The trend of change in catheter ablation versus antiarrhythmic drugs for the management of atrial fibrillation over time: a meta-analysis and meta-regression
2018-08-17WeiLIUQiangWUXiaoJieYANGJingHuang
Wei LIU, Qiang WU, Xiao-Jie YANG, Jing Huang
The trend of change in catheter ablation versus antiarrhythmic drugs for the management of atrial fibrillation over time: a meta-analysis and meta-regression
Wei LIU1, Qiang WU1, Xiao-Jie YANG2, Jing Huang1
1Department of Cardiology, Guizhou Provincial People’s Hospital, Guiyang, China2Department of Endocrinology, Guizhou Provincial People’s Hospital, Guiyang, China
To evaluate the trend of change in the efficacy and safety of catheter ablation compared with antiarrhythmic drug therapy (ADT) for rhythm control in patients with atrial fibrillation (AF) over time.The online databases PubMed and EMBASE were searched for relevant studies. STATA software (version 12.0) was used to perform the meta-analysis and meta-regression.Fifteen randomized controlled trials including 2249 patients with AF were identified. The pooled results showed that catheter ablation was associated with a 52% reduction in the risk of AF recurrence compared with ADT [risk ratio (RR) = 0.48, 95% confidence interval (CI): 0.40-0.57,2= 70.7%). Subgroup analyses showed that catheter ablation exhibited less efficacy in studies after 2011 compared to studies before 2011 (RR = 0.61, 95% CI: 0.54-0.68,2= 9.3% and RR = 0.34, 95% CI: 0.24-0.47,2= 69.9%, respectively), and the safety outcome showed a 1.08-fold higher incidence of adverse events (14.2%. 7.3%; RR = 1.08, 95% CI: 1.04–1.13) in studies after 2011.Catheter ablation appears to be superior to ADT for rhythm control. However, less efficacy and a higher rate of adverse events were observed in studies after 2011 compared to studies before 2011.
J Geriatr Cardiol 2018; 15: 441450. doi:10.11909/j.issn.1671-5411.2018.06.011
Antiarrhythmic drug therapy; Atrial fibrillation; Catheter ablation; Rhythm
1 Introduction
Atrial fibrillation (AF) is the most common type of cardiac arrhythmia in clinical practice, and it increases in prevalence with advancing age.[1]Furthermore, AF is also related to various clinical events, such as frequent hospitalizations, hemodynamic abnormalities and stroke, and results in significant morbidity and mortality.[2]
Rhythm control is a strategy for the management of AF, and it has the potential to restore and maintain sinus rhythm.[3]Antiarrhythmic drug therapy (ADT) and catheter ablation are commonly used strategies for rhythm control.[3]Currently, the guidelines suggest ADT as the first-line therapy when rhythm control is desired.[3,4]Nonetheless, the efficacy and safety of ADT remain an area of immense concern due to high rates of AF recurrence and long-term adverse drug reactions, which may mask the benefits of maintaining sinus rhythm.[5]Catheter ablation has been recognized as an alternative therapeutic modality for patients with AF refractory or intolerant to at least one class I or III antiarrhythmic medication in the recent guidelines.[3]Several clinical trials have compared the efficacy of ADT and catheter ablation on rhythm control in patients with AF.[6–10]However, there was a very confusing phenomenon in which early trials seemed to report higher success rates for catheter ablation than later studies.[6,7,10,11]Previous reviews that mainly included early studies also showed inconsistent results between catheter ablation and ADT, especially regarding the safety outcomes.[12,13]However, the different reports on efficacy and safety by early and later studies have not yet been completely investigated.
Therefore, we performed a systematic review and meta- analysis with more evidence from available randomized controlled trials (RCTs) to evaluate the trend of change in the efficacy and safety of catheter ablation compared with those of ADT for rhythm control in patients with AF over time.
2 Methods
2.1 Search strategy
The PubMed and EMBASE online databases were searched (up to March 12, 2017) to identify all publications associated with catheter ablation and ADT for rhythm control in patients with AF. The following terms were used with proper logical connectors: “rhythm”, “ablation”, “antiarrhythmics”, “amiodarone”, “randomized”, “randomised”, “randomly” and “atrial fibrillation”. Additionally, a manual search was performed by scanning the references of the identified articles to find studies that may have been missed by the electronic searches.
2.2 Study selection and data collection
The inclusion criteria of the present systematic review and meta-analysis were as follows: (1) the study must be a RCT; (2) patients were diagnosed with persistent or paroxysmal AF; (3) the studies compared ADT with catheter ablation techniques; and (4) relevant outcome data to be assessed in this systematic review and meta-analysis were reported in the article.
The article selection was performed strictly in compliance with the inclusion criteria. Two authors (LIU W and WU Q) independently assessed all potentially relevant studies. The selection process was carried out by crude screening of the title and abstract to exclude most of the irrelevant studies, and the full-texts of the remaining studies were examined twice to reach a final decision. A consensus was reached between the two screening authors on all eligible studies. Any discrepancies were resolved by discussion.
Two authors (LIU W and YANG XY) independently extracted all relevant information from eligible studies. A prespecified table containing the relevant items was used to help with data collection.
2.3 Endpoints
In the present systematic review and meta-analysis, efficacy and safety outcomes were analyzed to assess the differences of catheter ablation and ADT on rhythm control in patients with AF. The efficacy analysis was based on AF recurrence, and the safety outcome was based on complications and adverse events.
2.4 Evaluation of study quality and publication bias
The quality of the included studies was evaluated by the Jadad scale.[14]The Jadad scale consists of three items pertaining to descriptions of randomization (0–2 points), double blinding (0–2 points), and dropouts and withdrawals (0–1 point) for a total score of five, with a higher score indicating better quality. Trials scored 3 or greater were considered to be high quality.[14]
Publication bias was evaluated by Egger’s tests. In addition, a funnel plot was generated to visually inspect the symmetry.
2.5 Data synthesis and statistical analysis
We separately conducted meta-analyses on the efficacy and safety of catheter ablation and ADT. The2statistic was used to test statistical heterogeneity, with values > 50% representing important heterogeneity. A random-effects model was used to pool the effect sizes in all of the meta- analyses.
For the efficacy analysis, the risk ratio (RR) was calculated as the effect size. Subgroup analysis was performed to evaluate the efficacy of catheter ablation as a first- or second-line therapy. The role of study-level and aggregated individual-level parameters that might affect heterogeneity was assessed by meta-regression. The potential factors provided were types of AF (paroxysmal versus persistent), study design (single-center RCT versus multi-center RCT), intention-to-treat (ITT) analysis (ITT. non-ITT analysis studies), duration of follow-up (≤ 1 year. > 1 year), ablation approach [pulmonary vein isolation (PVI) only versus PVI + adjunctive ablation], year of publication (before 2011 versus after 2011) and electrical cardioversion during the blanking period. For the safety analysis, risk differences (RDs) were calculated to assess the differences in complications and adverse events between catheter ablation and ADT. Subgroup analyses were performed to compare studies before 2011 and studies after 2011.
The present systematic review and meta-analysis was performed in compliance with the recommendations of the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).[15]All meta-analyses in the present study were pooled in accordance with the[16]All analyses were conducted using STATA software (version 12.0).
3 Results
Figure 1 details the study search and selection process. A total of 525 potential literature citations were identified through a systematic search. Finally, fifteen randomized trials with 2249 patients were included in this systematic review and meta-analysis.[6–11,17–25]Among these 15 trials, four were conducted at a single center,[7,18,22,25]while the remaining 11 trials were performed at multiple centers.[6,8–11,17,19–21,23,24]The year of publication of these trials ranged from 2003 to 2016, with 8 trials published before 2011 and 7 trials after 2011. Seven trials focused on patients with paroxysmal AF, five trials enrolled patients with persistent AF, and three trials included both paroxysmal and persistent AF patients. Adjunctive ablations, such as linear atrial lesions, and complex fractionated electrogram abla- tions were used in almost all the trials, depending on the investigators’ decision, except for the earliest two trials.[11,25]The follow-up duration was 12 months or more in thirteen of the included trials, except for studies from Hummel,.[8]and Wilber,[20]ITT analysis was used in 13 out of 15 trials. Tables 1 and 2 show the baseline characteristics of the included studies.
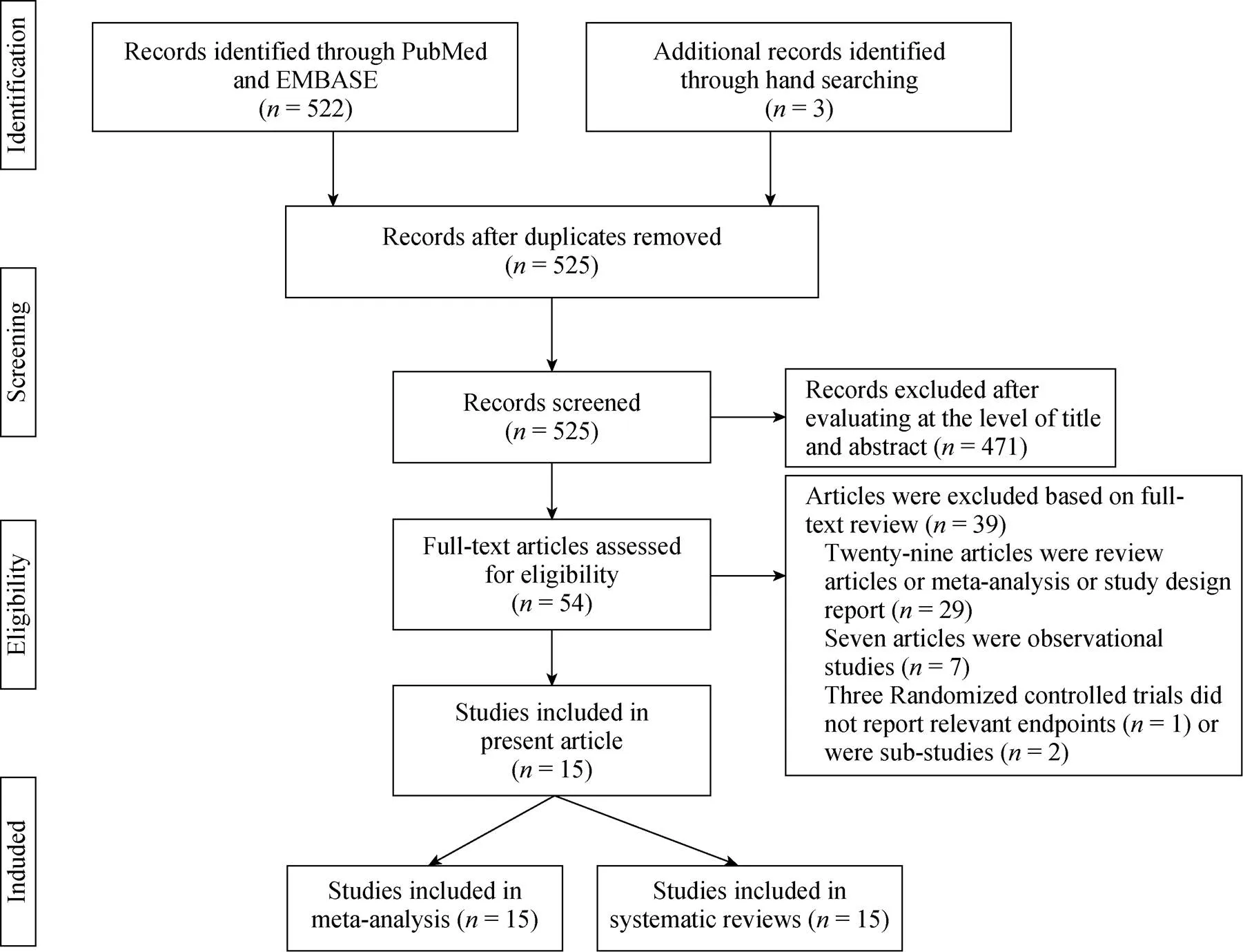
Figure 1. Flow chart of study selection.
3.1 The quality of included studies
Table 2 provides a detailed assessment of study quality. Notably, all included studies were open-label designs. Based on the Jadad score, 11 studies with a score of three were high quality, while the other four studies with a score of two were low quality.
3.2 AF recurrence
Fifteen studies compared AF recurrence between patients treated with catheter ablation and those treated with ADT. A total of 2249 patients were included in these studies, of which 1209 and 1040 patients were in the catheter ablation and ADT groups, respectively. The incidence of AF recurrence was 31.6% in patients who underwent catheter ablation and 63.0% in patients who underwent ADT. Catheter ablation was associated with a significantly lower risk of AF recurrence, as shown by the pooled results using a random-effects model [pooled RR = 0.48, 95% confidence interval (CI): 0.40-0.57,2=70.7%; Figure 2].
Subgroup analysis showed that there was a 50% reduction in the risk of AF recurrence in patients who underwent catheter ablation as a first-line therapy compared with patients who underwent ADT (pooled RR = 0.50, 95% CI: 0.34-0.75,2= 72.3%). In addition, our meta-analysis revealed a 54% reduction in the risk of AF recurrence in patients who underwent catheter ablation as a second-line therapy (pooled RR = 0.46, 95% CI: 0.37-0.57,2= 72.2%) compared to patients who underwent ADT.
3.2.1 Meta-regression
The2test revealed significant heterogeneity among the studies. Therefore, we performed a meta-regression to explore the source of heterogeneity. Meta-regression showed that year of publication had a significant effect on the observed heterogeneity (= 0.014, adjusted2= 55.41%; Figure 3). There were eight studies published before 2011 and seven studies published after 2011.
In the studies before 2011, the rates of AF recurrence were 25.4% and 70.7% in the catheter ablation and ADT groups, respectively. The pooled result showed a 66% reduction in AF recurrence in the catheter ablation group compared with the ADT group with significant heterogeneity (pooled RR = 0.34, 95% CI: 0.24–0.47,2= 69.9%; Figure 4).

Table 1. Baseline characteristics of the patients in the included studies.
ADT: antiarrhythmic drug therapy; AF: atrial fibrillation; CA: catheter ablation; CHD: coronary heart disease; EF: ejection fraction; HTN: hypertension; LAD: left atrial diameter; LVEF: left ventricular ejection fraction; NR: not reported.*Combined data for structural heart disease and hypertension.**Mean LVEF was not provided, all patients had an EF > 40% with majority having an EF > 60%.
In studies after 2011, there was still a 39% reduction in the risk of AF recurrence following catheter ablation compared with that following ADT (pooled RR = 0.61, 95% CI: 0.54–0.68,2= 9.3%). There was also nonsignificant between-study heterogeneity (2= 9.3%,= 0.358; Figure 4). The effect on AF recurrence was less apparent in studies after 2011 compared with studies before 2011.
The funnel plot and Egger’s test did not reveal any presence of publication bias (= 0.982).
3.3 Safety outcome
The incidence of complications and adverse events varied among studies, with the highest rate of complications and adverse events in the study by Krittayaphong,.[25]Pooled data did not indicate any significant difference in the incidence of complications and adverse events between the ablation group and the ADT group (RD = –0.00, 95% CI: –0.04 to 0.04; Figure 5). Notably, sensitivity analysis indicated a 1.08-fold increase in the incidence of complications and adverse events in patients who underwent catheter ablation in studies after 2011 when compared with studies before 2011 (14.2%. 7.3%, RR = 1.08, 95% CI: 1.04–1.13).

Table 2. Characteristics and quality of the included studies.
*The Jadad scale scores a maximum of five, with a higher score indicating better quality. Trials scored 3 or more are considered to be high-quality. ADT: antiarrhythmic drug therapy; CA: catheter ablation; CFAE: complex fractionated atrial electrograms; ECV: electrical cardioversion; ITT: intention-to-treat; PVI: circumferential pulmonary-vein isolation.
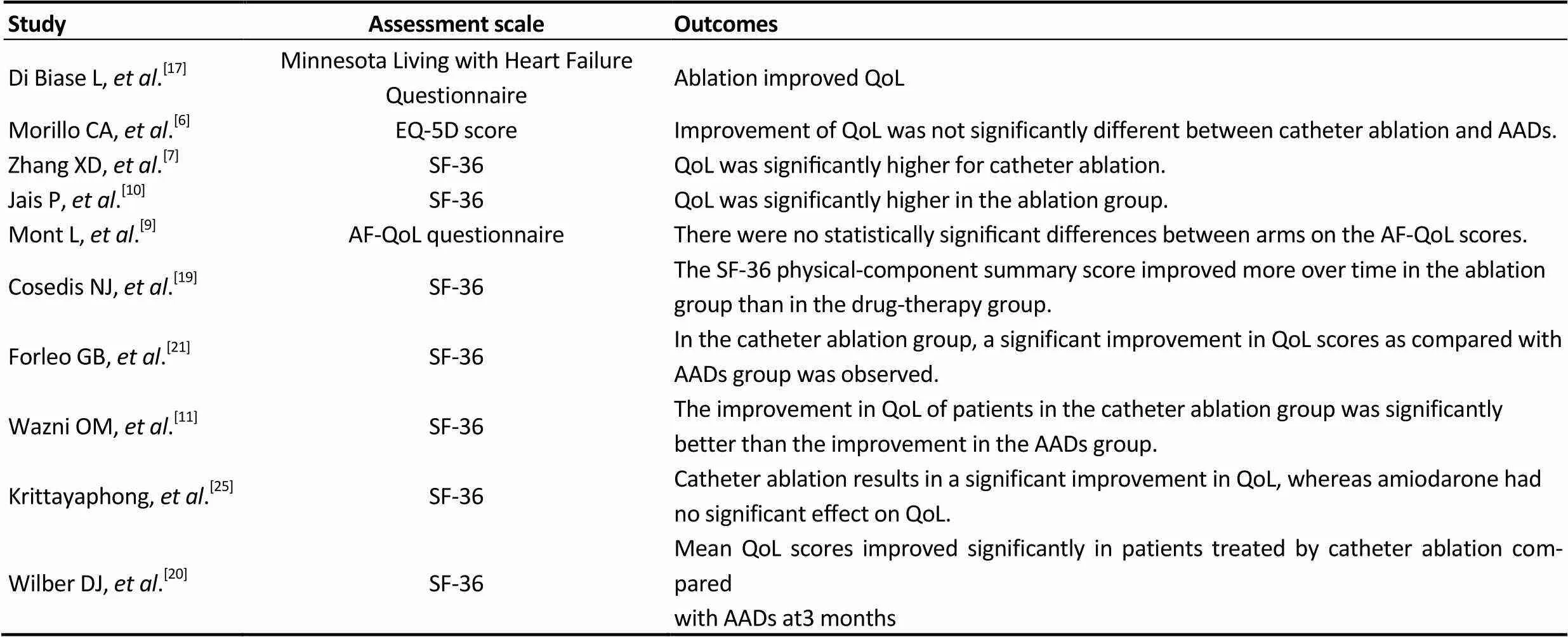
Table 3. Summary of the quality of life.
AF-QoL questionnaire: atrial fibrillation quality of life questionnaire; QoL: quality of life; SF-36: the medical outcomes study short-form36 health survey.

Figure 2. The pooled outcome of AF recurrence. Subgroup analysis was performed based on catheter ablation as a first- or second-line therapy. ADT: antiarrhythmic drug therapy; AF: atrial fibrillation; CA: catheter ablation.
Figure 3. Meta-regression of included studies based on the year of publication.
4 Discussion
In the present systematic review and meta-analysis, a total of 15 RCTs with 2249 patients were included. Our result showed that compared with ADT, catheter ablation was associated with a 52% reduction in the recurrence of AF. This result was stable when catheter ablation was considered both as a first- and second-line therapy. Notably, the efficacy was less apparent in studies after 2011, with a 1.08-fold higher rate of adverse events compared to studies before 2011. This difference might indicate that the efficacy was exaggerated and that the adverse events were overlooked among patients who underwent catheter ablation in studies before 2011.
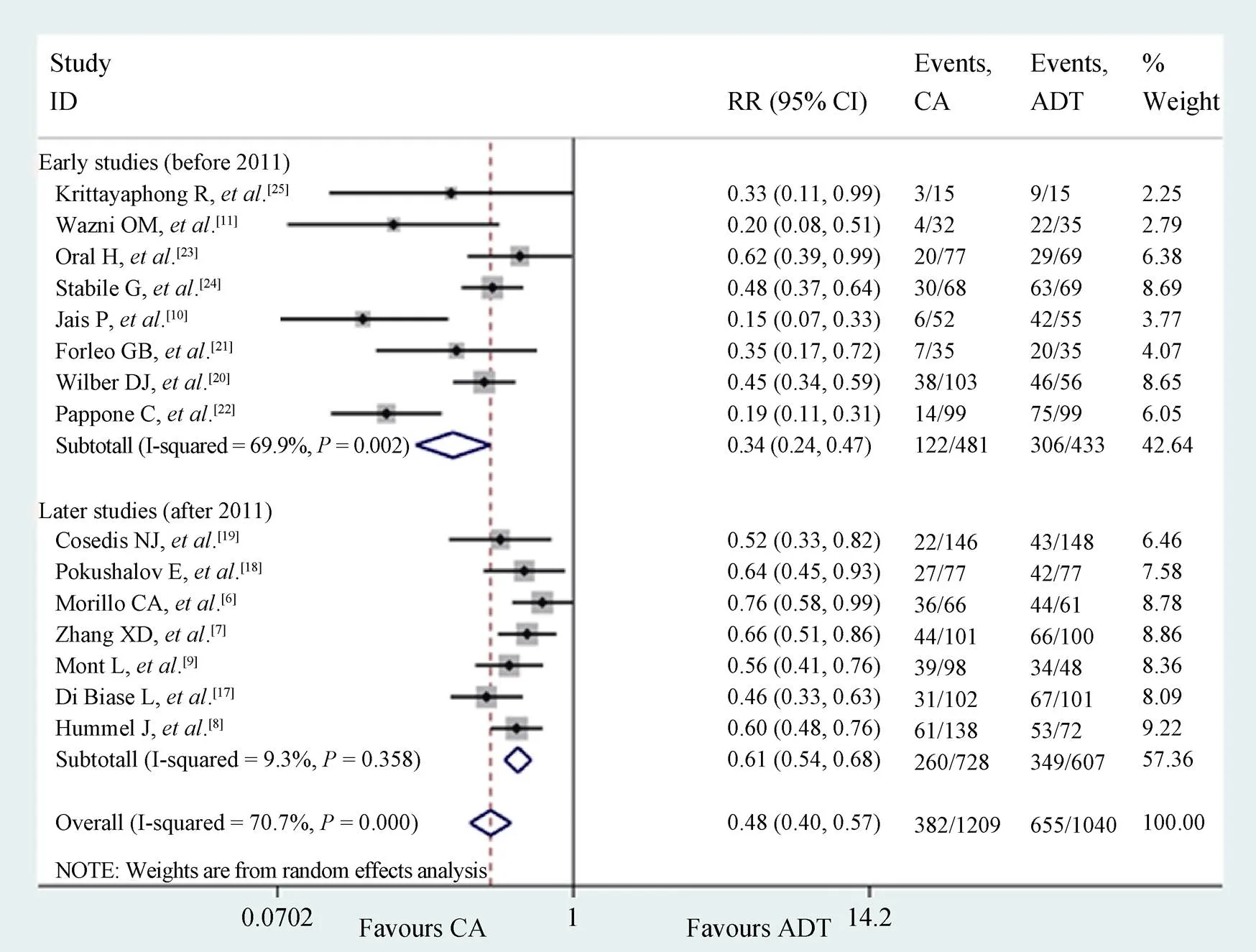
Figure 4. The pooled outcome of AF recurrence. Subgroup analysis based on the year of publication. ADT: antiarrhythmic drug therapy; AF: atrial fibrillation; CA: catheter ablation.
Figure 5. The pooled outcome of complications and adverse events between catheter ablation and ADT groups. Subgroup analysis was performed based on the year of publication. ADT: antiarrhythmic drug therapy; CA: catheter ablation.
However, when interpreting the results of the meta- analysis, we should note that there were obvious differences in the following factors among the studies. First, the types of AF were different among the 15 included trials: seven trials focused on patients with paroxysmal AF, five trials enrolled patients with persistent AF, and three trials included both paroxysmal and persistent AF patients. In addition, the study by Di Biase,.[17]included persistent AF patients with congestive heart failure. Some studies reported that both catheter ablation and ADT were more effective for paroxysmal AF than for persistent AF.[26–28]Therefore, the types of AF might influence the results of our meta-analysis. Second, the ablation methods were diverse among studies. All the trials used PVI as the endpoint of the ablation procedure. However, there were differences in adjunctive ablation strategies (linear lines and sources of complex fractionated electrograms). Several studies have shown that adjunctive ablation strategies might affect the efficacy of ablation,[29,30]but recently, a large RCT indicated that there was no difference in the rate of AF recurrence when either linear ablation or complex fractionated electrogram ablation was performed in addition to PVI.[31]Therefore, the impact of ablation strategies has not been completely and systematically evaluated. Third, catheter ablation was performed as a first-line therapy in four trials and as a second-line therapy in 11 trials. Currently, catheter ablation is recommended by guidelines as a second-line therapy for patients with AF after treatment with at least one antiarrhythmic drug has failed.[3,4]At present, no study has directly compared first- line therapy with second-line therapy. An indirect comparison from a recent meta-analysis showed comparable results between first-line therapy and second-line therapy.[13]In the present meta-analysis, we also performed subgroup analyses to compare the different effects of catheter ablation treated as a first- or second-line therapy. The pooled data showed that catheter ablation as both a first- and second-line therapy was associated with a lower incidence of AF recurrence compared with ADT. In addition, different study designs and statistical analyses were used in the included studies (single-center versus multicenter studies, ITT analysis versus non-ITT analysis). These differences among studies might also impact the results. However, their influence cannot be completely and quantitatively evaluated in the present study.
These abovementioned differences were reflected in the present study as significant heterogeneity among studies. Therefore, we performed a meta-regression to explore the source of heterogeneity. Meta-regression did not reveal any significant effect from the types of AF, ablation strategies, study design, indication and ITT analysis on the observed heterogeneity (data not shown). However, the year of publication was observed to significantly affect heterogeneity. In studies after 2011, the heterogeneity was nonsignificant. Pooled results showed that studies after 2011 seemed to exhibit less efficacy with an increased trend of adverse events compared to early studies before 2011. The reasons for the differences between studies before and after 2011 were unclear. The possible reasons may be as follows: (1) as the recognition on catheter ablation was more and more sober, the nocebo effects were gradually decreased.[32]Similar to renal denervation, the early studies showed better results than the later studies.[32]A sham control trial may be required to further verify the result. (2) the supervision and study designs improved and more cases of recurrent AF, complications and adverse events were adjudicated in the later studies. In the present study, studies before 2011 showed an AF recurrent rate of 25.4% among patients who underwent catheter ablation, whereas studies after 2011 showed an AF recurrent rate of 35.7%. The rate of AF recurrence after ablation from studies after 2011 is closer to 40%-50%, as reported in a recent study.[31]These differences between early and later studies reflect advanced awareness of catheter ablation and reveal that catheter ablation is not as effective as previously thought. The actual efficacy and safety of catheter ablation still requires verification by double-blinded RCTs.
4.1 Limitations
Although our study included only prospective RCTs, there were also some important limitations. (1) The present systematic review and meta-analysis included studies that differed in the types of AF evaluated as well as antiarrhythmic drugs, catheter ablation approaches, definitions of AF recurrence, study designs, indications and the methods of surveillance used. These factors may have had an impact on the pooled effect estimate. (2) In most of the included studies, the adherence to ADT is unclear. Medication adherence is known as a critical factor that affects treatment outcomes. This may have influenced the results of our analyses but cannot be systematically evaluated. (3) In all the included trials, no sham procedures were used. Placebo and nocebo effects from catheter ablation and ADT may affect the results of the present study. A recent editorial by Ozeke.[32]raised the question and systematically elaborated the theory regarding placebo and nocebo effects from catheter ablation of AF;[32]and (4) the inherent limitations of meta-analyses cannot be ignored, such as publication bias. Although no obvious publication bias was observed from the funnel plot and Egger’s test, bias cannot be ruled out entirely.
4.2 Conclusions
Catheter ablation may be superior for rhythm control, as it is not associated with increased complications and adverse events compared with ADT. Subgroup analyses indicated stable results when catheter ablation was considered both as a first- and second-line therapy. Subgroup analyses showed that studies after 2011 with non-significant heterogeneity also showed a lower risk of AF recurrence in the catheter ablation group, but the effect appeared to be smaller than that in studies before 2011. Future high-quality, large-sample trials with sham control groups are required to verify these findings.
Acknowledgements
The author states that there is no conflict of interest. This work was supported by grants from the National Clinical Key Specialty Construction Project of China [No. (2013) 544] and Clinical Research Center Project of the Department of Science and Technology of Guizhou Province [No. (2017) 5405].
1 Lloyd-Jones DM, Wang TJ, Leip EP,. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study.2004; 110: 1042–1046.
2 Kannel WB, Wolf P A, Benjamin EJ,. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates.1998; 82: 2N–9N.
3 January CT, Wann LS, Alpert JS,. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society.2014; 130: e199–e267.
4 Camm AJ, Lip GY, De Caterina R,. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation--developed with the special contribution of the European Heart Rhythm Association.2012; 14: 1385–1413.
5 Wyse DG, Waldo AL, Dimarco JP,. A comparison of rate control and rhythm control in patients with atrial fibrillation.2002; 347: 1825–1833.
6 Morillo CA, Verma A, Connolly SJ,. Radiofrequency ablationantiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial.2014; 311: 692–700.
7 Zhang XD, Gu J, Jiang WF,. Optimal rhythm-control strategy for recurrent atrial tachycardia after catheter ablation of persistent atrial fibrillation: a randomized clinical trial.2014; 35: 1327–1334.
8 Hummel J, Michaud G, Hoyt R,. Phased RF ablation in persistent atrial fibrillation.2014; 11: 202–209.
9 Mont L, Bisbal F, Hernandez-Madrid A,. Catheter ablation. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study).2014; 35: 501–507.
10 Jais P, Cauchemez B, Macle L,. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study.2008; 118: 2498–2505.
11 Wazni OM, Marrouche NF, Martin DO,. Radiofrequency ablationantiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial.2005; 293: 2634–2640.
12 Cheng X, Li X, He Y,. Catheter ablation versus anti- arrhythmic drug therapy for the management of a trial fibrillation: a meta-analysis.2014; 41: 267–272.
13 Khan A R, Khan S, Sheikh MA,. Catheter ablation and antiarrhythmic drug therapy as first- or second-line therapy in the management of atrial fibrillation: systematic review and meta-analysis.2014; 7: 853–860.
14 Jadad AR, Moore RA, Carroll D,. Assessing the quality of reports of randomized clinical trials: is blinding necessary?1996; 17: 1–12.
15 Moher D, Liberati A, Tetzlaff J,. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.2009; 151: 264–269, W64.
16 Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). http://handbook-5–1. cochrane.org/ (accessed on July 28, 2017).
17 Di Biase L, Mohanty P, Mohanty S,. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial.2016; 133: 1637–1644.
18 Pokushalov E, Romanov A, De Melis M,. Progression of atrial fibrillation after a failed initial ablation procedure in patients with paroxysmal atrial fibrillation: a randomized comparison of drug therapy versus reablation.2013; 6: 754–760.
19 Cosedis NJ, Johannessen A, Raatikainen P,. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation.2012, 367: 1587–1595.
20 Wilber DJ, Pappone C, Neuzil P,. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial.2010; 303: 333–340.
21 Forleo GB, Mantica M, De Luca L,. Catheter ablation of atrial fibrillation in patients with diabetes mellitus type 2: results from a randomized study comparing pulmonary vein isolation versus antiarrhythmic drug therapy.2009; 20: 22–28.
22 Pappone C, Augello G, Sala S,. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study.2006; 48: 2340-2347.
23 Oral H, Pappone C, Chugh A,. Circumferential pulmonary-vein ablation for chronic atrial fibrillation.2006; 354: 934–941.
24 Stabile G, Bertaglia E, Senatore G,. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study).2006; 27: 216–221.
25 Krittayaphong R, Raungrattanaamporn O, Bhuripanyo K,. A randomized clinical trial of the efficacy of radiofrequency catheter ablation and amiodarone in the treatment of symptomatic atrial fibrillation.2003; 86 (Suppl 1): S8-S16.
26 Parkash R, Verma A, Tang AS. Persistent atrial fibrillation: current approach and controversies.2010; 25: 1–7.
27 Brooks A G, Stiles MK, Laborderie J,. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review.2010; 7: 835–846.
28 Arbelo E, Brugada J, Hindricks G,. The atrial fibrillation ablation pilot study: a European Survey on Methodology and results of catheter ablation for atrial fibrillation conducted by the European Heart Rhythm Association.2014, 35: 1466–1478.
29 Elayi CS, Verma A, Di Biase L,. Ablation for longstanding permanent atrial fibrillation: results from a randomized study comparing three different strategies.2008; 5: 1658–1664.
30 Willems S, Klemm H, Rostock T,. Substrate modification combined with pulmonary vein isolation improves outcome of catheter ablation in patients with persistent atrial fibrillation: a prospective randomized comparison.2006; 27: 2871–2878.
31 Verma A, Jiang CY, Betts TR,. Approaches to catheter ablation for persistent atrial fibrillation.2015; 372: 1812–1822.
32 Ozeke O, Cay S, Ozcan F,. Similarities between the renal artery and pulmonary vein denervation trials: do we have to use sham procedures for atrial fibrillation catheter ablation trials?2016; 211: 55–57.
Qiang WU, MD, Department of Cardiology, Guizhou Provincial People’s Hospital, East Zhongshan Road No. 83, Guiyang, China. E-mail: gzgywq@126.com
November 25, 2017
February 27, 2018
March 20, 2018
June 28, 2018
杂志排行
Journal of Geriatric Cardiology的其它文章
- “Malignant” right coronary artery presenting as an ST-segment elevation myocardial infarction—a case report
- Influenza vaccination in acute coronary syndromes patients in Thailand: the cost-effectiveness analysis of the prevention for cardiovascular events and pneumonia
- Early mortality and safety after transcatheter aortic valve replacement using the SAPIEN 3 in nonagenarians
- Depression and chronic heart failure in the elderly: an intriguing relationship
- CIED implantation in elderly patients: a single-center experience
- Use of the reported Edmonton frail scale in the assessment of patients for transcatheter aortic valve replacement: a possible selection tool in very high-risk patients?
