0.1 to 0.9 (avg. = 0.3 ± 0.1)with higher values mainly near the estuaries (modern and old Yellow River and Yangtze River) and along the coast,and a clear offshore decreasing trend from the coast in the BS and YS (Fig.4a).
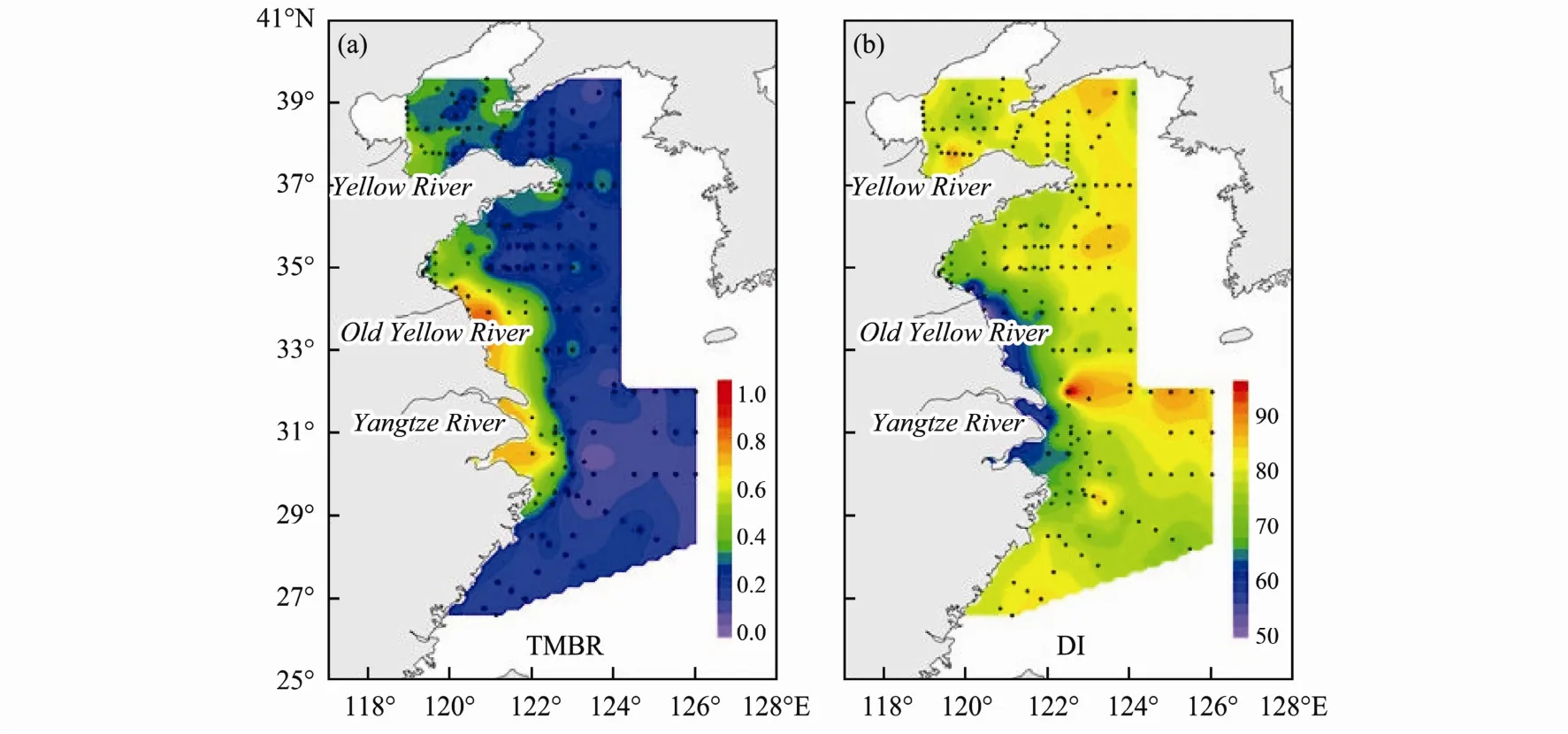
Fig.4 Spatial distribution of (a) TMBR; (b) Diol index proxies in eastern CMS.
Diol index (DI) varied from 48 to 89 with an average of 78 ± 6 (Fig.4b). The lowest values (< 67) were along the coast from the Old Yellow River to the Yangtze River.High values were in offshore sites in the YS and the ECS while in the BS high values occurred along the coast of the Shandong Peninsula. The general trend of diol index increased away from the coast in the YS and ECS.
4 Discussion
4.1 Spatial Distribution Patterns of Diols Revealing Potential Sources
The detection of C30and C321,15-diols in eastern CMS surface sediments with C30diol dominance was in agreement with previous studies in marine sediments (Versteegh et al., 1997). The contents of these diols were comparable with those in sediments from the Pearl River mouth basin (26- 4373 ng g-1dry weight, Zhu et al., 2014)and lacustrine sediments (Max. = 75 μg g-1TOC, Xu et al.,2007). The similar distribution pattern and strong linear correlation (Fig.5, R2= 0.96, p < 0.01) between C30and C32diols contents indicated that they had similar biological source in eastern CMS.
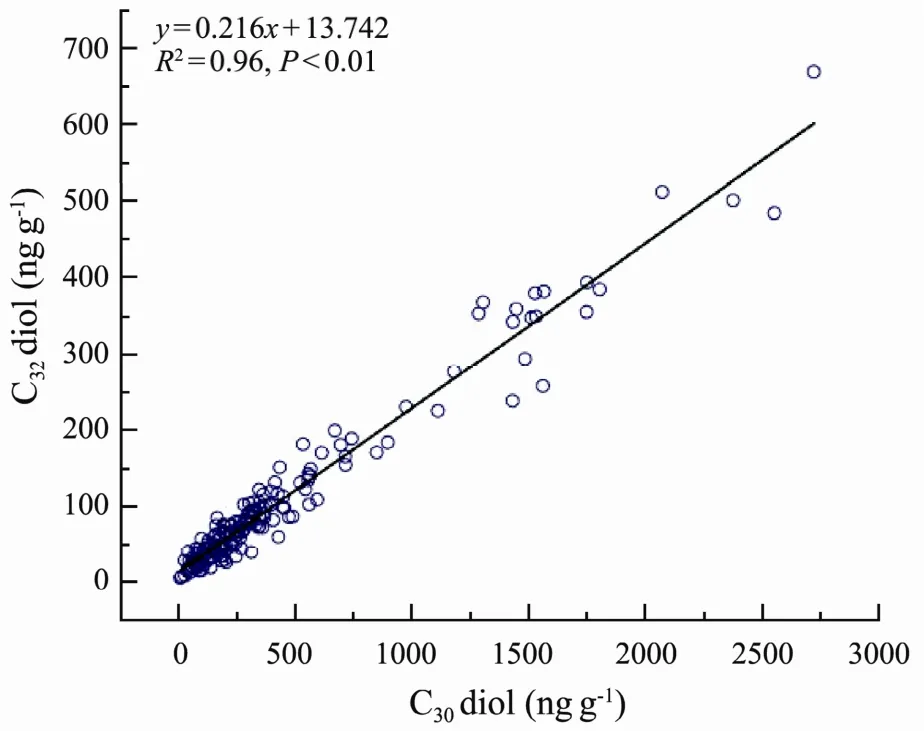
Fig.5 Correlation of contents of C30 and C32 diols in eastern CMS.
In marine system, brassicasterol (B), dinosterol (D) and C37alkenones (A) are major lipid biomarkers for diatoms,dino fl agellates and coccolithophorids respectively(Vlokman et al., 1998), and their contents and ratios in marine sediments can re fl ect the corresponding phytoplankton productivity and community structure in the euphotic layer (Zhao et al., 2006; Wu et al., 2015). Thus,the sum of these phytoplankton biomarkers (∑PB) are usually used as a proxy of marine productivity and marine organic matter (MOM) contribution. These phytoplankton biomarkers have been successfully applied to make palaeoenvironment reconstruction on productivity and community structure in eastern CMS from decadal to millennial timescales (Yuan et al., 2013; Duan et al.,2014). Long chain n-alkanes (C27, C29and C31) and nalkanols (C28, C30and C32) (Eglinton and Hamilton, 1967),have been widely used to trace terrestrial organic matter(TOM) input to marine sediments (Meyers, 1997; Xing et al., 2011). Previous studies have also confirmed that the C27, C29and C31n-alkanes and C28, C30and C32n-alkanols are useful TOM proxies in eastern CMS (Xing et al.,2011; Yuan et al., 2013). The mass normalized contents of TOM and MOM both had similar distribution pattern to the TOC% and had higher values in the mud area especially in the YS (Figs.2c-2f). The positive relationship between TOC% and grain size in the BS and YS suggested a general hydrodynamic constraint on organic carbon accumulation (Hu et al., 2011, 2016). After TOC normalization, the spatial distribution of marine biomarker content showed a pattern broadly opposite to that of terrestrial biomarker content (Figs.3c-3e). This different distribution pattern between TOM and MOM could also be reflected by the TMBR proxy. TMBR is the proxy based on the relative ratio of long chain odd alkanes and∑PB, which can quantify the percent of TOM in sedimentary OM (Xing et al., 2011a). Because the endmember of TMBR for TOM and MOM are 1 and 0 respectively, so the distribution pattern of TMBR could represent the distribution of %TOM. As shown in Fig.4a,TMBR showed a clear decreasing trend away from the coast under the influence of fluvial inputs. The spatial distribution of diol contents were similar to that of ∑PB(Figs.2a-2c), however, the TOC normalized diols contents showed more clearly offshore increasing trend than that of ∑PB (Figs.3a-3c).
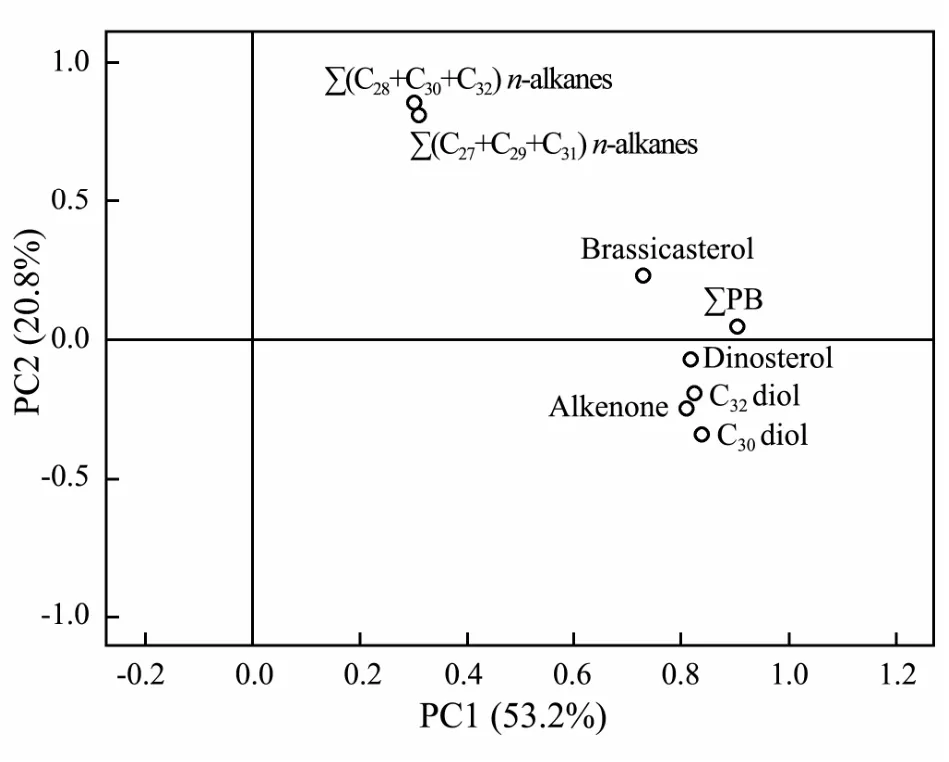
Fig.6 Plot of loadings on two principal components (PC1 and PC2).
Thus, to further evaluate the marine or terrestrial sources of C30and C32diols, principal component analysis (PCA) was performed on the datasets containing the TOC normalized contents of C30diol, C32diol, brassicasterol, dinosterol, C37alkenones, ∑PB, ∑(C27+ C29+C31) n-alkanes and ∑(C28+ C30+ C32) n-alkanols. As shown in Fig.6, two principal components (PC1 and PC2)explained 74.0% of the total variance. PC1 accounts for 53.2% of the total variance, with the high positive loadings of marine biomarker (brassicasterol, dinosterol, alkenones and ∑PB), suggesting that PC1 is a MOM indicator. PC2 accounts for 20.8% of the total variance, with high positive loadings of ∑(C27+ C29+ C31) n-alkanes and∑(C28+ C30+ C32) n-alkanols, which implied that PC2 is an indicator of TOM contribution. Because both C30and C32diol had high positive loadings on PC1 together with marine biomarkers, so this result strongly suggested the C30and C32diols were mainly from marine sources in eastern CMS. Although the LD distribution of cultured marine estigmatophytes is different from that in marine environment (Volkman et al., 1992), eustigmatophyte algae is the known potential source of both 1,13- and 1,15-diols (Volkman et al., 1992, 1999; Méjanelle et al.,2003). However, eustigmatophytes are rarely reported in the marine environment. A lacustrine sediment study founded that the δ13C value of C321,15-diol (-21.3‰)was substantially higher than those of the land plants produced long-chain n-alkanols (-29.6‰ ± 0.8‰) which suggested an autochthonous microbial source for the C32diol. But recent study in shelf seas with major river outflows revealed that the C321,15-diol was mainly produced in-situ in the river because of the high fractional abundance occurred in the river mouths and riverine suspended particulate matter and correlated strongly with the branched and isoprenoid tetraether (BIT) index which was used to trace the soil OM (de Bar et al., 2016; Lattaud et al., 2017). Since biomarker contents used in PCA have been normalized by TOC to minimize sedimentation rate and grain size effects, the sources should be the main factor on the spatial distributions of normalized biomarker contents. The offshore increasing diols distribution pattern in eastern CMS, and the significant Pearson correlations between the TOC normalized contents of∑LD and marine biomarkers (Table 1) further indicated that the C30and C321,15-diols in eastern CMS were mainly from marine algae. Further verification of their origins can be achieved by isotopic tracer measurements and laboratory cultures.
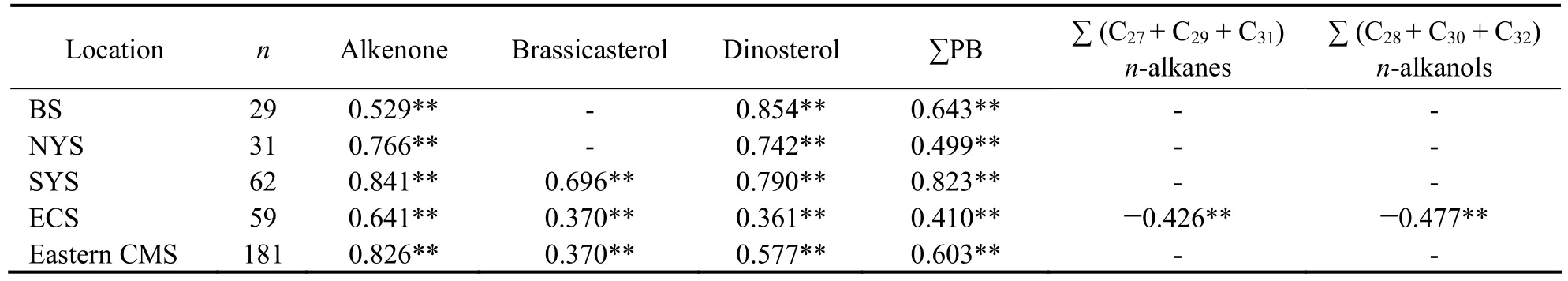
Table 1 Pearson correlations between the TOC normalized contents of ∑LD and biomarkers (marine and terrestrial)in different sections
4.2 Implications of Long Chain Diol Contents and Proxy
Because C30and C32diols had similar distribution pattern and source, so we use the sum of diols (∑LD) in the following discussion. ∑LD had comparable contents and significant positive correlation with marine biomarkers and ∑PB in eastern CMS (Table 1). The ratio of ∑LD/∑PB ranged from 0.1 to 1.8 (ave. = 0.5 ± 0.3) with higher values in the central and eastern SYS, south of the Shandong Peninsula, and the ECS outer shelf. The average∑LD/∑PB ratio was 0.4 ± 0.1 in the BS, 0.6 ± 0.3 in the YS and 0.3 ± 0.2 in the ECS. Therefore, the co-occurrence and good correlation of diols and marine productivity suggested diols could also represent the marine productivity, like those used in sediment cores (Li et al., 2014;Plancq et al., 2014). This also implied that combining the diols with other marine biomarkers could improve the understanding of marine productivity changes on both decadal and millennial timescales.
Although ∑LD had overall good correlation with other marine biomarkers and ∑PB, there were still some differences in specific subsection areas which indicated that there were some different factors controlling the distribu-tion of diols. In the BS and NYS, ∑LD had significant positive correlation with alkenone, dinosterol and ∑PB but not with brassicasterol (Table 1). Previous studies also found high brassicasterol content near estuaries and coastal area under the influence of riverine silicate to support diatom growth (Xing et al., 2016). ∑LD in the SYS had the higher Pearson correlation values with all marine biomarkers than other subsection areas (Table 1)and higher content values were found in the mud area.Marine organic matter had been suggested to be the major component of TOC in SYS especially the central basin with higher primary production and good preservation of organic matter (Xing et al., 2011b, 2014). Therefore, like other marine biomarkers, the high content of diols in the SYS was not only related to high productivity but also related to the high clay content which enhanced the preservation. Compared with those in the BS and YS, ∑LD in the ECS also had significant positive correlation with marine biomarkers but lower Pearson correlation value(Table 1). It is important to note that ∑LD had significant negative correlation with the two sets of terrestrial biomarkers. Under the direct influence of river discharge and hydrodynamic settings, TOM mainly accumulated in the coastal area and showed a rapidly decreasing trend offshore (Xing et al., 2011a; Zhu et al., 2013) which was in contrast with ∑LD. Thus, the different distribution pattern resulted in the negative relationship. The distribution of diols showed a broadly seaward increasing trend similar to that of alkenones (R = 0.641, p < 0.01), indicating 1,15-diols might be partially controlled by salinity in this area.
Diol index (DI) based on C30and C321,15-diols was first proposed to relate to salinity (Versteegh et al., 1997)and then used as indicators for tracing past sea surface water masses (Versteegh et al., 2000). However, little is known about the environmental controls on DI in eastern CMS. Thus, we compare these data with environmental parameters of the overlying surface waters such as annual sea surface temperature (SST), salinity (SSS) (data from the 1˚×1˚ grid 2009 WOA). Fig.7 clearly reveals the dramatically different distribution pattern between SST and DI, which implied that the SST was not the main factors controlling the distribution of DI in eastern CMS. For the SSS, only lower values near the Yangtze River estuary was similar to that of DI. The lower DI were along the coast from the Old Yellow River to the Yangtze River which implied the DI might be influenced by salinity;however, there was not the case near the Yellow River estuary. Based on the TMBR distribution pattern (Fig.4a),the higher values were also along the coast from Old Yellow River to Yangtze River. Thus, the lower DI might be most influenced by the terrestrial riverine input other than the salinity. Further study is needed to investigate how the environmental factors controlling on the proxies based on diol homologs.
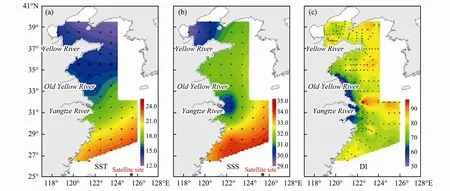
Fig.7 Distribution of 10-year annual average (a) SST, (b) SSS and (c) DI in the eastern CMS.
5 Conclusions
This study provides a comprehensive investigation of C30and C321,15-alkyl diols distribution patterns in eastern CMS by analyzing 181 surface sediment samples. The major conclusions are:
1) The contents of C30and C321,15-diols in eastern CMS ranged from 7 to 2726 ng g-1and 5 to 669 ng g-1dry weight, respectively. Their distribution both revealed a clear spatial trend with low values near the coasts and increasing seaward. Overall, diols were more abundant in the YS than those in the BS and ECS.
2) The similar distribution pattern between diols and the other well-known marine biomarkers indicated C30and C32diols in eastern CMS were mainly from marine algae, and this conclusion was further supported by PCA analysis.
3) Sediment diols contents were generally related to marine productivity, which suggested that they could be applied for marine productivity reconstruction.
4) Diol index based on C30and C321,15-diols was not applicable as indicators for SST and SSS in eastern CMS.The DI might be most influenced by the terrestrial inputs along the coast from Old Yellow River to Yangtze River.
This study laid the foundation of further diols study in eastern CMS. Future work is needed to provide detailed information about diol homologue distributions, which can be better used for plaleo-environmental reconstruction.
Acknowledgements
We would like to thank Drs. Yali Cao, Jiaokai Wang,Xingchen Wang, Lei Xing and Zongshan Zhao for help with sample analysis and for constructive suggestion on the manuscript. This study was supported by the National Natural Science Foundation of China (Nos. 41521064 and 41630966). This is MCTL (Key Laboratory of Marine Chemistry Theory and Technology) contribution #155.
杂志排行
Journal of Ocean University of China的其它文章
- Effect of Different Dietary Protein and Lipid Levels on the Growth, Body Composition, and Intestinal Digestive Enzyme Activities of Juvenile Yellow Drum Nibea albiflora (Richardson)
- Modelling Wave Transmission and Overtopping Based on Energy Balance Equation
- Extreme Sea Level Rise off the Northwest Coast of the South China Sea in 2012
- Spatial Distribution and Seasonal Variation of Explosive Cyclones over the North Atlantic
- The Influence of Two Kinds of El Niño Events on the Strong Tropical Cyclone Generation and Strength in the Pacific Ocean
- Sea State Bias Estimation with Least Absolute Shrinkage and Selection Operator (LASSO)
